Abstract
Objectives
This study aimed to examine the clinical effects of myofunctional treatment on children with functional mouth breathing by cephalometric radiographs and study models.
Methods
A total of 224 children (6–10 years old; 114 males and 110 females; SNA°: 82.24 ± 1.67°; ANB°: 2.79 ± 0.80°, 28° < SN-GoGn° < 37°) formed three groups: MB-M group (mouth breathers with myofunctional treatment,n = 75); MB-N group (mouth breathers with no treatment,n = 70); NB group (nasal breathers with no treatment, n = 79). A blind evaluation of cephalometric radiographs and study models was conducted at T1(pre-study) and T2 (post-study), respectively.
Results
Two hundred four children (MB-M:66, MB-N:68, NB:70) completed the present study. At T1, MB-M and MB-N groups, compared to their NB counterpart, had greater anterior lower facial height(P < 0.01) and overjet(P < 0.001) but shorter overbite and maxillary canines width (P < 0.001). At T2, the MB-N group exhibited a higher ANB angle, anterior lower facial height, and overjet, but shorter overbite and maxillary canines width (P < 0.001). From T1 to T2, the anterior lower facial height increased, overbite and the maxillary canines width further decreased in the MB-N group (P < 0.001). However, in the MB-M group, the incisors were retracted, overbite increased (P < 0.001), anterior lower facial height increased insignificantly (P > 0.05), and maxillary canines width increased slightly (P < 0.05). In the NB and MB-M groups, the mandible showed a normal tendency to grow forward, whereas, in the MB-N group, the mandible showed a tendency to grow downward (P < 0.001).
Conclusions
Mouth breathers demonstrated increased anterior facial height and overjet but reduced overbite and maxillary arch width, which improved significantly following myofunctional treatment.
Trial registration
TCTR: TCTR20220401001. Registered 1stApril 2022-Retrospectively registered.
Similar content being viewed by others
Introduction
Background and objective
Mouth breathing occurs when a patient substitutes nasal breathing with a pattern of oral or mixed breathing for more than 6 months [1, 2]. Mouth breathing has a complex etiology that may range from anatomic obstructions such as palatine and pharyngeal tonsil hypertrophy, septal deviation, nasal polyps, nasal turbinate hypertrophy, and allergic rhinitis to harmful oral habits [3,4,5].
Mouth breathers with no obstructive etiological factors are called functional mouth breathers [6, 7]. Functional mouth breathing is a harmful habit that may interfere with proper craniofacial development.
Mouth breathing jeopardizes maxillofacial muscle functioning and the upper and lower jaws, resulting in abnormal maxillofacial morphology and poor academic performance in children [8, 9]. As a result, proper interventions for mouth-breathing children are required. Before orthodontic treatment, those with nasal obstruction and upper respiratory infection should be treated as soon as possible [1]. It has been reported that adenotonsillectomy enhanced the myofunctional activity and nasopharyngeal airway for most mouth breathers with adenotonsillar hypertrophy [2].
Muscle weakness of the lip and tongue is one of the clinical manifestations of mouth breathers, which leads to abnormal craniofacial development [10, 11]. Kondo Etsuko reported that muscle training positively influenced the management of the different malocclusions and was crucial at the retention stage following orthodontic treatment [12]. Saccomanno et al. proposed that combining orthodontic therapy with functional muscle training might optimize orthodontic treatment stability in individuals with poor oral habits [13].
Oral Myofunctional Therapy (OMT) was described as the “therapy of dysfunctions of the muscles of the face and mouth to improve orofacial functions such as chewing and swallowing and encouraging nasal breathing [14]”. Dr. Farrell created pre-orthodontic trainers (Myobrace System appliances) to increase orofacial muscle training in the early 1990s”. These myofunctional therapies might aid in the correction of children’s tongue posture, swallowing habits, and mouth breathing [15, 16]. Furthermore, the functional orthodontic appliances significantly improved temporomandibular joint disorders (TMD) symptoms in individuals with juvenile idiopathic arthritis and TMD [17].
Although the effectiveness of myofunctional therapy has been questioned, some evidence has been published demonstrating the influence of myofunctional therapy on some dentoskeletal problems [18,19,20]. Investigating myofunctional treatment in children with functional mouth breathing may lead to a better understanding of myofunctional therapy’s clinical efficacy in individuals with dentofacial abnormalities induced by mouth breathing. It may give valuable information for orthodontic diagnosis and treatment plans in the clinical setting. Hence, this study aims to examine the clinical effects of myofunctional treatment on children with functional mouth breathing by cephalometric radiographs and study models.
Method
Trial design
This was a non-randomized concurrent controlled trial involving children who attended the orthodontic clinic of the Stomatological Hospital of Xi’an Jiaotong University, China. This study was carried out following the Helsinki Declaration on medical protocol and ethics, and it was approved by the Medical Ethics Committee of the Hospital of Stomatology, Xi’an Jiaotong University, Registration number: Xjkqll [2018] No.17.
Participants
Eligibility criteria for participants
This study involved 224 young patients from the Orthodontic Department, Stomatological Hospital of Xi’an Jiaotong University. Inclusion criteria: subjects aged 5–10 years; normal body mass index subjects: 18.5<BMI<24.9 [21, 22], Class I molar relationship; Skeletal Class I: ANB°:1–4°, SNA°: 79–85° and normal vertical facial growth: 28° < SN-GoGn° < 37°. Exclusion criteria: subjects with confirmed syndromes and neurologic disorders; subjects who previously received orthodontic therapies, and subjects diagnosed with the following conditions: temporomandibular joint disorders; hypotonia or hyperactivity of the jaw muscles; sleep-disordered breathing (SDB); allergy problems; tongue-tie problems; adenotonsillar hyperplasia, turbinate hyperplasia.
The subjects of this study were grouped into three groups: the MB-M group (functional mouth breathers with myofunctional treatment, n = 75) and the MB-N group (functional mouth breathers with no treatment, n = 70). The third group was the NB group (nasal breathers with no treatment, n = 79). Baseline descriptive data of the three groups were used to check whether the three groups matched age and craniofacial measurements Table 1.
The functional mouth breathers in MB-M and MB-N groups had no upper airways obstructive etiological factors. The absence of upper airway obstruction was established preliminarily by the findings of the otolaryngologist consultations, and habitual mouth breathing was confirmed by clinical history taken from the child’s parents describing the child’s sleeping behavior, such as sleeping with mouth open. An experienced otolaryngologist checked all individuals and confirmed that they were habitual mouth breathers. A complete evaluation by an otolaryngologist comprised a nasopharyngeal x-ray, rhinoscopy, and flexible nasopharyngoscopy; no blockage of the upper airway was observed in all mouth breathing participants. The children in the MB-N and MB-M groups were informed of clinical intervention in the same way by the orthodontist. Still, those in the MB-N group refused any treatments, so orthodontists advised the regular visits. Those in the MB-M group accepted clinical intervention (myofunctional treatment). Although the children in the NB group breathed through their noses, clinical and X-ray tests revealed mild malocclusions; thus, orthodontists also recommended regular visits.
The patients’ baseline characteristics are described in Table 1. The age, ANB°, and SNA° were analyzed with Oneway Anova. The sexes were analyzed with a Chi-square test (α = 5%). There were no statistical differences in the average age, sex, ANB°, and SNA° among the three groups (P > 0.05).
Interventions
All participants were given the information sheet about our study’s purpose and methods to read, and every child’s parent/guardian signed informed consent to participate.
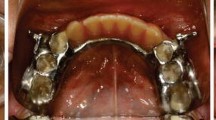
The children in the MB-M group received orofacial muscle training from one experienced orthodontic nurse: lip sealing training with a lip trainer that tension was 250 g for10 minutes, three times per day; tongue flipping training(the tip of the tongue bouncing at the palate strongly), 100 times per day; chewing gum training (spreading out chewing gum at the palate),15 times per day; swallowing training (pushing 15 ml water on the tip of their tongue up against the hard palate and swallowing with lips closed), 15 times per day. Parents were required to help their children fill out the daily training books. Moreover, children in the MB-M group were asked to wear Myobrace (Myofunctional Research Co. Queensland, Australia) (Fig. 1). The children were instructed to use the trainer by single orthodontist. Children were asked to wear the trainer every day for 2 h during the daytime and night while sleeping. Initial checks were conducted after 2 weeks, with subsequent checks every 4 weeks. The treatment process for MB-M children was 1–1.5 years when the severity of mouthing breathing was decreased. Children in the MB-N and NB groups were advised the regular visits by orthodontists over the same period.
Outcome measures
Cephalometric analysis
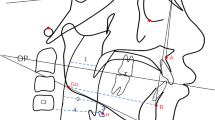
The cephalometric radiographs were analyzed by a calibrated investigator with Dolphin 11.5 (Dolphin Imaging and Management Solutions, Chatsworth, Calif). The investigator was blinded to the information of patients. The landmarks of cephalometric radiographs are shown in Fig. 2.
Landmarks of Cephalometric X-ray. Abbreviations of fig. 2: PNS: Posterior nasal spine, ANS: Anterior nasal spine, S: Sella, N: nasion, A: A-point, B: B-point, GO: Gonion, GN: Gnathion, Me: Menton, Po: Porion, Or: Orbitale, CO: condylion FH: Frankfort horizontal plane, PP: Palatal plane, MP: Mandibular plane, OP: Occlusal
Study model analysis
The study models and cephalometric radiographs were collected at T1 and T2(after 13.0 ± 1.1 months). Study models were blocked to avoid the risk of bias, and the landmark of the study models are shown in Fig. 3. The study models were measured with digital calipers (Tesa Technology, Renens, Switzerland; resolution 0.01 mm).
Sample size
The sample size was calculated using a formula proposed by Suresh KP [23]. The average standard deviation of 2.9 mm and the mean difference of 1.47 mm of overjet between the mouth and nasal breathing children were adopted from previous research by Harari et al. [24].
n is the sample size; Zα is the normal deviate at a level of significance (Zα is 1.96 for 5% level of significance and 2.58 for 1% level of significance), and Z1-β is the normal deviate at 1-β% power with β% of type II error (0.84 at 80% power and 1.28 at 90% statistical power). r = n1/n2 is the Ratio of the sample size required for groups, r = 1 (equal sample size); or r = 0.5(unequal sample size).σ is the pooled standard deviation, and d is the difference of means between groups. Researchers can obtain these values (σ, d) from prior research with comparable hypotheses or by performing a pilot study [23]. In the present study, those values (σ, d) were adopted from a previous study by Harari et al. [24].
Statistical method
All cephalometric and study model measurements were repeated for 30 randomly chosen participants at three-week intervals to verify reliability using the intra-class correlation coefficient (ICC). The error was calculated according to Dahlberg [25].
The data were processed with SPSS19.0 (IBM Corp, Armonk, NY). All variables pre-and post-study and intergroup variables were analyzed with paired t-test and Oneway Anova, respectively. All data were collected and processed by the same orthodontist.
Results
9 (12%) children in the MB-M group who did not do the muscle training or wear myobraces; 2 (2.8%) in the MB-N group, and 9 (11.4%) in the NB group lost to follow-up. The final data of these 20 patients were not analyzed at T2
Our study lasted for 13.0 ± 1.1 months, starting in September 2018 and ending in August 2020. Depending on our research power, the study was terminated once a sufficient sample size was obtained, with 66 children in the MB-M group, 68 in the MB-N group, and 70 in the NB group.
The intraclass correlation coefficient (ICC) ranged between 0.90–0.95 for landmark identification of cephalometric radiographs and study models, confirming the reproducibility and reliability of the method. According to all repeated analyses, the method error was negligible (less than 0.5 mm for linear measurements and less than 0.33° for angular measurements).
The average age was 7.41 ± 1.21 years for the MB-M group, 7.30 ± 1.21 years for the MB-N group, and 7.25 ± 1.05 years for the NB group Table 1.
Angular, ratio measurement results of cephalometric radiographs and linear measurement results of the intragroup and intergroup analysis were shown in Tables 2 and 3.
At T2, compared with the NB group, children in MB-M and MB-N groups had greater N-Me, ANS-Me, SN-GoGn, and ANS-Me, but lower S-Go/N-Me ratio (P < 0.001). Compared with the other two groups, children in the MB-N group had greater U1-NA(P < 0.01); ANB, FH-MP, and L1-NB angles; wider U1-NA, L1-NB, and overjet linear distances; lower S-Me/N-Me Ratio; shorter overbite and C-C linear distances(P < 0.001). The results indicated that ANB angle, anterior lower facial height, the inclination of incisors, and overjet were greater, while overbite and maxillary canine width were less for children in the MB-N group.
From T1 to T2, significant changes were observed in all groups. SNA angle and N-Me, ANS-Me linear distances increased significantly in all three groups (P < 0.001). It implied that the maxillary grew forward, and the anterior facial heights increased. However, some different changes are shown below:
There were significant increases in SN-GoGn, FH-MP angles, overjet, and ANS-Me/N-Me ratio in the MB-N group, but a significant decrease in S-Go/N-Me ratio, overbite, and C-C linear distances (P < 0.001). U1-NA and L1-NB angles and linear distances slightly increased (P < 0.05). SNB angle increased significantly in NB and MB-M groups (P < 0.001), but there was no significant difference in the MB-N group. It indicated the anterior lower facial heights increased and overbite decreased more in the MB-N group. Moreover, the widths of the maxillary canines were further decreased, and mandibles showed a downward growth rather than a forward growth.
In the MB-M group, U1-NA, L1-NB angles, and U1-NA, L1-NB linear distances decreased significantly; overjet decreased significantly; overbite increased, and C-C linear distances increased slightly (P < 0.05). It indicated the mandibles showed significant forward growth in the MB-M group. The incisors were retracted, overjet decreased, and overbite increased. Moreover, excessive increases of the anterior lower facial height and further decrease of the maxillary canine width were corrected.
Discussion
Interpretation
This study found that oral myofunctional treatment benefited children with dental malocclusion caused by functional mouth breathing. Previous research has shown that oral myofunctional therapy improves oral muscle function and eliminates oral behaviors, including thumb sucking, nail biting, tongue thrusting, mouth breathing, and poor tongue and lip posture [26,27,28]. Oral habits are considered a major etiologic factor of temporomandibular disorders (TMD) because they produce traumatic dental occlusion, which may affect the teeth, masticatory muscles, and temporomandibular joints, producing disturbance of the stomatognathic system’s functional balance [29]. The use of functional orthodontic appliances has been shown to benefit growing children with some TMD-related issues [17].
Research has shown that mouth breathing impairs dentoskeletal development and masticatory function and reduces the degree and duration of vertical occlusal force on the posterior teeth in developing children [30]. According to a previous systematic review and meta-analysis research, the major interventions used to correct musculoskeletal problems in children were nasopharyngeal lymphoid tissue removal, orthodontic appliances, muscle training programs, or combinations of the above [31]. In our study, orofacial muscle training included lip sealing, tongue flipping, chewing gum, and swallowing. Moreover, parents helped their children fill out the daily training books, and children wore myobraces to reinforce oral muscle training. Myobraces were pre-fabricated, removable, flexible appliances designed to stimulate the masticatory and facial muscles by lip sealing, training, and restoring the tongue to its correct position.
According to our study, children in the MB-N group were more likely to have increased anterior lower facial height, overjet, and proclination of upper incisors, which were consistent with some research results [32, 33]. Mouth-breathing children were likely to have an increased ratio of anterior lower facial height to posterior height with the clockwise rotation of the mandible [24, 34]. Some researchers have found that the facial characteristics of mouth breathers were related to altered breathing patterns. The isolated tonsil hypertrophy could lead to forwarding and upward rotation of the mandible. The isolated adenoid hypertrophy could cause the mandible to rotate downward and backward, resulting in a significantly decreased ratio of posterior height to anterior height [35]. There are also reports that mouth breathing was not directly related to facial discrepancies [36]. The research inconsistencies may be associated with the types (obstructive or functional), courses, and severity of mouth breathing.
The current study’s control group was the MB-N group, while the nasal breathing group was the blank control group. Individuals in the first group (MB-M) improved to resemble those with nasal breathing in their craniofacial measurements.
In our study, from T1 to T2, the lower facial height of children in the MB-M group did not increase significantly. The upper anterior teeth were retroclined, the overjet reduced, and the overbite increased. There was a slight increase in the width of maxillary canines, suggesting that myofunctional treatment played a role in controlling the lower facial height and promoting the transversal development of the maxillary arch. After passive myofunctional therapy, Chuang et al. reported changes in craniofacial parameters and life quality for children with sleep apnea. They also found improvements in nasal breathing, mandible linear growth, and airway morphology [18]. Unlike our study, Chuang et al. discovered more vertical growth in the anterior facial height in the treatment group subjects, indicating the mandible clockwise rotation trend [18]. These inconsistent findings might be related to the differences in patient characteristics and methodological techniques between our study and Chuang et al’ study.
Myobrace trainer, as an oral muscular trainer, could promote the lateral development of the dental arch for kids with insufficient lateral development of the maxillary arch [37]. It has also been reported that a myobrace trainer could reduce overjet while increasing facial height for patients with ANB angle > 4° [38]. However, our research found that vertical development was controlled after mouth breathers wore myobrace trainers. The different results were probably caused by subjects with different sagittal and vertical skeletal discrepancies. In the MB-M group, the ANB angle was 1–4°, and the SN-GoGn angle was 28–37°, indicating that myofunctional treatment combined with myobrace trainer might have some control over the vertical development of mouth breathers with normal sagittal and vertical growth.
With a treatment of 13.0 ± 1.1 months, 66 children in the MB-M group had improved lip sealing and nasal breathing, consistent with those reports on the improvement of peripheral muscle functions of children with the myofunctional treatment [39,40,41].
Myofunctional treatment could improve myoelectric activities of the perioral and masticatory muscles, especially for Angle Class II Division I patients. Atypical swallowing was corrected, bruxism was reduced, and aptitude for nasal breathing improved. A significant reduction of open bite and reduction of ANB angle were observed, along with a significant increase in inter-molar width [39,40,41]. In our study, myofunctional treatment corrected abnormal positions of tongue and mouth breathing habits and improved lip sealing. Meanwhile, myofunctional treatment inhibited vertical facial growth of mouth breathers with skeletal Class I.
Some researchers compared myobrace trainers with functional appliances, concluding that myobrace trainers induced less significant soft tissue and hard tissue changes than activators for patients with Skeletal Class II between ages 8 to 12. Compared to activators, fewer changes in ANB angle, nasolabial angle, overjet, and facial convexity angle were observed for patients with myobrace trainers [42]. However, in a multicenter, prospective randomized clinical research, myobrace trainers were as effective as Andresen activators in correcting overjet, overbite, sagittal molar relation, and lip sealing for patients aged 6–14 years with large overjet [43]. Moreover, a recent systematic review showed that myofunctional treatment improved snoring and mouth breathing habits [44]. The debate over whether myobrace trainers could treat patients with skeletal disharmonies and upper airway problems should be further explored.
As compliance is a key factor of successful myofunctional treatment, the low success rate in treatment with myobrace trainers was mainly due to poor compliance [43]. Another study found that the overall success rate of both myofunctional appliances (myobrace) and activator appliances was relatively low owing to poor compliance, even though their costs were considered inexpensive [45]. In our research, the myofunctional treatments were executed under parents’ supervision, and parents filled out the training books to ensure high compliance. The treatment would be unsuccessful if children refused to follow doctors’ advice. Myofunctional therapies were shown to be effective for mouth-breathing children in our research. Furthermore, greater outcomes might be obtained if other orthodontic therapy could be administered in addition to the myofunctional therapies.
Limitation
Due to ethical factors, we could not perform randomized controlled, double-blind clinical trials in our study. The risks of selection bias could not be eliminated, which is the present study’s limitation. Therefore, a randomized clinical controlled study on the efficacy of myofunctional treatments in functional mouth breathing participants is recommended.
Conclusion
After myofunctional treatment, mouth-breathing children showed better dentofacial growth. The excessive increase of the lower facial height was controlled, and the transverse restriction of the maxillary arch was relieved. Simultaneously, the myofunctional treatment resulted in the retraction of upper incisors, which increased the overbite of anterior incisors. It might be helpful for open-bite correction. The impact of myofunctional treatment on three-dimensional face development in children with functional mouth breathing should be validated using 3D data. Furthermore, the impact of myofunctional therapy on fixed appliance treatment and the long-term stability of myofunctional therapy should be investigated.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its Supplementary information files]
References
Ramos VM, Nader CM, Meira ZM, Capanema FD, Franco LP, Tinano MM, et al. Impact of adenotonsilectomy on nasal airflow and pulmonary blood pressure in mouth breathing children. Int J Pediatr Otorhinolaryngol. 2019;125:82–6 Elsevier.
Neiva PD, Franco LP, Kirkwood RN, Becker HG. The effect of adenotonsillectomy on the position of head, cervical and thoracic spine and scapular girdle of mouth breathing children. Int J Pediatr Otorhinolaryngol. 2018;107:101–6 Elsevier.
Zhao Z, Zheng L, Huang X, Li C, Liu J, Hu Y. Effects of mouth breathing on facial skeletal development in children: a systematic review and meta-analysis. BMC Oral Health. 2021;21:1–14 BioMed Central.
Paw K, Um W, Resler K. Craniofacial proportions in children with adenoid or adenotonsillar hypertrophy are related to disease duration and nasopharyngeal obstruction. Int J Pediatr Otorhinolaryngol. 2020;132:109911.
Mohamed AS, Habumugisha J, Cheng B, Zhao M, Guo Y, Zou R, et al. Three - dimensional evaluation of hyoid bone position in nasal and mouth breathing subjects with skeletal Class I , and Class II. BMC Oral Health. 2022;22(1):1–12 BioMed Central.
de Barbiero EFF, Vanderlei LCM, Neto AS, Nascimento PC. Influence of respiratory biofeedback associated to re-expansive ventilation patterns in individuals with functional mouth breathing. Int J Pediatr Otorhinolaryngol. 2008;72:1683–91.
Costa JG, Costa GS, Costa C, de Vilella OV, Mattos CT, de Cury-Saramago AA. Clinical recognition of mouth breathers by orthodontists: A preliminary study. Am J Orthod Dentofac Orthop. 2017;152:646–53 American Association of Orthodontists.
Ribeiro GCA, dos Santos ID, Santos ACN, Paranhos LR, César CPHAR. Influence of the breathing pattern on the learning process: a systematic review of literature. Braz J Otorhinolaryngol. 2016;82:466–78 Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.
Morais-almeida M, Wandalsen GF, Solé D. Growth and mouth breathers. J Pediatr. 2019;95:66–71 Sociedade Brasileira de Pediatria.
Sabashi K, Washino K, Saitoh I, Yamasaki Y, Kawabata A, Mukai Y, et al. Nasal obstruction causes a decrease in lip-closing force. Angle Orthod. 2011;81:750–3.
Nishiura M, Ono T, Yoshinaka M, Fujiwara S, Yoshinaka M, Maeda Y. Pressure production in oral vestibule during gum chewing. J Oral Rehabil. 2015;42:900–5.
Kondo E. Muscle wins! Treatment in orthodontics. Tokyo: DaehanNarae; 2007.
Saccomanno S, Antonini G, D’Alatri L, D’Angelantonio M, Fiorita A, Deli R. Causal relationship between malocclusion and oral muscles dysfunction: A model of approach. Eur J Paediatr Dent. 2012;13:321–3.
Wishney M. Myofunctional therapy and prefabricated functional appliances: an overview of the history and evidence. Aust Dent J. 2019;64:135–44.
Yagci A, Uysal T, Kara S, Okkesim S. The effects of Myofunctional appliance treatment on the perioral and masticatory muscles in class II division I patients. World J Orthod. 2010;11:117–22.
Owman-Moll P, Ingervall B. Effect of oral screen treatment on dentition, lip morphology, and function in children with incompetent lips. Am J Orthod. 1984;85:37–46.
Isola G, Ramaglia L, Cordasco G, Lucchese A, Fiorillo LMG. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiopathic arthritis. Minerva Stomatol. 2017;66:1–8.
Chuang L, Hwang Y, Lian Y, Hervy-auboiron M, Pirelli P, Huang Y. Changes in craniofacial and airway morphology as well as quality of life after passive myofunctional therapy in children with obstructive sleep apnea: a comparative cohort study. Sleep Breath. 2019;23:1359–69.
Chen L, Lai C, Chang I, Hsu C. ScienceDirect evaluation of skeletal and dentoalveolar changes in class II division I pediatric patients receiving myofunctional appliance therapy: A preliminary study. J Formos Med Assoc. 2022;22:1–7 Formosan Medical Association.
Homem MA, Vieira-Andrade RG, Moreira Falci SG, Ramos-Jorge ML, Marques LS. Effectiveness of orofacial myofunctional therapy in orthodontic patients: a systematic review. Dental Press J Orthod. 2014;19:94–9.
Wu S, Wang T, Kang X, Wang X, Jiao Y, Du X, et al. Hyoid bone position in subjects with different facial growth patterns of different dental ages. Cranio. 2021;00:1–7 J Craniomandib Pract Taylor & Francis.
Tee E-S. Obesity in Asia: prevalence and issues in assessment methodologies. Asia Pac J Clin Nutr. 2002;11:S694–701.
Suresh KP. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5:7–13.
Harari D, Redlich M, Miri S, Hamud T, Gross M. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patient. Laryngoscope. 2010;120:2089–93.
Dahlberg G. Statistical methods for medical and biological students. New York: Interscience Publications; 1940.
Moeller JL, Licia CP, Gelb ML. Myofunctional therapy: A novel treatment of pediatric sleep-disordered breathing. Clin Sleep Med. 2014;9:235–43 Elsevier Inc.
Levrini L, Sara G, German S. Efficacy of a pre-fabricated Myofunctional appliance for the treatment of mild to moderate pediatric obstructive sleep apnea: a preliminary report. J Clin Pediatr Dent. 2018;42:475–8.
Ciftci V, Uzel A. Dento-skeletal effects of myofunctional appliance on patients with class II div 1 in mixed dentition stage: A cephalometric study. Pediatr Dent J. 2021;31:235–41 Elsevier Ltd.
Perrotta S, Bucci R, Simeon V, Martina S, Michelotti A, Valletta R. Prevalence of malocclusion, oral parafunctions and temporomandibular disorder - pain in Italian schoolchildren: an epidemiological study. J Oral Rehabil. 2019;46:611–6.
Ikenaga N, Yamaguchi K, Daimon S. Effect of mouth breathing on masticatory muscle activity during chewing food. J Oral Rehabil. 2013;40:429–35.
Koletsi D, Makou M, Pandis N. Effect of orthodontic management and orofacial muscle training protocols on the correction of myofunctional and myoskeletal problems in developing dentition. A systematic review and meta-analysis. Orthod Craniofacial Res. 2018;21(4):202–15.
Chung Leng Muñoz I, Beltri OP. Comparison of cephalometric patterns in mouth breathing and nose breathing children. Int J Pediatr Otorhinolaryngol. 2014;78:1167–72 Elsevier Ireland Ltd.
de Cabrera LC, Retamoso LB, Mei RMS, Tanaka O. Sagittal and vertical aspects of class II division 1 subjects according to the respiratory pattern. Dental Press J Orthod. 2013;18:30–5.
Farronato M, Lanteri V, Fama A, Maspero C. Correlation between malocclusion and allergic rhinitis in pediatric patients: a systematic review. Children. 2020;7:1–11.
Franco LP, Souki BQ, Cheib PL, Abrão M, Pereira TBJ, Becker HMG, et al. Are distinct etiologies of upper airway obstruction in mouth-breathing children associated with different cephalometric patterns? Int J Pediatr Otorhinolaryngol. 2015;79:223–8.
Bianchini AP, Guedes ZCF, Vieira MM. A study on the relationship between mouth breathing and facial morphological pattern. Braz J Otorhinolaryngol. 2007;73:500–5 Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.
Ramirez-Yañez GO, Sidlauskas A, Junior E, Fluter J. Dimensional changes in dental arches after treatment with a prefabricated functional appliance. J Clin Pediatr Dent. 2007;31:279–83.
Usumez S, Uysal T, Sari Z, Basciftci FA, Karaman AI, Guray E. The effects of early preorthodontic trainer treatment on class II, division 1 patients. Angle Orthod. 2004;74:605–9.
Satygo EA, Silin AV, Ramirez-Yañez GO. Electromyographic muscular activity improvement in class II patients treated with the pre-orthodontic trainer. J Clin Pediatr Dent. 2014;38:380–4.
Quadrelli C, Gheorgiu M, Marchetti C, Ghiglione V. Early myofunctional approach to skeletal class II. Mondo Ortod. 2002;27:109–22.
Uysal T, Yagci A, Kara S, Okkesim S. Influence of pre-orthodontic trainer treatment on the perioral and masticatory muscles in patients with class II division 1 malocclusion. Eur J Orthod. 2012;34:96–101.
Idris G, Hajeer MY, Al-jundi A. Soft- and hard-tissue changes following treatment of class II division 1 malocclusion with activator versus trainer: a randomized controlled trial. Eur J Orthod. 2019;41:21–8.
Cirgić E, Kjellberg H, Hansen K. Treatment of large overjet in angle class II: division 1 malocclusion with Andresen activators versus prefabricated functional appliances- A multicenter, randomized, controlled trial. Eur J Orthod. 2016;38:516–24.
Carrasco-llatas M, Connor-reina CO. The role of Myofunctional therapy in treating sleep-disordered breathing: a state-of-the-art review. Int J Environ Res Public Health. 2021;18(14):7291.
Emina Č, Kjellberg H, Petzold M, Hansen K. Randomized controlled trial A cost-minimization analysis of large overjet reduction with two removable functional appliances based on a randomized controlled trial. Eur J Orthod. 2018;40:437–43.
Acknowledgements
Not applicable
Funding
This work was supported by:
1. General project from the field of Social Development, in the Department of Science and Technology of Shaanxi Province, Grant/Award Number: 2019SF-081
2. Science and Technology Project of Xi ‘an, Grant/Award Number: 20YXYJ0010(3)
3. Open projects of Clinical Research Center Dental of Shaanxi Province for Dental and Maxillofacial Diseases, Grant/Award Number: 2017YHJB01
4. Clinical New Technology from Stomatological Hospital of Xi ‘an Jiaotong University in 2018
Author information
Authors and Affiliations
Contributions
JH drafted the manuscript under the supervision of FW and RZ, BC, SH-Y.M and M-Y.Z contributed to the data collection. W-Q.B, G-L.W performed data analysis. LQ and JH did the study design, FW and RZ revised and supervised all the works. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Stomatological Hospital of Xi’an Jiaotong University, Ethics approval number: Xjkqll [2018] No.17. All patients’ parents or guardians were informed and signed written consent forms to participate in the research. All procedures performed in this study involving human participants were following the ethical standards of the institutional and/or research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from the parents/guardians of the children for their personal or clinical details and any identifying images to be published in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Age distribution data in MB-M,MB-N and NB groups. Supplementary Table 2. Sex distribution data in MB-M,MB-N and NB groups. Supplementary Table 3. ANB(°) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 4. SNA(°) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 5. SNB(°) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 6. SN-GoGn (°) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 7. FH-MP (°) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 8. S-Go /N-Me data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 9. ANS-Me/N-Me data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 10. U1-NA (°)data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 11. U1-NA (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 12. L1-NB (°)data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 13. L1-NB (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 14. Corpus length (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 15. N-Me (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 16. S-Go (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 17. ANS-Me (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 18. Co-Go (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 19. Ramus height (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 20. Overjet (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 21. Overbite (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 22. C-C (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 23. C′- C′(mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 24. M-M (mm) data in MB-M,MB-N and NB groups for T1 and T2. Supplementary Table 25. M’- M’(mm) data in MB-M,MB-N and NB groups for T1 and T2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Habumugisha, J., Cheng, B., Ma, SY. et al. A non-randomized concurrent controlled trial of myofunctional treatment in the mixed dentition children with functional mouth breathing assessed by cephalometric radiographs and study models. BMC Pediatr 22, 506 (2022). https://doi.org/10.1186/s12887-022-03559-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03559-w