Abstract
Background
Inflammatory bowel disease (IBD) is a heterogeneous group of disorders associated with environmental triggers and dysregulated immune responses resulting in chronic, recurrent intestinal inflammation. Very early-onset IBD (VEO-IBD) refers to patients with symptoms or diagnosis before the age of 6 years and is widely thought to be associated with monogenic mutations. Traditional drug therapy is often ineffective in this patient population, while hematopoietic stem cell transplantation (HSCT) represents the definitive cure for patients with gene mutations.
Case presentation
We report a case of VEO-IBD associated with a monogenic mutation in a 2-year-old girl presenting mainly with gastrointestinal symptoms, including recurrent hematochezia and abdominal pain for more than 3 months. A gastroscopy revealed erosive gastritis and bulbar duodenitis, while a colonoscopy indicated erosive colitis. Abnormal results were obtained from the dihydrohodamine (DHR) assay and immunoglobulin testing. Whole-exome sequencing identified a heterozygous and de novo nonsense mutation (c.388 C > T; p.R130X) in the CYBB gene leading to deficiency of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) (encoded by CYBB), a critical component of phagocytes. HSCT was performed successfully, and the DHR assay showed that normal neutrophil function was restored. Six months after HSCT, clinical remission was observed, and a repeat colonoscopy revealed intestinal mucosal healing was attained.
Conclusions
Patients with CYBB mutations often develop recurrent or severe bacterial or fungal infections, mostly in the lungs, skin, lymph nodes, and liver. Here, we report on a young female child with CYBB mutations presenting predominantly with gastrointestinal symptoms. This study explores the mechanisms of inflammatory bowel disease caused by a monogenic mutation in CYBB to improve early diagnosis and effective treatment rates of this patient population.
Similar content being viewed by others
Background
Inflammatory bowel disease (IBD) is a group of chronic, non-specific intestinal inflammatory diseases that can occur at any age with unknown etiology. IBD can be divided into ulcerative colitis (UC) and Crohn’s disease (CD). UC mainly involves the colorectal mucosa, presenting with diarrhea, mucous and bloody stools, and abdominal pain. CD can involve any part of the digestive tract from the mouth to the anus, manifesting as abdominal pain, diarrhea, and anal lesions. Very early-onset IBD (VEO-IBD) in patients with symptoms before 2 years are more likely to be associated with monogenic mutations that alter immune function and present with more severe disease [1]. Due to the abundance of variants in primary immunodeficiency genes in patients with VEO-IBD, some genes involved in immunodeficiency cause severe intestinal disease and systemic autoimmunity [2]. Several monogenic mutations have been identified in children with VEO-IBD, including IL‐10, IL‐10RA/B, XIAP, and TTC37 [3]. The patients often respond poorly to standard therapies, including biological agents [4]. Hematopoietic stem cell transplantation (HSCT) represents a definitive cure for diseases associated with gene mutations. For individuals indicated for HSCT, active infections should be treated prior to transplantation due to the increased risk of mortality.
CYBB (Cytochrome B-245 Beta Chain) is a protein-coding gene, and its mutation results in the deficiency of NADPH oxidase 2 (encoded by CYBB), which form the complex of nicotinamide adenine dinucleotide phosphate (NADPH) in phagocytes. NADPH represents a source of reducing equivalents for neutrophil respiratory burst oxidase [5]. Current evidence suggests that NADPH defects cause respiratory burst dysfunction in phagocytes (neutrophils, monocytes and macrophages), leading to the inability to produce reactive oxygen species (ROS), which activate granule proteases to destruct phagocytosed microorganisms. The dihydrorhodamine (DHR) flow cytometry assay is a useful diagnostic tool that can detect absent or reduced NADPH oxidase activity in stimulated phagocytes. Defective production of ROS leads to increased expression of nuclear factor (NF) kappa-B-regulated inflammatory genes, the hyperactivation of NF-ĸB and inflammasome in phagocytes lead to long-lasting production of pro-inflammatory cytokines and inflammatory manifestations, such as inflammatory bowel disease [6].
In this report, we identified a heterozygous and de novo nonsense mutation in the CYBB gene in a 2-year-old girl with IBD features. CYBB mutations are usually associated with X-linked chronic granulomatosis (X-CGD). Unlike most patients with this disease presenting with recurrent bacterial or fungal infections, our patient mainly exhibited gastrointestinal symptoms, such as hematochezia and abdominal pain. This report provides preliminary evidence of a de novo mutation (c.388 C > T; p.R130X) in the CYBB gene as a new disease-causing mechanism for VEO-IBD.
Case presentation
A 28-month-old girl was admitted to a local medical institution due to repeated hematochezia for more than 3 months. She experienced hematochezia for no obvious reasons at the age of 25 months, with small to moderate amounts of dark red or bright red paste-like stool 3–5 times a day, accompanied by abdominal pain, mainly around the umbilical area, which had no obvious relationship with eating and bowel function, and was not accompanied by vomiting, diarrhea, fever, and cough. Routine stool analysis showed red and white blood cells, but the pathological culture was negative. The complete blood count indicated that the white blood cell count was 16.3 × 109/L, and the hemoglobin concentration was 123 g/L. Abdominal ultrasound showed active intestinal peristalsis, multiple hypoechoic nodules around the umbilical area, and enlarged mesenteric lymph nodes. Bacterial enteritis was suspected and treated with anti-infection and hemostatic drugs. During the period, she was fed with deeply hydrolyzed formula which yielded poor therapeutic effects. Eventually, the patient was transferred to our hospital.
The patient was born to a healthy and non-consanguineous Chinese couple after a normal pregnancy with an unremarkable family history. The patient was delivered naturally and was generally in good condition after delivery. The patient previously underwent a cervical lymph node abscess biopsy at a local hospital, but the parents could provide no relevant clinical documentation. No abnormalities in growth and development were observed.
Physical and Laboratory examination During the physical examination upon admission, a small rash was observed on the neck. The complete blood count revealed a white blood cell count of 11.70 × 109/L (reference range: 5.0–9.0 × 109/L), hemoglobin 110 g/L (105–145 g/L), platelet count 38 × 109/L (140–440 × 109/L), and neutrophil percentage 31% (40-60%). Serum immunoglobulin testing revealed an immunoglobulin G (IgG) of 16.7 g/L (3.83 ~ 10.58 g/L), IgM 1.66 g/L (0.4 ~ 1.28 g/L), and IgE 74 IU/ML (0 ~ 60IU/ML). The absolute counts of B cells, T cells and NK cells were 1688.52 cells/ul (90–660 cells/ul), 4833.95 cells/ul (690–2540 cells/ul), and 877.12 cells/ul (90–590 cells/ul), respectively. Antiprotease 3 antibody (ELISA) 64.5 (less than 18). Food allergen IgE: milk 1.59 (less than 0.35 IU/ml). C-reactive protein, procalcitonin, blood gas analysis, biochemical indexes, coagulation index, autoimmune antibody, rheumatoid factor, and T-SPOT.TB test yielded no abnormal findings. No abnormality was found on chest and abdominal radiographs.
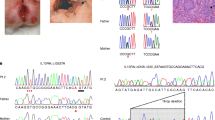
Diagnosis and treatment process and follow-up Based on the medical history and test results, an initial diagnosis of cow milk protein allergy and congenital immunodeficiency disease was established. A gastroscopy was conducted and indicated erosive gastritis and erosive bulbar duodenitis. The gastric antrum and duodenal bulbar mucosa were biopsied, and the histopathological results showed mild chronic inflammation. A colonoscopy indicated erosive colitis (Fig. 1A). Pathological examination of mucosal surface tissues of each segment of the colon showed that the mucosa presented mild chronic inflammation, a large number of lymphocytes and eosinophils infiltrated in lamina propria, with a maximum of 85 cells per high power field, and crypt abscesses in the ascending and transverse colon. Next, the neutrophil oxidative burst test was performed using the DHR assay. The DHR assay of the patient’s granulocytes (Fig. 2A) revealed almost absence of fluorescence upon granulocyte stimulation. The stimulation index (SI) was 5.82 which was compatible with X-CGD. The DHR assay of granulocytes from the patient’s mother (Fig. 2B) and the patient’s father (Fig. 2C) showed normal histogram with the SI of 277.05 and 364.03, respectively. Therefore, peripheral venous blood was collected from the patient and her parents for whole exome sequencing analysis. A heterozygous de novo mutation c.388 C > T (p.R130X) in CYBB gene (Fig. 3) was identified and validated by Sanger sequencing. Based on the above findings, we established a diagnosis of very early onset inflammatory bowel disease (VEO-IBD) with neutrophil dysfunction caused by CYBB gene mutation. Hematopoietic stem cell transplantation (HSCT) was conducted using peripheral blood stem cells (20.47 × 108/kg) from the father of the child. Mycophenolate Mofetil combined with Tacrolimus Capsules were used to prevent graft-versus-host disease (GVHD) and Voriconazole against fungal infections. Two months after HSCT, bone marrow and peripheral blood chimerism rates were 100% complete donor type, DHR assay of granulocytes from the patient at 8 weeks after HSCT (Fig. 4A) showed abnormal histogram with the SI of 28.85, 16 weeks after HSCT (Fig. 4B) showed normal histogram with the SI of 408.90, the normal neutrophil function was restored. A repeat colonoscopy six months after HSCT (Fig. 1B) showed complete intestinal mucosal healing. After 18 months of follow-up, there was no severe infection or acute or chronic GVHD-related manifestations.
The represented endoscopy images before and after hematopoietic stem cell transplantation. (A) Endoscopy images characteristics as mucosal erythema and erosions in gastric antrum and colon before hematopoietic stem cell transplantation. (B) Endoscopy images showed gastric antrum and colonic mucosa erythema and erosion disappear after hematopoietic stem cell transplantation
Discussion and conclusions
In this case, the heterozygous and de novo mutation c.388 C > T(p.R130X)was located in the second exon of CYBB gene and generated a premature stop codon, which was determined to be a pathogenic variant according to American College of Medical Genetics and Genomics guidelines. CYBB encodes the gp91 subunit of NADPH oxidase, and mutations impair the respiratory burst of all types of phagocytes [7]. The mutation was absent from her parents. and her parents had the normal neutrophil function. No mutation could be found on the patient’s other CYBB allele. Nevertheless, we had to consider two possibilities: The patient could have been compound heterozygous with a mutation on the second CYBB allele outside the sequenced regions or the patient’s cells could have had an extremely skewed X chromosome inactivation pattern. An example was described before a female with a de novo mutation in gp91-phox coinciding with an extreme X chromosome inactivation ratio resulted in X-linked CGD [8]. However, X chromosome inactivation pattern was not assessed due to the lack of recipient’s blood stored before HSCT. At present, X chromosome inactivation pattern might be performed on other somatic tissues, but the parents are not willing to do any more tests. Mutations in CYBB have been documented in multiple patients with X-CGD [9]. Patients with CGD usually present with recurrent or severe infections; the most common sites are the lungs, skin, and lymph nodes [10]. In our case, a mutation was identified in our patient, who presented with disease onset at a young age. The clinical manifestations were recurrent hematochezia and abdominal pain, and abnormal findings were found during the diagnostic workup (respiratory burst test and immunoglobulin test). The patient had a history of lymph node abscess prior to admission, and the white blood cell count was slightly higher, although the inflammatory markers were within normal range, probably related to antibiotic use prior to admission at our institution. Indeed, CYBB deficiency is an X-recessive disease, usually not clinically expressed in females. However, it is also plausible that common single nucleotide polymorphism in CYBB alter the expression or function of gp91-phox, determining differences in susceptibility to complex disorders such as autoimmune or infectious diseases [11]. In contrast, Shahram and colleagues [12] reported a case with a mutation at the same site. The male child with consanguinous parents was diagnosed with chronic granulomatosis when he was 3 years old. He developed recurrent infections such as disseminated bacillus Calmette-Guérin (BCG) infection, otitis media, perianal abscess, pneumonia, and pulmonary abscess. The respiratory burst test showed the inability of phagocytes to generate ROS. He also had a maternal cousin with CGD. Therefore, CYBB mutations lead to clinical heterogeneity. Interestingly, although mutations of the same gene and site yield different clinical phenotypes, similarities prevail, including elevated inflammatory markers, abscess formation and dysfunction of phagocytes.
VEO-IBD accounts for 6-15% of children’s IBD [13]. Recent epidemiologic evidence suggests that the incidence of pediatric IBD is increasing, especially in VEO-IBD [14]. Early diagnosis is often challenging since the symptoms are atypical with younger age. Our case was initially misdiagnosed as cow’s milk protein allergy, leading to delayed diagnosis and treatment. It has been established that IBD has a multifactorial pathogenesis. Recent studies have suggested that VEO-IBD is associated with monogenic mutations [15]. Currently, IL-10 receptor deficiencies are the most common in China, and patients often present with severe ileocolonic inflammation and are often complicated with severe anal fistula [16]. A study in China [17] reported 39 cases of infant IBD, including 33 cases (85%) with moderate or severe disease activity index scores, resulting in 10 cases of death during the neonatal period. Accordingly, emphasis should be placed on avoiding misdiagnosis of allergies or infections in cases with recurrent diarrhea, hematochezia and malnutrition, especially those with early-onset age and VEO-IBD should be suspected. Indeed, further research is warranted to elucidate the features and mechanisms of this disease to improve the diagnostic and therapeutic efficacy rates.
Current evidence suggests that HSCT can heal intestinal mucosa, relieve clinical symptoms, and restore mitochondrial activity in phagocytes [18], consistent with our findings. HSCT represents a definitive cure for patients with CYBB mutations and IL-10 receptor deficiencies [19]. However, HSCT in IBD patients with IKBKG and TTC7A mutations can cause GVHD, severe infection, and even intestinal atresia [20]. Therefore, to achieve accurate treatment, it is necessary to ascertain whether gene mutations are present in VEO-IBD patients before treatment.
Moreover, genetic syndromes, such as Turner syndrome, Down syndrome, and glycogen storage disease type Ib, mainly characterized by chromosomal abnormalities, can present with IBD or are at high risk of developing IBD [21]. Therefore, the diagnosis should not be limited to clinical, endoscopic and pathological findings for patients with recurrent gastrointestinal symptoms in the early postnatal period. Indeed, a genetic analysis should be conducted to determine monogenic mutations, and early diagnosis and treatment should be performed. Importantly, HSCT can achieve a good therapeutic effect for patients with VEO-IBD caused by CYBB gene mutation. Our study contributes to the understanding of the rare disease and provides the foothold for further studies to improve the long-term prognosis of this patient population by improving the diagnosis and treatment efficacy rates. Only one patient was discussed in the present study. Further studies with more patients and longer follow-ups are warranted to improve current knowledge on such rare diseases.
Data Availability
The datasets generated and/or analysed during the current study are available in the Genome Sequence Archive(GSA) for human repository with accession number : HRA004357 (https://ngdc.cncb.ac.cn/search/?dbId=hra&q=HRA004357). But the data isn’t release now, we provide with reviewer link (https://ngdc.cncb.ac.cn/gsa-human/s/i7Q29AdB).
Abbreviations
- IBD:
-
Inflammatory bowel disease
- VEO-IBD:
-
Very early-onset inflammatory bowel disease
- HSCT:
-
Hematopoietic stem cell transplantation
- X-CGD:
-
X-linked chronic granulomatosis
- DHR:
-
Dihydrorhodamine
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- UC:
-
Ulcerative colitis
- CD:
-
Crohn’s disease
- ROS:
-
Reactive oxygen species
- NF-ĸB:
-
Nuclear factor kappa-B
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IgE:
-
Immunoglobulin E
- GVHD:
-
Graft-versus-host disease
References
Kelsen JR, Conrad MA, Dawany N, Patel T, Shraim R, Merz A et al. The Unique Disease Course of Children with Very Early onset-Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020 May 12;26(6):909 – 18.
Kelsen JR, Dawany N, Moran CJ, Petersen BS, Sarmady M, Sasson A, et al. Exome sequencing analysis reveals variants in primary immunodeficiency genes in patients with very early onset inflammatory bowel disease. Gastroenterology. 2015 Nov;149(6):1415–24.
Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009 Nov;19(21):2033–45.
Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005 Jan;146(1):35–40.
Flannagan RS, Jaumouillé V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98.
Trevelin SC, Shah AM, Lombardi G. Beyond bacterial killing: NADPH oxidase 2 is an immunomodulator. Immunol Lett. 2020 May;221:39–48.
Yu HH, Yang YH, Chiang BL. Chronic Granulomatous Disease: a Comprehensive Review. Clin Rev Allergy Immunol. 2021;61(2):101–13.
Anderson-Cohen M, Holland SM, Kuhns DB, Fleisher TA, Ding L, Brenner S, et al. Severe phenotype of chronic granulomatous disease presenting in a female with a de novo mutation in gp91-phox and a non familial, extremely skewed X chromosome inactivation. Clin Immunol. 2003 Dec;109(3):308–17.
Kulkarni M, Hule G, de Boer M, van Leeuwen K, Kambli P, Aluri J, et al. Approach to Molecular diagnosis of chronic granulomatous disease (CGD): an experience from a large cohort of 90 indian patients. J Clin Immunol. 2018;38(8):898–916.
Winkelstein JA, Marino MC, Johnston RB, Boyle J, Gurnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Med (Baltim). 2000 May;79(3):155–69.
Tarazona-Santos E, Bernig T, Burdett L, Magalhaes WCS, Fabbri C, Liao J, et al. CYBB, an NADPH-oxidase gene: restricted diversity in humans and evidence for differential long-term purifying selection on transmembrane and cytosolic domains. Hum Mutat. 2008 May;29(5):623–32.
Teimourian S, Sazgara F, de Boer M, van Leeuwen K, Roos D, Lashkary S, et al. Characterization of 4 new mutations in the CYBB Gene in 10 iranian families with X-linked chronic Granulomatous Disease. J Pediatr Hematol Oncol. 2018 Jul;40(5):e268–72.
Kelsen JR, Baldassano RN, Artis D, Sonnenberg GF. Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2015 Sep 1;1(5):462 – 76.
Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. 2017 Jul;112(7):1120–34.
Chandrakasan S, Venkateswaran S, Kugathasan S. Nonclassic inflammatory bowel disease in Young Infants: Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked syndrome, and other Disorders. Pediatr Clin North Am. 2017 Feb;64(1):139–60.
Huang Z, Peng K, Li X, Zhao R, You J, Cheng X, et al. Mutations in Interleukin-10 receptor and clinical phenotypes in patients with very early onset inflammatory bowel disease: a chinese VEO-IBD collaboration Group Survey. Inflamm Bowel Dis. 2017 Apr;23(4):578–90.
Gong YZ, Ning HJ, Ma X, Zhu D, Wang FP, Zhang R, et al. [Clinical and genotypic characteristics of infantile inflammatory bowel disease]. Zhonghua Er Ke Za Zhi. 2019 Jul;2(7):520–5.
Migliavacca M, Basso Ricci L, Farinelli G, Calbi V, Tucci F, Barzaghi F, et al. A novel assay in whole blood demonstrates restoration of mitochondrial activity in phagocytes after successful HSCT in Hyperinflamed X-Linked chronic Granulomatous Disease. J Clin Immunol. 2022 Nov;42(8):1742–7.
Connelly JA, Marsh R, Parikh S, Talano JA. Allogeneic hematopoietic cell transplantation for chronic Granulomatous Disease: controversies and state of the art. J Pediatr Infect Dis Soc 2018 May 9;7(suppl_1):S31–9.
Samuels ME, Majewski J, Alirezaie N, Fernandez I, Casals F, Patey N, et al. Exome sequencing identifies mutations in the gene TTC7A in french-canadian cases with hereditary multiple intestinal atresia. J Med Genet. 2013 May;50(5):324–9.
Gatti S, Gelzoni G, Catassi GN, Catassi C. The clinical spectrum of inflammatory bowel Disease Associated with Specific Genetic Syndromes: two Novel Pediatric cases and a systematic review. Front Pediatr. 2021 Oct;26:9:742830.
Acknowledgements
We would like to thank the patient and her family for their participation.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MY has made substantial contributions to conception and design, has been involved in revising the manuscript. ZLL has been involved in the collection of clinical data of the patient and in drafting the manuscript. HC has been involved in the collection of clinical data of the patient. XQF has been involved in the patient’s treatment. YSR has been involved in the patient’s treatment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Medical Ethics Committee of Guangdong Provincial People’s Hospital approved the study procedures (ID: 202240501). The study was conducted in concordance with the International Ethical Guidelines for Research Involving Human Subjects as stated in the Helsinki Declaration and its later amendments. Informed written consent was obtained from the legal guardians of the participant.
Consent for publication
Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Z., Chen, H., Feng, X. et al. Hematopoietic stem cell transplantation for CYBB heterozygous mutation resulting in very early onset inflammatory bowel disease in children: a case report. BMC Pediatr 23, 348 (2023). https://doi.org/10.1186/s12887-023-04158-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04158-z