Abstract
Background
The association between fetal growth restriction (FGR) and childhood neurodevelopmental delay is unclear and the evidence available to the present date shows conflicting results. Our aim was to analyse the impact of early-onset FGR on the neurodevelopmental outcome at 24 months of corrected age in very preterm infants.
Methods
Retrospective cohort study of very preterm infants (≤ 32 weeks’ gestation) admitted to a neonatal intensive care unit between 1 January 2013–31 December 2019. The control group comprised appropriate for gestational age (AGA) newborns. Griffiths III Mental Development Scale was performed at 24 months of corrected age.
Results
132 infants were included: 44 FGR and 88 AGA. Mean Global Development Quotient (GDQ) was lower for FGR infants (p = 0.004) even after adjusting for maternal and perinatal factors (βadjusted -16.703; p = 0.009). The average scores for the neurodevelopmental domains were highest for personal-social-emotional skills (107.02 ± 16.34), followed by eye/hand coordination (105.61 ± 14.20) and foundation of learning skills (102.23 ± 13.74) and were lowest for gross motor (97.90 ± 11.88) and language/communication skills (96.39 ± 18.88). FGR had a significant negative impact on all domains except for gross motor skills. After adjustment, FGR continued to have a significant adverse impact on language/communication (βadjusted -21.924; p = 0.013), eye/hand coordination (βadjusted -15.446; p = 0.015) and foundation of learning skills (βadjusted -15.211; p = 0.013).
Conclusions
In very preterm infants, FGR was associated with a significantly increased risk of poor neurodevelopmental outcome at 24 months of corrected age compared to age-matched AGA infants.
Similar content being viewed by others
Introduction
Advances in neonatal intensive care have improved the survival of severe preterm infants, including those near the limit of viability. [1,2,3,4] Nevertheless, the impact on neonatal and long-term morbidity continues to be limited and neurodevelopmental impairment among the survivors appears to have remained stable. [3, 4].
Fetal growth restriction (FGR) is the failure of a fetus to achieve its biological growth potential due to impaired placental function. [5] The definition of FGR varies between different guidelines. The criteria proposed by an international Delphi consensus in 2016 is currently the most accepted definition. [5,6,7].
FGR occurs in 5–7% of all pregnancies [8, 9] and is estimated to be present in 15–20% of the infants admitted to Neonatal Intensive Care Units (NICU). [10] FGR is a proven risk factor for poor prognosis in very preterm infants, with higher morbidity and mortality rates during the perinatal and neonatal periods. [5,6,7,8,9,10].
The association between FGR and childhood neurodevelopmental delay is less clear. [10,11,12] Previous studies have yielded conflicting results. While some papers have reported worse neurodevelopment in FGR infants, [8, 11, 13,14,15] other authors have reported no link between the two. [9, 10, 12, 16, 17] This discrepancy may be justified by the significant heterogeneity in the studies evaluating neurodevelopment in this population, [16, 18] that ranges from inconsistencies in the definition of FGR and its frequent interchangeability with small for gestational age (SGA) infants, to different methods used to evaluate neurodevelopment. [8, 9] Moreover, some studies do not take into account several perinatal and neonatal characteristics that could also influence neurodevelopment and should be considered as possible confounding factors. [10, 13, 19].
This study aims to analyse the impact of well-defined early-onset FGR on the neurodevelopmental outcome at 24 months of corrected age in very preterm infants and to investigate if its effect is similar across the different neurodevelopmental areas.
Materials and methods
Study design and patient selection
We conducted a retrospective cohort study of all preterm infants born with gestational age equal to or under 32 weeks admitted consecutively to the NICU in a tertiary maternity hospital from January 2013 to December 2019. Sample size was calculated using the Fleiss formula with continuity correction and taking into account the results of previous studies. [20, 21] Using an alpha of 0.05 and 80% power, we calculated a minimum sample size of 65 (minimum of 22 with FGR and 43 without FGR).
According to the institution’s protocol, all very preterm infants were included in a follow-up program with frequent reassessment after discharge. This follow-up program was conducted by a specialized multidisciplinary team that includes pediatricians, nurses, and trained educators. At 24 months of corrected age, all children were evaluated for neurodevelopmental outcomes using the Griffiths III Mental Development Scale (GMDS-III). [22] This scale was applied to all very preterm infants by the same two educators who were blind to the diagnosis of FGR and is validated for the Portuguese population. [23].
The control group comprised two appropriate for gestational age (AGA) newborns for every FGR infant. These neonates were matched for gestational age and were admitted to the NICU immediately before or after the FGR infant. Twin infants with FGR were paired with neonates with the same chorionicity and amnionicity. In the event only one twin had FGR, the other one was automatically included in the control group.
Neonates with major congenital malformations or genetic diagnoses that cause lifelong impact were excluded. Children who were lost to follow-up or died before 24 months of corrected age were also excluded.
Clinical data was obtained through the review of the perinatal and neonatal medical records included in the NICU database, the prospective National Registry of Very Preterm Newborns and the eNewborn European Network database, and through the follow-up assessment registered in the personal clinical file.
Data collection
FGR was diagnosed using the international Delphi consensus: onset before 32 weeks of gestation of an absent end-diastolic flow in the umbilical artery or a fetal abdominal circumference or estimated fetal weight below the 3rd centile or below the 10th centile combined with abnormal Doppler findings in uterine or umbilical arteries. [5].
Sociodemographic characteristics (age, parity, education level) and maternal morbidity factors (placenta previa, chorioamnionitis, hypertension/preeclampsia, HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, gestational diabetes, TORCH (toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus) screening, thrombophilia, autoimmune disease and tobacco use) were included in the analysis. Gestational age was estimated using the first trimester ultrasound.
Perinatal factors such as pregnancy surveillance, multifetal gestation, antenatal corticosteroid therapy for pulmonary fetal maturation, antenatal magnesium sulphate administration for fetal neuroprotection, labor induction, prolonged premature rupture of membranes (PPRM), cesarean delivery, outborn status, sex, gestational age, birthweight, five-minute Apgar score less than 7 and endotracheal intubation during neonatal resuscitation were also retrieved.
Neonatal characteristics and morbidity were also explored. Clinical risk index for babies (CRIB) was assessed. [24] Late-onset sepsis (LOS) was defined as clinical sepsis and abnormal laboratory findings (leukocyte count above 30,000/µL or under 5000/µL and C-reactive protein above 2 mg/dL), irrespective of blood culture results. [25] Bronchopulmonary dysplasia (BPD) was defined as oxygen need at 36 weeks postmenstrual age. [26] Patent arterial duct was systematically evaluated by echocardiogram according to protocol or in case of clinical suspicion. [27] Hypotension was diagnosed when mean blood pressure was lower than gestational age in weeks. [28] Necrotizing enterocolitis was classified according to the Modified Bell’s staging system. [29] Retinopathy of prematurity (ROP) was graded using the International Classification of ROP. [30] Periventricular leukomalacia was classified according to De Vries et al.. [31] Periventricular-intraventricular hemorrhage was graded using Volpe’s classification. [32] Hyaline membrane disease, pulmonary hemorrhage, neonatal seizures, mechanical ventilation and postnatal surfactant or corticosteroid administration were also assessed.
At 24 months of corrected age all children were evaluated for neurodevelopmental outcomes using the GMDS-III. [22] A global development quotient (GDQ) and development quotients for each specific area were calculated. Neuropsychomotor developmental delay was considered when GDQ was equal to or smaller than 70.
Cerebral palsy (CP) and vision and hearing impairment were also investigated. CP was diagnosed by a neuropaediatrician using the definition of the European Cerebral Palsy Network. [33] Vision and hearing impairment were systematically evaluated by a pediatric ophthalmologist and otolaryngologist.
Severe neurodevelopmental impairment was considered in the presence of at least one of the following: neuropsychomotor developmental delay, CP, and neurossensorial hearing impairment with need of implantable hearing device or blindness.
Data analysis
Statistical analysis was performed using IBM®SPSS® Statistics version 26. Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations (SD) if normally distributed. Normal distribution was verified through the Kolmogorov-Smirnov test or skewness and kurtosis (maximum tolerated interval of -1 to 1). Bivariate analysis was performed using the χ2 test (or Fisher exact test as appropriate) for categorical variables and t test for continuous variables.
Logistic regression was performed to identify the predictors and outcomes of FGR. Quality of fit was verified by the Hosmer and Lemeshow test and significance by the Omnibus test. Linear regression was used to evaluate the impact of FGR on the neurodevelopmental outcome and to adjust for confounding variables with analysis of covariance. We constructed a model by adjusting for statistically significant and relevant maternal and perinatal factors. In this model we excluded variables with significant collinearity and with a very small number of cases (placenta previa, chorioamnionitis and cesarean delivery). Neonatal variables were not included in our model as they may be on the pathway between FGR and neurodevelopmental outcomes.
All reported p values are two-tailed with values inferior to 0.05 indicating statistical significance.
Approval was obtained from the local Ethics Committee (process number OBS.SF.40/2021).
Results
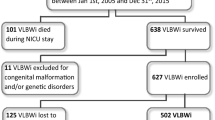
During the study period 323 very preterm infants were admitted in the NICU. Of these, 56 (17.3%) were FGR infants. Twelve FGR infants were excluded, 5 (8.9%) due to death before discharge. Final sample size was 132 infants: 44 (33.3%) with FGR and 88 (77.7%) in the control group (Fig. 1).
Flowchart of patient selection. AGA, appropriate for gestational age; FGR, fetal growth restriction; NICU, neonatal intensive care unit. *Selected with a proportion of 2 controls for 1 case and matched for gestational age and date of admission in the NICU. The remaining very preterm infants were not included in the study
Maternal, perinatal and neonatal characteristics
Mean gestational age at birth was 29.09 ± 1.36 weeks in the FGR group and 29.15 ± 1.56 weeks in the control group (p = 0.837). Average birthweight was lower in the study group (856.48 ± 201.66 gram (g) vs. 1310.86 ± 280.14 g in the control group, p < 0.001). Remaining baseline characteristics are shown in Table 1.
Pregnant women carrying a FGR infant had a higher odd of having hypertension/preeclampsia during the pregnancy (OR 7.11, 95% confidence interval (CI), 3.16 to 16.03; p < 0.001). Additionally, these infants had a higher odd of having a CRIB score at NICU admission higher than 5 (OR 25.87, 95% CI, 7.09 to 94.40; p < 0.001), LOS (OR 3.30, 95% CI, 1.41 to 7.72; p = 0.006) and BPD (OR 11.15, 95% CI, 1.26 to 98.67; p = 0.030) (Fig. 2).
Neurodevelopmental follow-up at 24 months of corrected age
Overall risk of severe neurodevelopmental impairment in the FGR infants was 11.4% and 3.4% in the control group (p = 0.116). No FGR infant was diagnosed with CP (vs.2.3% control, p = 0.842). Only one FGR child (2.3%) had neurosensorial hearing impairment with need of implantable hearing device (vs. none in control group, p = 0.671). No blindness was present in both groups.
Mean GDQ was lower for FGR infants, and after adjusting for confounding, FGR maintained its negative impact on the GDQ (adjusted coefficient: -16.703; p = 0.009). The overall scores for the five neurodevelopmental domains were highest for personal-social-emotional skills (107.02 ± 16.34), followed by eye and hand coordination skills (105.61 ± 14.20) and foundation of learning skills (102.23 ± 13.74), and were lowest for gross motor skills (97.90 ± 11.88) and language and communication skills (96.39 ± 18.88). FGR had a significant impact on all domains except for gross motor skills, even though the score was numerically lower than the average. After adjusting for both maternal and perinatal factors, FGR continues to have a significant adverse impact on language and communication skills, eye and hand coordination skills and foundation of learning skills (Table 2).
Discussion
Main findings
Our findings suggest that (1) in severe preterm infants, FGR is associated with a statistically significant increased risk of poor neurodevelopmental outcome at 24 months old of corrected age and (2) its impact on the different neurodevelopmental domains varies, with a greater impact in language and communication, eye and hand coordination and foundation of learning skills.
The impact of FGR on neurodevelopment is in line with those of some previous studies. [15, 20, 34,35,36] Of note, a meta-analysis by Sacchi et al. showed poorer cognitive function during the first 12 years of life in children who had FGR and were SGA compared with AGA children matched for gestational age. [13] Nevertheless, the criteria of FGR have been extensively debated and as such, the criteria used in previous studies differ. We believe that by using the definition established in the most recent consensus using a Delphi procedure we were able to exclusively study FGR infants and exclude SGA infants that were previously wrongly included in the first group. [19, 37,38,39,40,41].
Regarding the impact on the different domains, few studies have addressed the influence of FGR. One study showed that children born before 27 weeks of gestation with birthweight lower than the 10th centile had worse fine motor and social interaction skills at 2 years of corrected age than age-matched AGA infants. [11] Another study demonstrated that children born at 27–34 weeks of gestation with FGR were particularly impaired in cognitive, behavioural and hearing development domains during the first 12 years of life compared with age-matched non-FGR infants. Nevertheless, the aforementioned papers significantly differ from our study regarding the FGR definition, the selected control group, the assessment ages, and the follow-up assessment tools. Hence, the comparison between our results and the findings of these other papers must be interpreted with caution.
The mechanisms behind FGR are not fully understood. Maternal vascular malperfusion as a consequence of anomalous remodelling of the uterine spiral arteries is believed to be the most frequent factor leading to placental insufficiency, with subsequent hypoxia-reoxygenation damage. [42] Inflammatory lesions and villitis of unknown origin may also play a role. [42, 43].
The pathophysiology of FGR appears to impact only specific neurodevelopmental domains. Placental insufficiency inherent to FGR has been associated with metabolic and structural changes in the fetal and postnatal brain [19, 37,38,39,40,41] mainly due to oxidative stress and an adaptive response to malperfusion with shunt to specific organs such as the brain. [44, 45] This abnormal brain flow persists in the first days of the postnatal period, potentiating hyperoxia and perpetuating oxidative stress. [44, 46] Some authors have observed smaller head circumferences in children born with FGR, [9, 10, 19, 40] a finding that has been correlated with worse cognitive and language outcomes. [19, 20] Decreased brain volumes in FGR infants in utero, [37] at preschool [38, 39] and early school ages [40] have also been reported as well as an altered distribution pattern of grey and white matter. [34, 37, 38] We postulate that our findings may be justified by these asymmetries in brain development that may preferentially affect specific brain structures.
Finally, we found no association between FGR and the risk of having CP, neurosensorial hearing impairment with need of implantable hearing device or blindness. Other studies were also unable to show this association. [11, 12, 15, 34] Our results could be explained by the small sample size and the low prevalence of these outcomes in our sample.
Strengths and limitations
To the best of our knowledge, this is the first study that aims to determine the impact of FGR on neurodevelopmental outcomes using the criteria defined by the international Delphi consensus. [5] Thus, by using fetal growth references instead of postnatal references (as most available studies have done) we believe we estimated the real impact of FGR on child health and neurodevelopmental outcomes.
Our study also included neurodevelopment assessments using GMDS-III performed by the same two trained educators throughout the study period who were blind to the FGR diagnosis, ensuring uniform evaluations. These scales have been extensively validated to assess the psychomotor development of preterm infants at an early age and are regarded as one of the most accurate infant developmental tests in Europe and Portugal, particularly in the follow-up of at-risk infants. [23, 47,48,49].
This study also has some limitations. The study was conducted in a single tertiary maternity, and as such, these findings may be skewed and must be interpreted with caution. Nevertheless, given the high rates of stillbirths and the elevated neonatal mortality among very preterm FGR infants, this limitation is practically unsurmountable in all studies. [7, 8, 34, 50] As a retrospective study, diagnoses and comorbidities may have been underreported. However, since our institution participates in the prospective National Registry of Very Preterm Newborns and the eNewborn European Network database, [51] we believe the data included in our study was precise and accurate. In addition, the non-random selection of controls and FGR infants may have led to bias. Nevertheless, we believe the methodology used for selecting the controls and studying population differences by adjusting for confounding variables minimized this issue to the extent possible. While we believe the study was adequately powered for the primary endpoint, the small sample size and low frequency of some of the outcomes (such as cerebral palsy and blindness) may facilitate type II error when analysing comorbidities. Finally, although we adjusted our results for confounding variables, other unmeasured confounders may have influenced the final results.
In conclusion, FGR constitutes a significant risk for neurodevelopmental impairment during childhood. Both maternal and perinatal factors play an essential role in its development. Efforts should be made to ensure early and correct diagnosis of FGR and all contributing factors, with the aim of reducing their adverse impact on neurodevelopmental outcomes. Further prospective and multicentric follow-up studies with standardized definitions are crucial to expand our understanding of the impact of FGR on the neurodevelopment outcome of very preterm infants and the underlying mechanisms.
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- AGA:
-
Appropriate for gestational age
- BPD:
-
Bronchopulmonary dysplasia
- CP:
-
Cerebral palsy
- CRIB:
-
Clinical risk index for babies
- FGR:
-
Fetal growth restriction
- GDQ:
-
Global development quotient
- GMDS-III:
-
Griffiths III Mental Development Scale
- HELLP:
-
Hemolysis, elevated liver enzymes, and low platelets
- HMD:
-
Hyaline membrane disease
- LOS:
-
Late-onset sepsis
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatal Intensive Care Units
- PIVH:
-
Periventricular-intraventricular hemorrhage
- PPRM:
-
Prolonged premature rupture of membranes
- PVHI:
-
Periventricular hemorrhagic infarction
- PVL:
-
Periventricular leukomalacia
- ROP:
-
Retinopathy of prematurity
- SD:
-
Standard deviation
- SGA:
-
Small for gestational age
- TORCH:
-
Toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus
References
Chung EH, Chou J, Brown KA. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr. 2020;9:3–8.
Hollanders JJ, Schaëfer N, Van Der Pal SM, Oosterlaan J, Rotteveel J, Finken MJJ. Long-term neurodevelopmental and functional outcomes of infants born very Preterm and/or with a very low Birth Weight. Neonatology. 2019;115:310–9.
Twilhaar ES, Wade RM, De Kieviet JF, Van Goudoever JB, Van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172:361–7.
Resende C, Martins D, Faria D, Taborda A. Neurodesenvolvimento em Crianças Nascidas Pré-Termo de Muito Baixo Peso: Fatores de Risco Ambientais e Biológicos. Acta Pediatr Port. 2017;48:212–1.
Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48:333–9.
Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, et al. ISUOG Practice guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. 2020;56:298–312.
Pels A, Beune IM, van Wassenaer-Leemhuis AG, Limpens J, Ganzevoort W. Early-onset fetal growth restriction: a systematic review on mortality and morbidity. Acta Obstet Gynecol Scand. 2020;99:153–66.
Levine TA, Grunau RE, McAuliffe FM, Pinnamaneni RM, Foran A, Alderdice FA. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics. 2015;135:126–41.
Padilla N, Perapoch J, Carrascosa A, Acosta-Rojas R, Botet F, Gratacós E. Twelve-month neurodevelopmental outcome in preterm infants with and without intrauterine growth restriction. Acta Paediatr. 2010;99:1498–503.
Pau JC, López JP, Salinas FC, Garcia OS, Hoyos SP, Olivé EL. Neurodevelopment in preterm infants with and without placenta-related intrauterine growth restriction and its relation to perinatal and postnatal factors. J Matern Fetal Neonatal Med. 2016;29:2268–74.
El Ayoubi M, Patkai J, Bordarier C, Desfrere L, Moriette G, Jarreau PH, et al. Impact of fetal growth restriction on neurodevelopmental outcome at 2 years for extremely preterm infants: a single institution study. Dev Med Child Neurol. 2016;58:1249–56.
Streimish IG, Ehrenkranz RA, Allred EN, O’Shea TM, Kuban KCK, Paneth N, et al. Birth weight- and fetal weight-growth restriction: impact on neurodevelopment. Early Hum Dev. 2012;88:765–71.
Sacchi C, Marino C, Nosarti C, Vieno A, Visentin S, Simonelli A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr. 2020;174:772–81.
Murray E, Fernandes M, Fazel M, Kennedy SH, Villar J, Stein A. Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG. 2015;122:1062–72.
Morsing E, Åsard M, Ley D, Stjernqvist K, Maršál K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127:874–82.
Mazarico E, Llurba E, Cabero L, Sánchez O, Valls A, Martín-Ancel A, et al. Associations between neural injury markers of intrauterine growth-restricted infants and neurodevelopment at 2 years of age. J Matern Fetal Neonatal Med. 2019;32:3197–203.
Kan E, Roberts G, Anderson PJ, Doyle LW. The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev. 2008;84:409–16.
Baschat AA. Neurodevelopment after fetal growth restriction. Fetal Diagn Ther. 2014;36:136–42.
Wang Y, Fu W, Liu J. Neurodevelopment in children with intrauterine growth restriction: adverse effects and interventions. J Matern Fetal Neonatal Med. 2016;29:660–8.
Marín MJB, Alonso MB, Mesa EG. Prenatal predictors of neurobehavioral outcome in children with fetal growth restriction at 6 years of age: a retrospective cohort study. Children. 2023;10(6):997.
Frondas-Chauty A, Simon L, Branger B, Gascoin G, Flamant C, Ancel PY, et al. Early growth and neurodevelopmental outcome in very preterm infants: impact of gender. Arch Dis Child Fetal Neonatal Ed. 2014;99:66–72.
Green E, Stroud L, Bloomfield S, Cronje J, Foxcroft C, Hurter K, et al. Griffiths Scales of Child Development. 3rd ed. Oxford, UK: Hogrefe Ltd.; 2016.
Borges P, Costa IAMP, Ferreira CT, Gil IMCLC, Carvalhão I, Fernandes S, et al. Escalas de Desenvolvimento Mental de Ruth Griffiths - Adaptação para a população portuguesa. Actas do 12.º Colóquio Internacional de Psicologia e Educação. 2012;922–32.
The International Neonatal Network. The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet. 1993;342:193–8.
Sociedade Portuguesa de Neonatologia. Consenso Clínico: Procedimento no recém-nascido com risco infeccioso. 2014;1–15.
Jobe AH, Bancalari E. Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9.
Direção Geral da Saúde. Tratamento médico e cirúrgico do canal arterial no pré-termo. Norma DGS nº 021/2012. 2012.
Fanaroff JM, Fanaroff AA. Blood pressure disorders in the neonate: hypotension and hypertension. Semin Fetal Neonatal Med. 2006;11:174–81.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201.
International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
de Vries LS, Eken P, Dubowitz LMS. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49:1–6.
Inder TE, Perlman JM, Volpe JJ. Chapter 24 - Preterm intraventricular hemorrhage/posthemorrhagic hydrocephalus. In: Volpe JJ (ed) Volpe’s Neurology of the Newborn, 6th edn. Elsevier, 2017;637–98.
Cans C, Dolk H, Platt MJ, Colver A, Prasauskiene A, Krägeloh-Mann I. Recommendations from the SCPE collaborative group for defining and classifying cerebral palsy. Dev Med Child Neurol. 2007;49:35–8.
Von Beckerath AK, Kollmann M, Rotky-Fast C, Karpf E, Lang U, Klaritsch P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol. 2013;208:130.e1–6.
Olga L, Sovio U, Wong H, Smith G, Aiken C. Association between antenatal diagnosis of late fetal growth restriction and educational outcomes in mid-childhood: a UK prospective cohort study with long-term data linkage study. PLoS Med. 2023;20.
Groene SG, Stegmeijer KJJ, Tan RNGB, Steggerda SJ, Haak MC, Slaghekke F et al. Long-term effects of selective fetal growth restriction (LEMON): a cohort study of neurodevelopmental outcome in growth discordant identical twins in the Netherlands. Lancet Child Adolesc Health. 2022;6.
Polat A, Barlow S, Ber R, Achiron R, Katorza E. Volumetric MRI study of the intrauterine growth restriction fetal brain. Eur Radiol. 2017;27:2110–8.
Padilla N, Falcón C, Sanz-Cortés M, Figueras F, Bargallo N, Crispi F, et al. Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res. 2011;1382:98–108.
Simões RV, Muñoz-Moreno E, Cruz-Lemini M, Eixarch E, Bargalló N, Sanz-Cortés M et al. Brain metabolite alterations in infants born preterm with intrauterine growth restriction: association with structural changes and neurodevelopmental outcome. Am J Obstet Gynecol. 2017;216:62.e1–14.
Korkalainen N, Ilvesmäki T, Parkkola R, Perhomaa M, Mäkikallio K. Brain volumes and white matter microstructure in 8- to 10-year-old children born with fetal growth restriction. Pediatr Radiol. 2022;52:2388–400.
Della Gatta AN, Aceti A, Spinedi SF, Martini S, Corvaglia L, Sansavini A, et al. Neurodevelopmental outcomes of very preterm infants born following early foetal growth restriction with absent end-diastolic umbilical flow. Eur J Pediatr. 2023.
Kamphof HD, Posthuma S, Gordijn SJ, Ganzevoort W. Fetal growth restriction: mechanisms, epidemiology, and management. Maternal-Fetal Med. 2022;4.
Arsène M, Kolanska K, Cheloufi M, Coulomb A, Cohen J, Abisror N et al. Chronic villitis of unknown etiology (VUE): obstetrical features, outcome and treatment. J Reprod Immunol. 2021;148.
Malhotra A, Allison BJ, Castillo-Melendez M, Jenkin G, Polglase GR, Miller SL. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Frontiers in Endocrinology. 2019;10:55.
Giussani DA. The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol. 2016;594.
Ishii H, Takami T, Fujioka T, Mizukaki N, Kondo A, Sunohara D et al. Comparison of changes in cerebral and systemic perfusion between appropriate- and small-for-gestational-age infants during the first three days after birth. Brain Dev. 2014;36.
Romeo DM, Ricci M, Mirra F, Venezia I, Mallardi M, Pede E, et al. Longitudinal Cognitive Assessment in Low-Risk very Preterm infants. Med (Lithuania). 2022;58:6–13.
Picciolini O, Giannì ML, Messina L, Pesenti N, Fumagalli M, Gardon L et al. Development of a new scoring method in the neurofunctional assessment of preterm infants. Sci Rep. 2022;12.
Dicanio D, Spoto G, Alibrandi A, Minutoli R, Nicotera AG, Di Rosa G. Long-term predictivity of early neurological assessment and developmental trajectories in low-risk preterm infants. Front Neurol. 2022;13.
Pankiewicz K, Maciejewski T. Perinatal mortality and morbidity of growth restricted fetuses and newborns (own experience) - first report. Dev Period Med. 2017;21:29–34.
Haumont D, Modi N, Saugstad OD, Antetere R, NguyenBa C, Turner M et al. Evaluating preterm care across Europe using the eNewborn European Network database. Pediatr Res. 2020;88.
Acknowledgements
None.
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
All authors contributed to the concept and design of the article. MCF and JM also collected the data and all authors performed its analysis and interpretation. MCF drafted the original article. All authors reviewed it. All authors approve this version of the article.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Ethics approval
Approval was obtained from the Ethics Committee of Centro Hospitalar e Universitário de Coimbra (process number OBS.SF.40/2021). Due to the retrospective nature of the study, informed consent was waived by the Ethics Committee of Centro Hospitalar e Universitário de Coimbra. All the methods included in this study are in accordance with the declaration of Helsinki.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cortez Ferreira, M., Mafra, J., Dias, A. et al. Impact of early-onset fetal growth restriction on the neurodevelopmental outcome of very preterm infants at 24 months: a retrospective cohort study. BMC Pediatr 23, 533 (2023). https://doi.org/10.1186/s12887-023-04361-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04361-y