Abstract
Background
Sickle cell disease (SCD) is associated with an increased risk of cardiovascular disease that may be due to a variety of possible risk factors, including abnormal blood pressure. Blood pressure (BP) of children and adolescents with SCD has been reported to be lower compared to the BP of the general pediatric population.
Methods
To confirm this prior observation, we compared reference BP values for children with SCD with reference BP values of the general pediatric population. We hypothesized that children with SCD do not have lower BPs than children without SCD.
Results
Systolic BP differed for both males and females, over the different age groups between pediatric subjects with and without SCD. Systolic BP was higher in children with SCD, in both obese and non-obese populations. Diastolic BP did not differ between the groups.
Conclusions
Our analysis demonstrated that systolic BP values are indeed higher in children with SCD than in the general pediatric population. This finding is consistent with the most recent literature showing abnormal BP patterns in the SCD pediatric population utilizing 24-hour BP monitoring devices. This is an important step for recognizing abnormal BP as a risk factor for cardio- and neurovascular events in SCD.
Similar content being viewed by others
Background
Sickle cell disease (SCD) is associated with an increased risk of cardiovascular disease that may be due to a variety of possible risk factors, including hemolytic anemia and abnormal blood pressure [1,2,3,4,5]. Blood pressure (BP) of children and adolescents with SCD has been reported to be lower compared to the BP of healthy children without SCD [6, 7]. Based on this assumption, “relative” hypertension had been proposed as a risk factor for vaso-occlusive complications in SCD, including both overt and silent cerebral infarcts [6, 8, 9].
More recent studies have questioned the “low BP” hypothesis in SCD [10]. With the increased use of 24-hour ambulatory blood pressure monitoring (ABPM), children with SCD have been found to have abnormal BP patterns, including ambulatory hypertension, decreased nocturnal BP dipping, and masked hypertension [11,12,13]. Confirming whether children with SCD have lower reference BP values than children without SCD is an important step for defining BP abnormalities that are risk factors for cardiovascular events. Our analysis compared reference BP values of children with SCD with reference BP values of children without SCD. We hypothesized that children with SCD do not have lower BPs than children without SCD, contrary to current belief.
Methods
We compared BP values for children with SCD with reference BP values for general population children without SCD. In this study, we did not evaluate determinants of BP.
Data
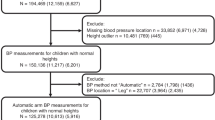
Reference BP values for children without SCD were obtained from the most recent BP tables in the 2017 clinical practice guideline (CPG) for screening and Management of High Blood Pressure in children and adolescents (secondary data analysis, data publicly available) [14]. These normative data are based on the first BP measured during screening of 63,227 male and female children for each age and height. Like the older tables from the fourth report published in 2004, the new tables include systolic BP and diastolic BP values arranged by age, sex, and height percentile. However, in contrast to the fourth report, the new BP tables are based on normal-weight youth BP measurements from children and adolescents with body mass index (BMI) < 85th percentile, below the overweight cut-off. We also compared the SCD BP charts tables with the 2004 fourth report on BP that included children of all BMI percentiles as a robustness check [15].
For children with SCD, we used BP measurement data from the Silent Cerebral Infarct Multicenter Clinical Trial (SIT) [16, 17]. The SIT trial is a multicenter randomized controlled clinical trial that assessed whether blood transfusion can prevent recurrence of cerebral infarcts in children with prior silent infarcts. Elegibility included subjects 5 to 15 years of age with a confirmed diagnosis of HbSS or HbSβ°, with no history of focal neurologic deficits and no treatment with hydroxyurea or chronic transfusions [17]. Data available through 2012 were included from academic centers in North America (23 in USA, one in Canada) and Europe (4 in United Kingdom and 1 in France) Serial systolic and diastolic BPs were obtained from 944 and 943 pediatric subjects, respectively. Participants had a median of 5 BP measurements over a median follow-up of 3.3 years.
For this trial, quantile regression was used to generate estimates of the percentiles of height, weight, systolic and diastolic BP by age and gender, to construct the growth curves from the recorded data. More specifically, restricted cubic splines were used for the variable age to obtain smoothed curves, and cluster bootstrap methods were used to adjust the model-based standard errors for repeated measures. New tables that included the 50th, 90th and 95th percentiles of systolic and diastolic BPs for different height percentiles were constructed for males and females, based on these mathematically derived BPs [16]. Some children were on corticosteroids (8%), and 24% had asthma that could be associated with sleep disturbances at night and higher BP. We did not match the SIT trial data and CPG data by race.
Supplementary Table 1 presents BP measurements (50th, 90th and 95th percentiles) of children with SCD and of children without SCD (2004 Fourth report and 2017 CPG) for different heights (5th, 50th, 90th and 95th percentiles) [14,15,16].
Statistical analysis
In this study, we assess whether children with SCD had higher BP than children without SCD. The BP data are matched by age, gender, and height percentile. For each combination of age, gender, and height percentile, we have diastolic and systolic readings for the SCD group, 2004 reference data, and 2017 reference data. Within each matched height percentile, we used the Wilcoxon signed-rank test to evaluate whether the distribution of BP differed between SCD and each set of reference children (2004 and 2017). Under the null hypothesis, on average the difference of systolic and diastolic BP between SCD and reference children for each combination of age, gender, and height percentile would be 0, which is tested by the Wilcoxon signed-rank test. We analyzed the data in R version 4.0.2 using a two-sided significance level of 0.05.
Results
Figure 1 compares BP of children with and without SCD by sex and height percentile. Systolic BP differed for both males and females, over the different age groups between pediatric subjects with and without SCD. Systolic BP was higher in children with SCD, in both obese and non-obese populations. Diastolic BP did not differ between the groups.
In addition to the differences found in BPs, height percentiles were lower in children with SCD in the SIT trial compared to children without SCD in the 2017 CPG [14, 16]. For example, the 95th percentile of height was 118.3 cm vs. 120.3 cm for a 5-year-old male and 163.8 cm vs. 173.4 cm at 13 years in the SCD and non-SCD groups, respectively. In girls, the 95th height percentile was 115.9 cm vs. 120 cm for a 5-year-old and 164.8 cm vs. 170 cm at 13 years, respectively.
Discussion
Our analysis showed that BPs of children and adolescents with SCD are higher than of children without SCD, in contrast to prior studies. In addition, height percentiles were lower in children with SCD in the SIT trial compared to children without SCD in the 2017 CPG [14, 16]. Because height percentiles were lower in SCD than general populations, the BP elevation in these children is even higher: our results are likely biased away from the null of no effect because short children have lower BPs than tall children. These findings have important clinical implications for the interpretation of research studies as well in the decision to treat children with SCD and elevated BP.
Early studies reported lower BPs in children with SCD [6, 7]. Pegelow et al. compared 3317 subjects with SCD to those reported by NHANES I and II and found that BP was lower in SCD at median and 90th percentile. The BP difference between these populations increased with age [6]. Homi et al. studying adolescents aged 16–18 years showed that the lower BP described in SCD compared to non SCD subjects could be accounted by weight differences [7]. In another small study, Rodgers et al. reported no significant differences in BP values prior to age 17 in a small cross-sectional cohort (n = 13) [8].
More recently, children with SCD have been found to have abnormal BP patterns [10,11,12]. Bodas et al., in a cross-sectional review, found that 8/48 (16.7%) children with SCD had abnormal BP (elevated BP or hypertension) [10]. The authors suggested that hypertension may be underdiagnosed in children with SCD using standard clinic based assessments. Utilizing 24-hour BP monitoring criteria [18], Shatat et al. reported that 18/52 (35%) of patients had previously unrecognized hypertension, 9/52 (17%) had elevated BP and 29/52 (56%) lacked the normal nocturnal dip in BP [11]. In addition, masked hypertension was found to be prevalent in children with SCD: while only 4/38 (10.3%) of these children were hypertensive based on clinic BP, 17/38 (43.6%) had ambulatory hypertension confirmed by 24-hour BP measurements [11].
Strengths and limitations
This secondary analysis has notable limitations. First, the SIT trial BP measurement methods were not standardized across clinical sites without specification whether auscultatory or oscillometric measurement, although BPs were obtained by auscultatory methods in the 2017 CPG [9, 17]. Second, a median of 5 BP measurements was recorded in children with SCD over 3 years. This repeated measurement further supports our hypothesis because multiple measurements should have resulted in regression to the mean and lower BP values in SCD. However, they could also represent increasing BPs over time in SCD; therefore, the median of 5 measurements was higher.
Third, this analysis is premised on the assumption that BP measurements from the SIT trial represent the SCD population without comorbidities, to the extent that any comorbidities are not consequences of SCD, rather than general SCD population with all comorbidities.
Finally, the original trial report included little information on the characteristics of the SCD population, which included both children with and without silent strokes [16]. In addition, the trial only included children with HbSS or HbSβ°. Because the SIT trial is not representative of all children with SCD, our study may have limited external validity.
Conclusions
Our comparative analysis compared an SCD population with reference BP values and demonstrated that systolic BP values are higher in children with SCD than in the general pediatric population, in contrast to previously reported. This finding is also consistent with the most recent literature showing abnormal BP patterns in the SCD pediatric population utilizing 24-hour BP monitoring devices. These are important steps for recognizing abnormal BP as a risk factor for cardio- and neurovascular events in SCD.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
The data and R code are available at: https://github.com/Misreporting/sickle-cell-disease-blood-pressure.
Abbreviations
- ABPM:
-
Ambulatory blood pressure monitoring
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CPG:
-
Clinical Practice Guideline
- SCD:
-
Sickle cell disease
- SIT:
-
Silent Cerebral Infarct Multicenter Clinical Trial
References
Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–94.
Strouse JJ, Hulbert ML, DeBaun MR, Jordan LC, Casella JF. Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics. 2006;118:1916–24. https://doi.org/10.1542/peds.2006-1241.
Gladwin MT, Sachdev V. Cardiovascular abnormalities in sickle cell disease. J Am Coll Cardiol. 2012;59:1123–33. https://doi.org/10.1016/j.jacc.2011.10.900.
Kupferman JC, Zafeiriou DI, Lande MB, Kirkham FJ, Pavlakis SG. Stroke and hypertension in children and adolescents. J Child Neurol. 2017;32:408–17. https://doi.org/10.1177/0883073816685240.
Kupferman JC, Lande MB, Stabouli S, Zafeiriou DI, Pavlakis SG. Hypertension and childhood stroke. Pediatr Nephrol. 2020. https://doi.org/10.1007/s00467-020-04550-2.
Pegelow CH, Colangelo L, Steinberg M, et al. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102:171–7.
Homi J, Homi-Levee L, Gentles S, Thomas P, Serjeant G. Adolescent blood pressure in a cohort study of sickle cell disease. Arch Intern Med. 1993;153:1233–6.
Rodgers GP, Walker EC, Podgor MJ. Is "relative" hypertension a risk factor for vaso-occlusive complications in sickle cell disease? Am J Med Sci. 1993;305:150–6. https://doi.org/10.1097/00000441-199303000-00004.
DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119:3684–90. https://doi.org/10.1182/blood-2011-05-349621.
Bodas P, Huang A, O'Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease -a cross sectional review. BMC Nephrol. 2013;14:237. https://doi.org/10.1186/1471-2369-14-237.
Shatat IF, Jakson SM, Blue AE, Johnson MA, Orak JK, Kalpatthi R. Masked hypertension is prevalent in children with sickle cell disease: a Midwest pediatric nephrology consortium study. Pediatr Nephrol. 2013;28:115–20. https://doi.org/10.1007/s00467-012-2275-9.
Becker AM, Goldberg JH, Henson M, et al. Blood pressure abnormalities in children with sickle cell anemia. Pediatr Blood Cancer. 2014;61:518–22. https://doi.org/10.1002/pbc.24843.
Stabouli S, Antza C, Papadopoulou E, Teli A, Kotsis V, Economou M. Unmasking hypertension in children and adolescents with sickle/beta-thalassemia. J Clin Hypertens (Greenwich). 2020. https://doi.org/10.1111/jch.13957.
Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and Management of High Blood Pressure in children and adolescents. Pediatrics. 2017;140. https://doi.org/10.1542/peds.2017-1904.
National High Blood Pressure Education Program Working Group on high blood pressure in C, adolescents: the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76.
Wolf RB, Saville BR, Roberts DO, et al. Factors associated with growth and blood pressure patterns in children with sickle cell anemia: silent cerebral infarct multi-center clinical trial cohort. Am J Hematol. 2015;90:2–7. https://doi.org/10.1002/ajh.23854.
Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27:69–89. https://doi.org/10.3109/08880010903360367.
Flynn JT, Daniels SR, Hayman LL, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–35. https://doi.org/10.1161/HYP.0000000000000007.
Acknowledgments
We are grateful for helpful conversations with Dr. Michael DeBaun for clarifying data from the SIT trial.
Funding
None.
Author information
Authors and Affiliations
Contributions
JK made substantial contribution to conception, design, acquisition, interpretation, and drafting of the manuscript. JR made substantial contributions to design, analysis and interpretation of data, and substantially revised manuscript. ML, SS, and DZ made substantial contributions to interpretation of data, and substantially revising manuscript. YW and DF made substantial contributions to analysis and interpretation of data. SP made substantial contributions to conception/design, interpretation of data, and substantially revised manuscript. All authors have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study); and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations under the Declaration section; sub-section Ethical approval and consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary table 1
. Systolic and diastolic blood pressures for males and females ages 5-17 with and without Sickle Cell Disease.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kupferman, J.C., Rosenbaum, J.E., Lande, M.B. et al. Blood pressure in children with sickle cell disease is higher than in the general pediatric population. BMC Pediatr 22, 549 (2022). https://doi.org/10.1186/s12887-022-03584-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03584-9