Abstract
Preeclampsia is a hypertensive disorder of pregnancy with serious health implications for mother and their offspring. The uteroplacental vascular insufficiency caused by preeclampsia is associated with epigenetic and pathological changes in the mother and fetus. However, the impact of preeclampsia in infancy (birth to 2 years), a time of rapid development influenced by pre- and postnatal factors that can predict future health outcomes, remains inconclusive. This narrative review of 23 epidemiological and basic science studies assessed the measurement and impact of preeclampsia exposure on infant growth and psychomotor developmental outcomes from birth to 2 years. Studies assessing infant growth report that preeclampsia-exposed infants have lower weight, length and BMI at 2 years than their normotensive controls, or that they instead experience accelerated weight gain to catch up in growth by 2 years, which may have long-term implications for their cardiometabolic health. In contrast, clear discrepancies remain as to whether preeclampsia exposure impairs infant motor and cognitive development, or instead has no impact. It is additionally unknown whether any impacts of preeclampsia are independent of confounders including shared genetic factors that predispose to both preeclampsia and childhood morbidity, perinatal factors including small for gestational age or preterm birth and their sequelae, and postnatal environmental factors such childhood nutrition. Further research is required to account for these variables in larger cohorts born at term, to help elucidate the independent pathophysiological impact of this clinically heterogenous and dangerous disease.
Similar content being viewed by others
Introduction
Approximately 3–5% of women worldwide experience preeclampsia, a multisystem hypertensive disorder of pregnancy (Table 1) [1,2,3]. Preeclampsia represents a significant maternal health burden with complications including perinatal mortality and increased lifetime risks of cardiometabolic diseases such as hypertension, stroke, ischaemic heart disease and type 2 diabetes mellitus [2, 4,5,6,7,8,9].
In preeclampsia, pathological mechanisms such as uteroplacental vascular insufficiency create an unfavourable intrauterine environment [10, 11], which lead to many extensively studied fetal and neonatal complications [2, 7, 10,11,12,13,14,15]. In children and adults, intrauterine preeclampsia exposure is associated with an increased risk of cardiovascular, metabolic, immune, respiratory, and neurodevelopmental morbidities [10, 16,17,18,19,20,21]. One explanation is the Developmental Origins of Health and Disease (DOHaD) hypothesis, which suggests that the fetal adaptation to the adverse intrauterine environment increases future chronic disease risk [10]. Alternatively, others suggest that shared genetic or environmental risk factors predispose to future maternal and paediatric morbidity [16, 22].
There is some evidence for impaired growth and psychomotor neurodevelopment in infancy (birth to 2 years) after preeclampsia exposure [23, 24], but much of the existing data are limited by their minimal adjustment for perinatal confounders, the variable use of assessment tools for growth and development, and their specific study cohorts of preterm or very low birthweight (VLBW) infants (Table 1 and 2). Robust early detection of abnormal growth and development trajectories may aid the development of novel therapeutic interventions to improve childhood health outcomes for infants exposed to preeclampsia. We aimed to determine whether infants with intrauterine preeclampsia exposure, compared to infants born from normotensive pregnancies, have differing anthropometric growth outcomes and psychomotor developmental outcomes from birth to 2 years of age. Thus, we review the fetal, neonatal and long-term consequences of preeclampsia exposure, discuss differing ways to measure infant growth and developmental outcomes, and review studies of infant growth and psychomotor development associated with preeclampsia exposure.
Methods
We searched PubMed, Medline and Embase using search terms: preeclampsia AND (infant OR child) AND (growth OR weight OR length OR development OR neurodevelopment), Google Scholar with key words preeclampsia, hypertensive disorders of pregnancy, child, infant, growth, development, neurodevelopment, health, and the gray literature to identify cohort or case–control studies, published any date to 31st October, 2021, without language restriction or full-text restriction, that assessed infant growth or development after preeclampsia exposure.
Inclusion criteria were outcome data on infant growth (weight, length, BMI, weight for age, weight for length, growth trajectories and other anthropometric measures) and psychomotor neurodevelopment (gross and fine motor, expressive and receptive communication, social, personal and cognitive skills) from birth up to and including 2 years of age in infants with intrauterine preeclampsia exposure. Studies were also included if other hypertensive disorders of pregnancy such as gestational hypertension, were combined with preeclampsia or included as a separate exposure group in addition to preeclampsia exposure, or if preeclampsia exposure was stratified according to severity or timing of onset, for example, in the case that no normotensive group was compared. Studies were excluded if they only reported birth outcomes, did not include outcomes reported between birth to 2 years of age, but were included if they reported later outcomes in addition to this age range. Studies were also excluded if data were reported on other hypertensive disorders not including preeclampsia.
Eligible studies were critically appraised by two reviewers (PV and MLG) for methodological quality using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Cohort Studies and Case–Control Studies, with possible answers including “yes”, “no”, “unclear” or “not applicable” [36]. After discussion and agreeance between reviewers about cut-off values as suggested in the JBI Manual for Evidence Synthesis, the studies were categorised as either of low risk (≥ 70% “yes”), moderate risk (50–69% “yes”), or high risk (< 50% “yes”) of bias [36, 37].
Background
Intrauterine complications of preeclampsia and the DOHaD hypothesis
Barker et al. [38,39,40,41] were the first to suggest that a chronic, non-communicable disease in adulthood- ischaemic heart disease, was associated with exposure to an intrauterine environment that inhibited fetal growth and nutrition. Barker’s hypothesis was extended by studies that controlled for confounders including gestational age at birth, genetic risk factors and postnatal environmental factors [19, 42]. They found independent associations between fetal growth restriction (FGR) and a wider range of chronic diseases, resulting in the DOHaD hypothesis.
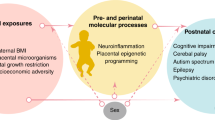
Epigenetics refers to phenotype changes caused by alterations in gene expression rather than hereditary changes in the DNA sequence itself. Epigenetic changes occur in both developing and differentiated tissue through mechanisms including DNA methylation, histone modification and the action of micro- and noncoding-RNAs [43,44,45]. These mechanisms can be influenced by the perinatal maternal, paternal and postnatal environment, and in line with the DOHaD hypothesis, may impact the offspring’s future health [45]. Although the pathogenesis of preeclampsia is incompletely understood, proposed mechanisms include immunological imbalances, pre-existing comorbidities including obesity and chronic hypertension, and epigenetic changes in the placenta and maternal circulation, which lead to defective placentation and incomplete trophoblast invasion into the myometrial spiral arteries in early pregnancy. Subsequent angiogenic imbalances, placental hypoperfusion and ischaemia, and systemic maternal inflammation and oxidative stress occur, with associated fetal endothelial dysfunction, hypoxia and malnutrition of varying severity [11, 43, 44]. It is hypothesised that the fetus undergoes ‘developmental programming’ as an adaptation to this adverse intrauterine environment, which may increase their future risk of morbidity [43,44,45] (Fig. 1).
The DOHaD hypothesis suggests that greater intrauterine preeclampsia-exposure, irrespective of shared genetic or lifestyle factors, has a programming effect that impacts the child’s development of morbidities. For example, a large population-based cohort study (n = 758,524) [46] demonstrated a higher relative risk of long-term morbidity in offspring the longer the intrauterine preeclampsia-exposure. However, the investigators were unable to control for maternal body mass index (BMI), smoking, lifestyle factors or diet, all possible contributors to childhood morbidity. In sibling studies, children exposed to preeclampsia had increased vascular dysfunction [19] and higher risks of developing neurodevelopmental morbidities [47] than their unexposed sibling, supporting an intrauterine programming effect of preeclampsia.
Conversely, others suggest that shared genetic or environmental risk factors that predispose to future paediatric morbidity, or even preeclampsia itself such as maternal cardiometabolic disease, are responsible for the increased disease risk observed in exposed children [16, 22] (Fig. 1). This may also explain why late-onset preeclampsia with uteroplacental disease of differing severity, or gestational hypertension, which does not typically demonstrate the intrauterine complications of preeclampsia, are also associated with increased risks of childhood morbidity [3, 48,49,50].
Figure 1: In the prenatal, perinatal, and postnatal periods, factors associated with preeclampsia such as genetic risk factors shared between parent and child, intrauterine changes, and external environmental influences including neonatal complications, parent health behaviours and the postnatal lifestyle, may contribute to altered childhood health outcomes. These factors can directly influence childhood growth and development, or may induce epigenetic reprogramming during fetal and neonatal development that can subsequently increase child future chronic disease risk. Created with BioRender.com
Perinatal and neonatal outcomes after preeclampsia exposure
Preeclampsia is associated with adverse fetal outcomes including FGR, placental abruption, stillbirth, and neonatal mortality [7, 51]. Approximately 12–33% of preeclampsia-exposed neonates are born small for gestational age (SGA, birthweight z-score corrected for sex and gestational age < 10th centile) [52,53,54,55]. Delivery is the only definitive management of preeclampsia to prevent progression to end-organ damage [56]. Subsequently, many neonates are born premature, with associated sequelae including nursery admission, respiratory distress syndrome, bronchopulmonary dysplasia, and sepsis [7, 12, 57].
Clinically, preeclampsia is a heterogenous disorder with poorer perinatal outcomes generally associated with early-onset (< 34 weeks’ gestation) and preterm (34 – 36 + 6 weeks’ gestation) preeclampsia compared to that diagnosed at term (≥ 37 weeks). This may be due to the complications of preterm delivery, or longer fetal exposure to the adverse intrauterine environment, resulting from the greater uteroplacental dysfunction in early-onset (versus term) preeclampsia [13, 49, 50, 58]. Nonetheless, the intrauterine and perinatal complications of preeclampsia are associated with adverse paediatric outcomes [59].
Long-term paediatric outcomes after preeclampsia exposure
Preeclampsia exposure has been associated with increased long-term paediatric cardiometabolic risk, including increased blood pressure [16, 60,61,62,63,64] and BMI [16, 17, 63], altered cardiac structure [65] and vascular function [19, 66], and increased stroke [67] and hypertension risk [21, 67]. While preeclampsia exposure has also been associated with increased risks of neurodevelopmental disorders including autism spectrum disorder [68,69,70,71], attention deficit hyperactivity disorder [72,73,74], epilepsy [75, 76], impaired motor development [77, 78], mild cognitive impairment or neurodevelopmental delay [79,80,81,82,83], cerebral palsy [84,85,86] and mood disorder symptoms [87], some studies suggest it has a neuroprotective effect [88]. Furthermore, preeclampsia is linked to immunological impairment in exposed offspring, including increased risk of asthma and other respiratory diseases [20, 75, 89, 90], atopy and allergic sensitisation [90,91,92], and allergic rhinoconjunctivitis [92].
The strengths of these studies are that most had relatively large sample sizes and adjusted for putative genetic and lifestyle confounders, including maternal demographic variables like BMI, prior comorbidities and ethnicity, and neonatal factors including prematurity status, gestational age, SGA status, and special care nursery stay [7, 10, 59, 93]. However, heterogenous findings between studies could be explained by the nonstandard adjustment of these potential confounders, and further replication of results is needed for lesser studied morbidities such as stroke [67] and allergic rhinoconjunctivitis [92]. Furthermore, few studies adjusted for confounding lifestyle factors such as childhood nutrition [10] which influence cardiometabolic health, and despite adjustment for maternal CVD, the genetic inheritability of chronic morbidities like CVD are difficult to exclude. Furthermore, few studies considered preeclampsia severity or onset, which, given the clinically heterogeneity of preeclampsia, may significantly alter paediatric outcomes [93]. Hence, while the longer-term paediatric consequences of preeclampsia have been investigated, more targeted research is needed to validate and replicate current findings, and disentangle the impact of genetic and lifestyle factors from preeclampsia exposure itself.
Infant growth after preeclampsia exposure
Growth in infancy (birth – 2 years) is rapid, non-linear, and a key indicator of health and nutritional status. Infant growth is influenced by many factors including genetics, feeding patterns, nutrient composition, metabolic and hormonal signals, environmental influences and underlying pathological processes [94,95,96]. Rapid growth in infancy can reflect underlying genetic, cardiovascular, metabolic, endocrine, or gastrointestinal morbidities including preeclampsia exposure, and is associated with increased future risks of obesity, metabolic syndrome, and CVD. Poor growth in infancy may indicate poor nutritional status, underlying genetic conditions or morbidity associated with FGR such as that experienced in preeclampsia, and is associated with later neurological, cardiovascular, renal, and respiratory morbidity [94,95,96,97,98]. Elucidating the impact of preeclampsia on growth is hence of utmost clinical significance.
Assessment of infant body composition
Body composition assessments, including anthropometric measurements of weight, length, head, abdominal and mid-upper arm circumferences, and triceps and subscapular skinfold thicknesses act as clinical screening tools to monitor infant growth and risk of future morbidity [99]. Body proportion metrics derived from height and weight measures include weight-for-length and BMI. Weight-for-length is currently recommended by the World Health Organisation (WHO) and has been adopted internationally to assess body proportionality in infants aged ≤ 2 years [100,101,102]. It considers the positive relationship between height and weight and is a useful indicator of nutritional status when infant age is unknown, however it is not adjusted for age-dependent variations and is a suboptimal indicator of adiposity [103,104,105]. In contrast, BMI (weight in kilograms/ height in metres squared) has a higher correlation with fat mass, fat-free mass and percent body fat z-scores than weight-for-length. It is also adjustable for infant age, including gestational age, to assess infant growth over time [104,105,106]. Although the ponderal index (weight in grams × 100/ length in centimetres cubed) has been considered a more appropriate measure of proportional growth in preterm infants in the past, BMI may have a stronger correlation with fat measures and is also a suitable measure of preterm infant body proportionality [107]. Considering BMI z-scores are currently recommended for assessing growth in children older than 2 years, measuring BMI in infancy may also provide a more consistent growth assessment in primary care settings [104,105,106]. However, one large prospective cohort study found the choice of weight-for-length compared to BMI z-scores did not greatly affect the association with future cardiometabolic outcomes, suggesting either are suitable measures of infant growth [106].
Assessment of longitudinal infant growth
The WHO Child Growth Standards charts are validated standards to calculate an infant’s age- and sex-adjusted growth relative to the population mean [100, 101]. The Fenton Preterm Growth Charts, revised in 2013, are established standards developed to assess the size of preterm infants at birth [108]. However, they do not consider the postnatal physiological weight loss experienced by infants in the first days of life, and thus are unsuitable for assessing the longitudinal growth of preterm infants [109]. The INTERGROWTH-21(st) Preterm Postnatal Growth Standards [110] may be more accurate for preterm populations as they consider the differing postnatal growth patterns in the first 6 months that preterm neonates experience. They were developed from the postnatal growth of preterm infants born without morbidity from uncomplicated pregnancies across 8 countries, and have high concordance with the Fenton Preterm Growth Charts, identifying slightly greater numbers of SGA infants at birth. Importantly, these additional infants identified had higher incidences of morbidity than those identified by the Fenton Charts, supporting the use of the INTERGROWTH-21(st) Standards in preterm populations [111, 112]. However, they were developed from only 201 infants and require further international validation in larger, ethnically and socioeconomically diverse populations. As the postnatal growth of preterm infants converges with term infants by 6 months, the WHO standards are appropriate for all infants 6 months onwards [110].
Weight-for-age z-scores assess longitudinal infant growth, and BMI or weight-for-length z-scores assess proportionality change [101]. Rapid weight gain is defined as a > 0.67 gain in weight-for-age z-score, corresponding to crossing two centile lines on respective growth charts, and is associated with future CVD risk [113]. Infants who suffered FGR and were subsequently born SGA, a common complication of preeclampsia, often experience necessary rapid weight gain as a recovery response to intrauterine undernutrition [114]. This is referred to as ‘rapid catch-up growth’; an example of how infants born on weight extremes may experience natural regression to the mean postpartum [115], and also how infant weight may vary dynamically relative to weight-for-age growth curves [113]. Current interpretations of WHO weight-for-age curves assume children may normally not deviate from their initial weight standard deviation (SD) score [101], and thus weight-for-age changes can represent pathological growth trajectories in otherwise healthy children.
For infants 0–6 months, this limitation of weight-for-age z-scores may be overcome using conditional weight gain z-scores. This compares current infant weight with that predicted from their previous weight to derive a weight gain SD score, and references this to a conditional reference which considers the tendency of infants on the extremes of weight to experience non-pathological regression to the mean [31, 115,116,117]. For infants 6–24 months, including those born premature or SGA, BMI or weight-for-length z-scores are alternative metrics to assess growth that may account for the limitations of weight-for-age z-scores [118].
Results: growth outcomes of infants exposed to preeclampsia
While is it well established that preeclampsia is associated with FGR and both premature and SGA birth, it is still unclear whether preeclampsia has an intrauterine programming effect impacting infant growth trajectories independent of these perinatal and other genetic and lifestyle confounders [15, 57, 119]. Furthermore, although all classifications of preeclampsia are considered clinically significant and potentially life-threatening for mother and child [50], early onset or more severe preeclampsia may reflect greater placental dysfunction that can impact fetal, neonatal and childhood growth differently to later onset, mild or moderate disease [49].
Our search identified 11 studies that assessed infant growth outcomes after preeclampsia exposure. All studies were assessed with the JBI tool to have a low risk of bias. (Table 2). Six of these reported that infants exposed to preeclampsia had lower weight and BMI throughout infancy, remaining smaller at multiple timepoints from birth to 2 years than infants of normotensive pregnancies [23, 24, 26,27,28, 31]. Two cohort studies of preterm, VLBW (< 1500 g) infants, found those exposed to preeclampsia had significantly lower absolute weight, weight z-scores and weight-for-length z-scores throughout infancy [23, 26]. In preterm infants, two studies also report an association with preeclampsia and lower weight [24, 28], however the latter study grouped preeclampsia and gestational hypertension exposure and found no difference in weight in term infants compared to those born from normotensive pregnancies. While this suggests that the impact of preeclampsia may vary across the gestational spectrum, it may instead reflect the impact of early-onset or more severe preeclampsia that is often the cause of premature birth [49, 50]. This is supported by Byberg et al. (2017), who reported lower BMI z-scores from infancy in those exposed to more severe preeclampsia [27]. However, although these studies demonstrate associations between preeclampsia and poor infant growth, they did not adjust for the confounding influence of premature or VLBW birth, which are independently associated with infant growth restriction [15, 24, 57, 119]. This limits the isolation of the specific pathophysiological implications of preeclampsia exposure independent of these confounders.
In contrast, three studies have reported no difference in weight or BMI in infants exposed to preeclampsia or normotensive pregnancies in late infancy [24, 25, 120]. Davis et al. (2015) [18] reported preeclampsia and gestational-hypertension-exposed neonates were not significantly smaller in birthweight when adjusted for gestational age and had no differences in weight z-score or BMI at 12 months compared to infants of normotensive pregnancies. However, Martikainen et al. (1989) [24] reported that preeclampsia-exposed infants who were born significantly smaller at term, similarly had no difference in weight to normotensive infants by 18 months, suggesting they had an accelerated growth trajectory that enabled ‘catch up’ growth. This may suggest a relationship of preeclampsia exposure with accelerated growth independently, or in conjunction with SGA birth that, although associated with impaired infant growth in some infants, is a cause of rapid weight gain in others as a response to intrauterine undernutrition [114, 115]. Both Gow et al. (2021) [31] and Jasper et al. (2021) [32] investigated this relationship, and while they reported associations between preeclampsia exposure and weight gain throughout infancy, preeclampsia exposure was no longer a significant contributor to this catch-up growth after full adjustment for confounders like SGA status and maternal BMI. Overall, this suggests that the pathological mechanisms of preeclampsia may have no independent impact on infant weight gain. However clinically, preeclampsia and its associated comorbidities have been associated with increased growth trajectories and rapid weight gain, leading to greater BMIs in late infancy [30], greater weight and BMI from school age onwards in females especially [27], and a threefold risk of being hypertensive by age 20 [18].
Similar discrepancies regarding infant length and length gain are present. Although Martikainen et al. (1989) [24] and Matić et al. (2017) [28] reported that in preterm infants, those exposed to preeclampsia continued to have lower lengths in late infancy, this trend did not persist for term infants, potentially reflective of the impact of more severe or early-onset PE that these preterm infants may have experienced. Five other studies reported no difference in length or length z-scores in late infancy [18, 23, 25, 26, 31], reflecting either minimal differences in length at birth between groups, or for preeclampsia-exposed infants born small, the catch-up growth they experienced. Interestingly, while Gunnarsdottir et al. (2018) [29] reported no length differences in infants exposed to mild or moderate preeclampsia versus normotensive pregnancies, those with severe preeclampsia exposure had lower length z-scores at 18 months. This supports the notion that preeclampsia may encompass pathologically diverse diseases grouped by onset or severity that impact infant growth heterogeneously. In infants exposed to severe preeclampsia, Gunnarsdottir et al. (2018) [29] additionally reported greater absolute length gain, while Gow et al. (2021) [31] reported no difference in length z-score gains, and Byberg et al. (2017) [27] lower length z-score gains. The heterogenous findings of these studies may be partially mediated by gestational age and SGA status.
Head circumference differences between infants exposed to preeclampsia versus normotensive pregnancies have also been explored [23,24,25,26, 28]. In preterm or VLBW infants, preeclampsia exposure seems to minimally contribute to differences in head circumference, or be associated with lower head circumferences throughout infancy [23,24,25,26, 28]. When considering only those born at term, Martikainen et al. (1989) [24] demonstrated that preeclampsia exposure was associated with larger head circumferences. While potentially confounded by the influence of SGA status, this finding may suggest an independent impact of preeclampsia, and support the differences between severe, earlier-onset preeclampsia more common in preterm infants, versus the moderate or later onset disease positively associated with growth in term infants.
In general, there are many discrepancies between the studies assessing the impact of preeclampsia exposure on growth. Despite demonstrating low risks of methodological bias, these studies had limitations such as differing adjustment for confounders due to a lack of collected data, specific cohorts of premature or VLBW infants, or deliberate choice to consider the intermediate relationship of the confounder with preeclampsia and growth [27]. Furthermore, lack of adjustment for postnatal infant nutrition and other environmental influences may lead to an overestimation of the impact of preeclampsia exposure. Also, certain studies had smaller sample sizes [23, 25, 30], were designed to assess multiple perinatal comorbidities rather than preeclampsia specifically [18, 30, 32], or compared differing subgroups of preeclampsia severity [18, 27, 29]. As such, the impact of intrauterine preeclampsia exposure on growth in infancy, either independent of confounders like SGA or prematurity status in line with the DOHaD hypothesis, or in conjunction with inherited genetics that predispose to both preeclampsia and cardiometabolic disease, remain uncertain. Nevertheless, preeclampsia exposure remains a clinically significant risk factor that highlights opportunities to monitor infants into later childhood, and may indicate a need for early clinical intervention.
Infant development after preeclampsia exposure
Infant psychomotor development refers to the maturation of the brain and central nervous system in four main domains: gross and fine motor skills, speech and language, performance and cognition, and social and personal skills [121]. Despite being a dynamic process influenced by genetic, perinatal, and environmental factors, normal development generally occurs in an ordered and sequential pattern correlating to age-dependent developmental milestones [121, 122].
Assessment of infant development
Developmental assessment is a longitudinal process involving joint surveillance by both clinicians and parents [123]. Developmental screening tools assist the identification of potential developmental delay, defined in infants as > 2SDs below the mean on age-appropriate standardised testing [124]. The Ages and Stages Questionnaire (ASQ) [125], Parents Evaluation of Developmental Status [126] and Survey of Well-being of Young Children [127] are commonly used, parent-completed screening surveys that assess many domains including fine and gross motor, receptive and expressive communication, problem solving, and personal or social skills. These tools consider parental observation which may increase their sensitivity [123, 128,129,130], however, they may not be suitable for infants younger than 4 months or those with special needs [125, 127, 129]. The Parent Report of Children’s Abilities-Revised [131, 132] is useful for screening preterm infants, while the parent-completed Child Development Inventory [133] can assist identification of children with special needs [129, 133]. Similarly, the child-administered Battelle Developmental Inventory Screening Tool, 2nd edition [134, 135] can be modified for special needs children to assess psychomotor development. The Denver Developmental Screening Test, 2nd edition [136] and the Brigance Screens [137] also assess infant psychomotor domains through direct elicitation and observation of the child, however are longer to administer (10–20 min) than parent-completed surveys [129, 137]. While these screening tools are generally simple, quick and cost effective to implement in a primary care setting, they are not diagnostic, so children identified at risk of developmental delay require specialist diagnostic developmental assessment [121, 123].
While no gold standard assessment tool exists, the Bayley Scales of Infant Development 2nd (BSID-II) [138] and 3rd (BSID-III) [139] editions are the most commonly used and validated psychometric assessments used in infancy for both clinical and research purposes [122, 123]. These assessments assist the identification, and for the BSID-III, quantification of developmental delay in infant psychomotor (PDI) and mental (MDI) developmental indices. While the BSID-II MDI score was additionally useful in determining cognitive function in preterm or low birthweight infants, the BSID-III may have reduced sensitivity in these populations [140], and both are long assessments which may provide more difficulty for clinician, parent and infant [122, 123, 140]. The Griffiths Mental Development Scale, 2nd Edition [141] is another assessment with concurrent validity to the BSID-II that may be more successful than the BSID-II at detecting motor delays in infancy, however it may not be as sensitive for detecting speech and language delay. It also has a subsequent scale from ages 2–8, which may be useful for longitudinal childhood developmental surveillance [122, 141]. Furthermore, assessment tools like the Mullen Scales of Early Learning [142] may be useful for assessing the cognitive development of infants without or with autism spectrum disorder or known developmental delay. While many developmental assessments measure similar domains and have concurrent validity, scores are often measured on differing scales and thus clinically, should not be interchanged between tools to prevent inaccurate approximation of infant ability [122, 143].
Results: developmental outcomes of infants exposed to preeclampsia
Although intrauterine preeclampsia exposure has been associated with impaired psychomotor development in older children, adolescents, and adults [83, 144], there are uncertainties regarding its effects on psychomotor development in infancy (birth – 2 years).
Our search identified 17 studies assessing infant psychomotor development after preeclampsia exposure. All studies were assessed with the JBI tool to have a low risk of bias. (Table 3). Most studies were conducted on specific populations of infants, such as those born preterm, of VLBW or SGA, comorbidities independently associated with poorer neurodevelopment [15, 145,146,147]. For example, in cohorts of preterm infants, Spinillo et al. (1994) [148] reported lower BSID mental and psychomotor developmental index scores, and Johnson et al. (2015) [149] poorer cognitive outcomes, in 2 year old infants exposed to preeclampsia after adjustment for SGA status and other covariates. Similarly, Martikainen et al. (1989) demonstrated infants exposed to preeclampsia born preterm had poorer fine motor skills and visuo-auditory perception at 18 months than normotensive controls, while term infants had better motor skills, visuo-auditory perception and social abilities. This may reflect the impact of more severe or early-onset preeclampsia, which are associated with greater uteroplacental deficiencies and are often the cause of preterm birth [49, 150]. In contrast, other studies in preterm populations reported no difference in infant neurodevelopmental outcomes after preeclampsia exposure alone [151, 152] or after grouped preeclampsia and gestational hypertension exposure [28] after adjustment for confounders. Schlapbach et al. (2010) [152] further demonstrated that postnatal complications of preterm birth including mechanical ventilation, bronchopulmonary dysplasia and sepsis had greater associations with poor neurodevelopment than the pathophysiological changes of preeclampsia exposure itself [152].
When considering infant populations born not only preterm, but also of VLBW or SGA, studies assessing the impact of preeclampsia have reported similarly discrepant findings. Two small studies in VLBW, preterm infants, found those exposed to preeclampsia had lower BSID-II MDI scores at 2 years but no difference in PDI scores, suggesting preeclampsia exposure itself may contribute to poor mental development [23, 25]. However, Cheng et al. (2004) [25] found these differences were only associated with mild neurodevelopmental delay (-1 to -2 SDs from the mean) rather than severe delay (> -2 SDs), and found no differences when controlling for SGA status. In FGR infants, Warshafsky et al. (2016) [80] demonstrated that those exposed to severe preeclampsia were more likely to have failed at least one ASQ category at 12 and 24 months, especially the gross motor category, than those exposed to mild preeclampsia or normotensive pregnancies. This may reflect the clinical variability of mild versus severe disease. Similar to Martikainen et al. (1989) [24] however, they also reported that lower gestational age significantly contributed to the impact of severe preeclampsia, and FGR increased the risk in all groups, suggesting the impacts of preeclampsia on infant neurodevelopment may not be independent of these intermediary morbidities.
Alternatively, studies in these VLBW, SGA or preterm cohorts have suggested preeclampsia exposure may be neuroprotective and associated with a reduced risk of neurodevelopmental delay in one or more subcategories [26, 80, 153,154,155]. Two large cohort studies in preterm cohorts found preeclampsia-exposed infants had higher BSID-II MDI scores at 2 years [154, 155]. Although Spinillo (2009) [154] reported preeclampsia overall was associated with reduced risk of neurodisability, this finding may be explained by their normotensive preterm group being predominantly exposed to spontaneous birth or preterm premature rupture of the membranes, which carry increased risks of infection or inflammation that may be greater associated with abnormal neurodevelopment than preeclampsia exposure itself [154]. Furthermore, Spinillo (2009) [154] reported that although preeclampsia exerted a protective effect overall, the impaired neurodevelopment associated with male sex was higher for preeclampsia-exposed infants than their normotensive counterparts, suggesting a greater vulnerability of male infants to the pathophysiological changes of preeclampsia. Furthermore, McCowan et al. (2002) [153] and Silveira et al. (2007) [26] in SGA or VLBW cohorts, reported that preeclampsia-exposed infants had higher MDI and PDI scores respectively at 18 months. Although suggestive of a neuroprotective effect of preeclampsia, McCowan et al. (2002) [153] grouped preeclampsia and gestational hypertension exposure, and the other causes of SGA birth that normotensive controls were exposed to may mediate this finding, as they may be more strongly associated with neurodevelopmental delay than preeclampsia itself, similar to the complications of preterm birth, [153].
Few studies assessing infant neurodevelopment after preeclampsia exposure have been conducted in mixed cohorts including infants born at term or of an appropriate birthweight. As previously described, Martikainen et al. (1989) [24] found term preeclampsia-exposed infants had greater motor performance, visuo-auditory skills and social abilities at 18 months than both term normotensive, or preterm preeclampsia-exposed infants. In contrast, Wade (2016) [156] reported infants exposed to preeclampsia and other hypertensive pregnancy disorders had poorer social cognition at 18 months after full adjustment for confounders, however their study was retrospective in design, and limited by a small sample of preeclampsia-exposed infants. Similarly, Bharadwaj et al. (2018) [157] using a comparably validated foreign language tool to the BSID-II, reported preeclampsia exposure was independently associated with poorer motor and cognitive development at 1 year. They additionally reported that a lower maternal antioxidant status was an independent predictor of poorer motor development in the preeclampsia-exposed group, suggesting the intrauterine maternal oxidative stress present in preeclampsia may potentially contribute to impaired infant neurodevelopment. However, in larger cohorts, Chen et al. (2020) [158] and Maher (2020) [159] reported no difference in psychomotor developmental outcomes after full adjustment for perinatal confounders.
As such, while preeclampsia may not be associated with neuroprotective impacts in infancy, it remains inconclusive as to whether its underlying pathophysiological mechanisms negatively impact infant neurodevelopment independent of common perinatal confounders. Subsequently, further prospective studies with larger sample sizes, that include term infants born at an appropriate birthweight, and that use validated psychometric assessment tools such as the BSID-II, are indicated to disentangle the relationships of these variables. While these pathophysiological relationships remain unclear, clinically, preterm and SGA birth are common complications experienced by preeclampsia-exposed infants, and hence exposed infants may be at greater risk of neurodevelopmental impairment overall [7, 10, 57]. Although not the focus of this review, preeclampsia exposure may be additionally associated with other neurosensory disabilities including cerebral palsy [85, 165], blindness, deafness and intellectual disabilities [81, 165], which are often studied concurrently to psychomotor development and can assist in providing a greater understanding of the impact of preeclampsia on infant neurodevelopment as a whole.
Limitations
The reviewed literature contains several limitations. Each study varied slightly in their definitions of preeclampsia, with most defining preeclampsia as new onset hypertension > 20 weeks gestation with varying degrees of proteinuria [18, 24,25,26,27, 29, 30, 32, 80, 148, 153,154,155, 158, 159], some using a broader definition of preeclampsia encompassing features of maternal end-organ dysfunction or uteroplacental insufficiency [23, 31, 151, 152, 157], one combining gestational hypertension and preeclampsia [28], and others poorly defining preeclampsia or relying on maternal report of preeclampsia during pregnancy [149, 156, 159]. Studies used a variety of local and international growth standards, including the WHO Growth Standards, to calculate z-scores for infant anthropometric measures which may not be appropriate for calculating the longitudinal growth outcomes of preterm populations. While studies were assessed as containing a low risk of bias based on methodological quality, the JBI tools do not consider sample size, and the aforementioned variances in study design, exposure definitions, growth assessments and control of confounders allow no definitive conclusions to be drawn, and we acknowledge that as a review article, our interpretation of the literature is subject to bias. However, our review aims to highlight trends in the literature and guide future study design rather than draw definitive conclusions regarding the impact of preeclampsia on child health.
Conclusions
Preeclampsia is a serious pregnancy complication with significant consequences for both maternal and paediatric health. It is well established that preeclampsia causes FGR, SGA and preterm birth, and is associated with increased risk of cardiometabolic, neurodevelopmental and immunological morbidity in later life. Preeclampsia-exposed infants born SGA either do not demonstrate catch-up, especially those exposed to more severe or early-onset preeclampsia, or alternatively may experience rapid weight gain and catch-up growth, however perinatal confounders such as maternal BMI and postnatal feeding may influence this association. While most data suggest preeclampsia exposure may not impair infant motor and cognitive development independent of the influence of preterm and SGA birth, further research is required in larger cohorts born at term, controlling for perinatal confounders, and using standardised and validated assessment measures appropriate for individual child health and demographic characteristics, including gestational age at birth, SGA status, language, and neuropsychological disabilities. These may elucidate how the underlying pathophysiological mechanisms of preeclampsia impact infant health outcomes, and highlight the opportunity for early monitoring of infant growth and development before school age and the onset of later childhood morbidity. These may also indicate the need for novel therapeutic intervention, or early lifestyle intervention such as improving infant feeding practices, to optimise the future cardiometabolic and neurodevelopmental health outcomes of exposed infants.
Availability of data and materials
No datasets generated or analysed for this review. All data reviewed is previously published. Figure created with BioRender.com.
Abbreviations
- ASQ:
-
Ages and Stages Questionnaire
- BMI:
-
Body Mass Index
- BSID-II/III:
-
Bayley Scales of Infant Development (2nd ed./3rd Edition)
- CVD:
-
Cardiovascular disease
- DOHaD:
-
Developmental Origins of Health and Disease
- FGR:
-
Fetal growth restriction
- JBI:
-
Joanna Briggs Institute
- MDI:
-
Mental developmental index
- PDI:
-
Psychomotor developmental index
- SD:
-
Standard deviation
- SGA:
-
Small for gestational age
- VLBW:
-
Very low birthweight
- WHO:
-
World Health Organisation
References
Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open. 2011;1(1):e000101.
Gestational Hypertension and Preeclampsia. ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237–60.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310.
Brown MA, Roberts L, Hoffman A, Henry A, Mangos G, O’Sullivan A, et al. Recognizing Cardiovascular Risk After Preeclampsia: The P4 Study. J Am Heart Assoc. 2020;9(22):e018604.
Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003497.
Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. The Lancet. 2016;387(10022):999–1011.
Bokslag A, van Weissenbruch M, Mol BW, de Groot CJM. Preeclampsia; short and long-term consequences for mother and neonate. Early Human Dev. 2016;102:47–50.
Hermes W, Franx A, van Pampus MG, Bloemenkamp KW, Bots ML, van der Post JA, et al. Cardiovascular risk factors in women who had hypertensive disorders late in pregnancy: a cohort study. Am J Obstet Gynecol. 2013;208(6):474.e1-8.
Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, et al. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: An Observational Cohort Study. Ann Intern Med. 2018;169(4):224–32.
Lu HQ, Hu R. Lasting Effects of Intrauterine Exposure to Preeclampsia on Offspring and the Underlying Mechanism. AJP Rep. 2019;9(3):e275–91.
Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275–89.
Nomura Y, John RM, Janssen AB, Davey C, Finik J, Buthmann J, et al. Neurodevelopmental consequences in offspring of mothers with preeclampsia during pregnancy: underlying biological mechanism via imprinting genes. Arch Gynecol Obstet. 2017;295(6):1319–29.
Pettit F, Mangos G, Davis G, Henry A, Brown MA. Pre-eclampsia causes adverse maternal outcomes across the gestational spectrum. Pregnancy Hypertens. 2015;5(2):198–204.
Harmon QE, Huang L, Umbach DM, Klungsøyr K, Engel SM, Magnus P, et al. Risk of fetal death with preeclampsia. Obstet Gynecol. 2015;125(3):628–35.
von Beckerath A-K, Kollmann M, Rotky-Fast C, Karpf E, Lang U, Klaritsch P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol. 2013;208(2):130.e1-e6.
Alsnes IV, Vatten LJ, Fraser A, Bjørngaard JH, Rich-Edwards J, Romundstad PR, et al. Hypertension in Pregnancy and Offspring Cardiovascular Risk in Young Adulthood: Prospective and Sibling Studies in the HUNT Study (Nord-Trøndelag Health Study) in Norway. Hypertens. 2017;69(4):591–8.
Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–61.
Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, et al. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5(6):e008136.
Jayet PY, Rimoldi SF, Stuber T, Salmòn CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122(5):488–94.
Nahum Sacks K, Friger M, Shoham-Vardi I, Sergienko R, Landau D, Sheiner E. In utero exposure to pre-eclampsia as an independent risk factor for long-term respiratory disease. Pediatr Pulmonol. 2020;55(3):723–8.
Nahum Sacks K, Friger M, Shoham-Vardi I, Spiegel E, Sergienko R, Landau D, et al. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy Hypertens. 2018;13:181–6.
Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Diseases in children born to mothers with preeclampsia: a population-based sibling cohort study. Am J Obstet Gynecol. 2011;204(2):157.e1-e5.
Szymonowicz W, Yu VY. Severe pre-eclampsia and infants of very low birth weight. Arch Dis Child. 1987;62(7):712.
Martikainen A. Growth and development at the age of 1.5 years in children with maternal hypertension. J Perinat Med. 1989;17(4):259–69.
Cheng S-W, Chou H-C, Tsou K-I, Fang L-J, Tsao P-N. Delivery before 32 weeks of gestation for maternal pre-eclampsia: neonatal outcome and 2-year developmental outcome. Early Human Dev. 2004;76(1):39–46.
Silveira RC, Procianoy RS, Koch MS, Benjamin ACW, Schlindwein CF. Growth and neurodevelopment outcome of very low birth weight infants delivered by preeclamptic mothers. Acta Paediatr. 2007;96(12):1738–42.
Byberg KK, Øymar K, Eide GE, Forman MR, Júlíusson PB. Exposure to preeclampsia in utero affects growth from birth to late childhood dependent on child’s sex and severity of exposure: Follow-up of a nested case-control study. PLoS One. 2017;12(5):e0176627.
Matić M, Inati V, Abdel-Latif ME, Kent AL. Maternal hypertensive disorders are associated with increased use of respiratory support but not chronic lung disease or poorer neurodevelopmental outcomes in preterm neonates at <29 weeks of gestation. J Paediatr Child Health. 2017;53(4):391–8.
Gunnarsdottir J, Cnattingius S, Lundgren M, Selling K, Högberg U, Wikström A-K. Prenatal exposure to preeclampsia is associated with accelerated height gain in early childhood. PLoS One. 2018;13(2):e0192514.
Huang Y, Zhang W, Go K, Tsuchiya KJ, Hu J, Skupski DW, et al. Altered growth trajectory in children born to mothers with gestational diabetes mellitus and preeclampsia. Arch Gynecol Obstet. 2020;301(1):151–9.
Gow ML, Roberts L, Henry A, Davis G, Mangos G, Pettit F, et al. Growth from birth to 6-months of infants with and without intrauterine preeclampsia exposure. J Dev Orig Health Dis. 2021;132(2):151–5.
Jasper EA, Cho H, Breheny PJ, Bao W, Dagle JM, Ryckman KK. Perinatal determinants of growth trajectories in children born preterm. PLoS ONE. 2021;16(1):e0245387.
CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. The Lancet. 1994;343(8898):619–29.
Alsnes IV, Janszky I, Forman MR, Vatten LJ, Økland I. A population-based study of associations between preeclampsia and later cardiovascular risk factors. AJOG. 2014;211(6):657.e1-.e7.
World Health Organisation. International statistical classification of diseases and related health problems. Geneva: World Health Organization; 2015.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R, et al. Chapter 7: Systematic Reviews of Etiology and Risk. In: Aromataris E, Munn Z (Editors). JBI: JBI Manual for Evidence Synthesis; 2020.
Melo G, Dutra KL, Rodrigues Filho R, Ortega AOL, Porporatti AL, Dick B, et al. Association between psychotropic medications and presence of sleep bruxism: A systematic review. J Oral Rehabil. 2018;45(7):545–54.
Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. The Lancet. 1986;1(8489):1077–81.
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. The Lancet. 1989;2(8663):577–80.
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. The Lancet. 1993;341(8850):938–41.
Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7.
Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–68.
Apicella C, Ruano CSM, Méhats C, Miralles F, Vaiman D. The Role of Epigenetics in Placental Development and the Etiology of Preeclampsia. Int J Mol Sci. 2019;20(11):2837.
Kamrani A, Alipourfard I, Ahmadi-Khiavi H, Yousefi M, Rostamzadeh D, Izadi M, et al. The role of epigenetic changes in preeclampsia. BioFactors. 2019;45(5):712–24.
Safi-Stibler S, Gabory A. Epigenetics and the Developmental Origins of Health and Disease: Parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin Cell Dev Biol. 2020;97:172–80.
Hollegaard B, Lykke JA, Boomsma JJ. Time from pre-eclampsia diagnosis to delivery affects future health prospects of children. Evol Med Public Health. 2017;2017(1):53–66.
Wang H, László KD, Gissler M, Li F, Zhang J, Yu Y, et al. Maternal hypertensive disorders and neurodevelopmental disorders in offspring: a population-based cohort in two Nordic countries. Eur J Epidemiol. 2021;36(5):519–30.
Braunthal S, Brateanu A. Hypertension in pregnancy: Pathophysiology and treatment. SAGE Open Med. 2019;7:2050312119843700-.
Staff AC, Redman CWG. The Differences Between Early- and Late-Onset Pre-eclampsia. In: Saito S, editor. Preeclampsia. Comprehensive Gynecology and Obstetrics. Singapore: Springe; 2018.
Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013;3(1):44–7.
Lausman A, Kingdom J. Intrauterine growth restriction: screening, diagnosis, and management. J Obstet Gynaecol Can. 2013;35(8):741–8.
de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64(4):650–8.
Report of a WHO Expert Committee. Physical status: the use and interpretation of anthropometry. World Health Organ Tech Rep Series. 1995;854:1–452.
Habli M, Levine RJ, Qian C, Sibai B. Neonatal outcomes in pregnancies with preeclampsia or gestational hypertension and in normotensive pregnancies that delivered at 35, 36, or 37 weeks of gestation. Am J Obstet Gynecol. 2007;197(4):406.e1-.e7.
Langenveld J, Ravelli AC, van Kaam AH, van der Ham DP, van Pampus MG, Porath M, et al. Neonatal outcome of pregnancies complicated by hypertensive disorders between 34 and 37 weeks of gestation: a 7 year retrospective analysis of a national registry. Am J Obstet Gynecol. 2011;205(6):540.e1-7.
Lowe SA, Bowyer L, Lust K, McMahon LP, Morton M, North RA, et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015;55(5):e1-29.
Gruslin A, Lemyre B. Pre-eclampsia: Fetal assessment and neonatal outcomes. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):491–507.
Rasmussen S, Irgens LM. Fetal growth and body proportion in preeclampsia. Obstet Gynecol. 2003;101(3):575–83.
Pinheiro TV, Brunetto S, Ramos JG, Bernardi JR, Goldani MZ. Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J Dev Orig Health Dis. 2016;7(4):391–407.
Staley JR, Bradley J, Silverwood RJ, Howe LD, Tilling K, Lawlor DA, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4(5):e001422.
Miliku K, Bergen NE, Bakker H, Hofman A, Steegers EAP, Gaillard R, et al. Associations of Maternal and Paternal Blood Pressure Patterns and Hypertensive Disorders during Pregnancy with Childhood Blood Pressure. J Am Heart Assoc. 2016;5(10):e003884.
Miettola S, Hartikainen AL, Vääräsmäki M, Bloigu A, Ruokonen A, Järvelin MR, et al. Offspring’s blood pressure and metabolic phenotype after exposure to gestational hypertension in utero. Eur J Epidemiol. 2013;28(1):87–98.
Fraser A, Nelson SM, Macdonald-Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62(3):614–20.
Lawlor DA, Macdonald-Wallis C, Fraser A, Nelson SM, Hingorani A, Davey Smith G, et al. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J. 2012;33(3):335–45.
Timpka S, Macdonald-Wallis C, Hughes AD, Chaturvedi N, Franks PW, Lawlor DA, et al. Hypertensive Disorders of Pregnancy and Offspring Cardiac Structure and Function in Adolescence. J Am Heart Assoc. 2016;5(11):e003906.
Lazdam M, Pitcher A, de la Horra A, Kylintireas I, Mannie Z, Diesch J, et al. Hypertension in offspring of pregnancies complicated by pre-eclampsia: unerdlying vascular mechanisms? Hypertension. 2010;56:159–65.
Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–80.
Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, Placental Insufficiency, and Autism Spectrum Disorder or Developmental Delay. JAMA Pediatr. 2015;169(2):154–62.
Mann JR, McDermott S, Bao H, Hardin J, Gregg A. Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord. 2010;40(5):548–54.
Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 2010;30(4):125–34.
Maher GM, O’Keeffe GW, Dalman C, Kearney PM, McCarthy FP, Kenny LC, et al. Association between preeclampsia and autism spectrum disorder: a population-based study. J Child Psychol Psychiatry. 2020;61(2):131–9.
Silva D, Colvin L, Hagemann E, Bower C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;133(1):e14-22.
Getahun D, Rhoads GG, Demissie K, Lu SE, Quinn VP, Fassett MJ, et al. In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics. 2013;131(1):e53-61.
Maher GM, Dalman C, O’Keeffe GW, Kearney PM, McCarthy FP, Kenny LC, et al. Association between preeclampsia and attention-deficit hyperactivity disorder: a population-based and sibling-matched cohort study. Acta Psychiatr Scand. 2020;142(4):275–83.
Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. 2009;201(3):269.e1-e.10.
Mann JR, McDermott S. Maternal pre-eclampsia is associated with childhood epilepsy in South Carolina children insured by Medicaid. Epilepsy Behav. 2011;20(3):506–11.
van Wassenaer AG, Westera J, van Schie PEM, Houtzager BA, Cranendonk A, de Groot L, et al. Outcome at 4.5 years of children born after expectant management of early-onset hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2011;204(6):510.e1-e9.
Grace T, Bulsara M, Pennell C, Hands B. Maternal hypertensive diseases negatively affect offspring motor development. Pregnancy Hypertens. 2014;4(3):209–14.
Rätsep MT, Hickman AF, Maser B, Pudwell J, Smith GN, Brien D, et al. Impact of preeclampsia on cognitive function in the offspring. Behav Brain Res. 2016;302:175–81.
Warshafsky C, Pudwell J, Walker M, Wen S-W, Smith GN. Prospective assessment of neurodevelopment in children following a pregnancy complicated by severe pre-eclampsia. BMJ Open. 2016;6(7):e010884.
Griffith MI, Mann JR, McDermott S. The risk of intellectual disability in children born to mothers with preeclampsia or eclampsia with partial mediation by low birth weight. Pregnancy Hypertension. 2011;30(1):108–15.
Ehrenstein V, Rothman KJ, Pedersen L, Hatch EE, Sørensen HT. Pregnancy-associated hypertensive disorders and adult cognitive function among Danish conscripts. Am J Epidemiol. 2009;170(8):1025–31.
Tuovinen S, Eriksson JG, Kajantie E, Räikkönen K. Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. J Am Soc Hypertens. 2014;8(11):832-47.e1.
Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Dev Med Child Neurol. 2014;56(8):779–85.
Strand KM, Heimstad R, Iversen AC, Austgulen R, Lydersen S, Andersen GL, et al. Mediators of the association between pre-eclampsia and cerebral palsy: population based cohort study. BMJ. 2013;347:f4089.
Mor O, Stavsky M, Yitshak-Sade M, Mastrolia SA, Beer-Weisel R, Rafaeli-Yehudai T, et al. Early onset preeclampsia and cerebral palsy: a double hit model? Am J Obstet Gynecol. 2016;214(1):105.e1-9.
Tuovinen S, Aalto-Viljakainen T, Eriksson JG, Kajantie E, Lahti J, Pesonen AK, et al. Maternal hypertensive disorders during pregnancy: adaptive functioning and psychiatric and psychological problems of the older offspring. Br J Obstet Gynaecol. 2014;121(12):1482–91.
Tuovinen S, Räikkönen K, Pesonen A-K, Lahti M, Heinonen K, Wahlbeck K, et al. Hypertensive disorders in pregnancy and risk of severe mental disorders in the offspring in adulthood: The Helsinki Birth Cohort Study. J Psychiatr Res. 2012;46(3):303–10.
Liu X, Olsen J, Agerbo E, Yuan W, Wu CS, Li J. Maternal preeclampsia and childhood asthma in the offspring. Pediatr Allergy Immunol. 2015;26(2):181–5.
Stokholm J, Sevelsted A, Anderson UD, Bisgaard H. Preeclampsia Associates with Asthma, Allergy, and Eczema in Childhood. Am J Respir Crit Care Med. 2017;195(5):614–21.
Keski-Nisula L, Heinonen S, Remes S, Pekkanen J. Pre-eclampsia, placental abruption and increased risk of atopic sensitization in male adolescent offspring. Am J Reprod Immunol. 2009;62(5):293–300.
Byberg KK, Ogland B, Eide GE, Oymar K. Birth after preeclamptic pregnancies: association with allergic sensitization and allergic rhinoconjunctivitis in late childhood; a historically matched cohort study. BMC Pediatr. 2014;14:101.
Goffin SM, Derraik JGB, Groom KM, Cutfield WS. Maternal pre-eclampsia and long-term offspring health: Is there a shadow cast? Pregnancy Hypertension. 2018;12:11–5.
Demerath EW, Fields DA. Body composition assessment in the infant. Am J Hum Biol. 2014;26(3):291–304.
Rodríguez-Cano AM, Mier-Cabrera J, Muñoz-Manrique C, Cardona-Pérez A, Villalobos-Alcázar G, Perichart-Perera O. Anthropometric and clinical correlates of fat mass in healthy term infants at 6 months of age. BMC Pediatr. 2019;19(1):60.
Barstow C, Rerucha C. Evaluation of Short and Tall Stature in Children. Am Fam Physician. 2015;92(1):43–50.
Wang G, Johnson S, Gong Y, Polk S, Divall S, Radovick S, et al. Weight Gain in Infancy and Overweight or Obesity in Childhood across the Gestational Spectrum: a Prospective Birth Cohort Study. Sci Rep. 2016;6(1):29867.
Singhal A. Long-Term Adverse Effects of Early Growth Acceleration or Catch-Up Growth. Ann Nutr Metab. 2017;70(3):236–40.
Nash A, Corey M, Sherwood K, Secker D, Saab J, O’Connor DL. Growth Assessment in Infants and Toddlers Using Three Different Reference Charts. J Pediatr Gastroenterol Nutr. 2005;40(3):283–8.
World Health Organisation: WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. 217. Geneva: World Health Organisation; 2006.
World Health Organisation: WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. 336. Geneva: World Health Organisation; 2006.
de Onis M, Onyango A, Borghi E, Siyam A, Blössner M, Lutter C. Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr. 2012;15(9):1603–10.
Woo JG, Daniels SR. Assessment of Body Mass Index in Infancy: It Is Time to Revise Our Guidelines. J Pediatr. 2019;204:10–1.
Roy SM, Fields DA, Mitchell JA, Hawkes CP, Kelly A, Wu GD, et al. Body Mass Index Is a Better Indicator of Body Composition than Weight-for-Length at Age 1 Month. J Pediatr. 2019;204:77-83.e1.
Roy SM, Spivack JG, Faith MS, Chesi A, Mitchell JA, Kelly A, et al. Infant BMI or Weight-for-Length and Obesity Risk in Early Childhood. Pediatrics. 2016;137(5):e20153492.
Aris IM, Rifas-Shiman SL, Li L-J, Yang S, Belfort MB, Thompson J, et al. Association of Weight for Length vs Body Mass Index During the First 2 Years of Life With Cardiometabolic Risk in Early Adolescence. JAMA Network Open. 2018;1(5):e182460-e.
Ferguson AN, Grabich SC, Olsen IE, Cantrell R, Clark RH, Ballew WN, et al. BMI Is a Better Body Proportionality Measure than the Ponderal Index and Weight-for-Length for Preterm Infants. Neonatology. 2018;113(2):108–16.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59.
González-García L, García-López E, Fernández-Colomer B, Mantecón-Fernández L, Lareu-Vidal S, Suárez-Rodríguez M, et al. Extrauterine Growth Restriction in Very Low Birth Weight Infants: Concordance Between Fenton 2013 and INTERGROWTH-21(st) Growth Charts. Front Pediatr. 2021;9:690788.
Villar J, Giuliani F, Bhutta ZA, Bertino E, Ohuma EO, Ismail LC, et al. Postnatal growth standards for preterm infants: the Preterm Postnatal Follow-up Study of the INTERGROWTH-21(st) Project. Lancet Global Health. 2015;3(11):e681–91.
Kim YJ, Shin SH, Cho H, Shin SH, Kim SH, Song IG, et al. Extrauterine growth restriction in extremely preterm infants based on the Intergrowth-21st Project Preterm Postnatal Follow-up Study growth charts and the Fenton growth charts. Eur J Pediatr. 2021;180(3):817–24.
Tuzun F, Yucesoy E, Baysal B, Kumral A, Duman N, Ozkan H. Comparison of INTERGROWTH-21 and Fenton growth standards to assess size at birth and extrauterine growth in very preterm infants. J Matern Fetal Neonatal Med. 2018;31(17):2252–7.
Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–71.
Cho WK, Suh B-K. Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr. 2016;59(1):1–7.
Cole TJ. Conditional reference charts to assess weight gain in British infants. Arch Dis Child. 1995;73(1):8–16.
Cole TJ. Presenting information on growth distance and conditional velocity in one chart: practical issues of chart design. Stat Med. 1998;17(23):2697–707.
Griffiths LJ, Smeeth L, Hawkins SS, Cole TJ, Dezateux C. Effects of infant feeding practice on weight gain from birth to 3 years. Arch Dis Child. 2009;94(8):577–82.
Woo JG. Infant Growth and Long-term Cardiometabolic Health: a Review of Recent Findings. Current Nutrition Reports. 2019;8(1):29–41.
Bocca-Tjeertes IFA, Reijneveld SA, Kerstjens JM, de Winter AF, Bos AF. Growth in Small-for-Gestational-Age Preterm-Born Children from 0 to 4 Years: The Role of both Prematurity and SGA Status. Neonatology. 2013;103(4):293–9.
Davis GK, Roberts L, Mangos G, Henry A, Pettit F, O’Sullivan A, et al. Postpartum physiology, psychology and paediatric follow up study (P4 Study) - Study protocol. Pregnancy Hypertension. 2016;6(4):374–9.
Bellman M, Byrne O, Sege R. Developmental assessment of children. BMJ. 2013;346:e8687.
Cirelli I, Bickle Graz M, Tolsa J-F. Comparison of Griffiths-II and Bayley-II tests for the developmental assessment of high-risk infants. Infant Behav Dev. 2015;41:17–25.
Stein MT, Lukasik MK. Chapter 79 - DEVELOPMENTAL SCREENING AND ASSESSMENT: INFANTS, TODDLERS, AND PRESCHOOLERS. In: Carey WB, Crocker AC, Coleman WL, Elias ER, Feldman HM, editors. Developmental-Behavioral Pediatrics. 4th ed. Philadelphia: W.B. Saunders; 2009. p. 785–96.
Choo YY, Agarwal P, How CH, Yeleswarapu SP. Developmental delay: identification and management at primary care level. Singapore Med J. 2019;60(3):119–23.
Squires J, Bricker D, Potter L. Revision of a parent-completed development screening tool: Ages and Stages Questionnaires. J Pediatr Psychol. 1997;22(3):313–28.
Glascoe FP. Collaborating with parents: Using Parents' Evaluation of Developmental Status to detect and address developmental and behavioral problems. Nashville, TN, US: Ellsworth & Vandermeer Press; 1998.
Perrin EC, Sheldrick RC, Visco Z, Mattern K. The survey of well-being of young children (SWYC) user’s manual (1.01). Available at: https://pediatrics.tuftsmedicalcenter.org/the-survey-of-wellbeing-of-young-children/manual-training-resources. Accessed 22 Aug 2022.
Sheldrick RC, Marakovitz S, Garfinkel D, Carter AS, Perrin EC. Comparative Accuracy of Developmental Screening Questionnaires. JAMA Pediatr. 2020;174(4):366–74.
National Child Health and Wellbeing subcommittee of the Australian Population Health Development Principal Committee of the Australian Health Ministers’ Conference (AHMC). Appendix 3: Tools to assist in health surveillance and monitoring. In: National Framework for Universal Child and Family Health Services, Australian Government Department of Health; 2011.
Moodie S, Daneri P, Goldhagen S, Halle T, Green K, LaMonte L. Early childhood developmental screening: A compendium of measures for children ages birth to five. Washington, DC: Office of Planning, Research and Evaluation, Administration for Children and Families; 2014. Report No.: (OPRE Report 2014 11).
Martin AJ, Darlow BA, Salt A, Hague W, Sebastian L, McNeill N, et al. Performance of the Parent Report of Children’s Abilities-Revised (PARCA-R) versus the Bayley Scales of Infant Development III. Arch Dis Child. 2013;98(12):955.
Johnson S, Bountziouka V, Brocklehurst P, Linsell L, Marlow N, Wolke D, et al. Standardisation of the Parent Report of Children’s Abilities-Revised (PARCA-R): a norm-referenced assessment of cognitive and language development at age 2 years. Lancet Child Adolesc Health. 2019;3(10):705–12.
Ireton H, Glascoe FP. Assessing children’s development using parents’ reports. Clin Pediatr (Phila). 1995;34(5):248–55.
Cunha A, Berkovits M, Albuquerque K. Developmental Assessment With Young Children: A Systematic Review of Battelle Studies. Infants Young Child. 2018;31(1):69–90.
Hilton-Mounger A. Battelle Developmental Inventory: 2nd Edition. In: Goldstein S, Naglieri JA, editors. Encyclopedia of Child Behavior and Development. Boston, MA: Springer, US; 2011. p. 210–2.
Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89(1):91–7.
Glascoe FP. The Brigance Infant and Toddler Screen: Standardization and Validation. J Dev Behav Pediatr. 2002;23(3):145–50.
Bayley N. Bayley Scales of Infant Development. 2nd ed. 1993.
Bayley N. Bayley Scales of Infant Development 3rd Edition (Bayley-III). San Antonio, TX: Harcourt; 2006.
Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW, Group tVIC. Underestimation of Developmental Delay by the New Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164(4):352–6.
Luiz DM, Faragher B, Barnard A, Knoesen N, Kotras N, Burns LE, et al. Griffiths Mental Development Scales – Extended Revised. Analysis manual. Association for Research in Infant and Child Development (ARICD). Oxford: Hogrefe – The Tests Agency Ltd; 2006.
Mullen EM, American Guidance S. Mullen Scales of Early Learning. Circle Pines, Minnesota: AGS; 1995.
Farmer C, Golden C, Thurm A. Concurrent validity of the differential ability scales, second edition with the Mullen Scales of Early Learning in young children with and without neurodevelopmental disorders. Child Neuropsychol. 2016;22(5):556–69.
Heikura U, Hartikainen A-L, Nordström T, Pouta A, Taanila A, Järvelin M-R. Maternal Hypertensive Disorders during Pregnancy and Mild Cognitive Limitations in the Offspring. Paediatr Perinat Epidemiol. 2013;27(2):188–98.
Jelliffe-Pawlowski LL, Hansen RL. Neurodevelopmental Outcome at 8 Months and 4 Years among Infants Born Full-Term Small-for-Gestational-Age. J Perinatol. 2004;24(8):505–14.
Thomaidis L, Zantopoulos GZ, Fouzas S, Mantagou L, Bakoula C, Konstantopoulos A. Predictors of severity and outcome of global developmental delay without definitive etiologic yield: a prospective observational study. BMC Pediatr. 2014;14:40.
Love ER, Crum J, Bhattacharya S. Independent effects of pregnancy induced hypertension on childhood development: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2012;165(2):219–24.
Spinillo A, Iasci A, Capuzzo E, Egbe TO, Colonna L, Fazzi E. Two-year infant neurodevelopmental outcome after expectant management and indicated preterm delivery in hypertensive pregnancies. Acta Obstet Gynecol Scand. 1994;73(8):625–9.
Johnson S, Evans TA, Draper ES, Field DJ, Manktelow BN, Marlow N, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. 2015;100(4):F301–8.
Li XL, Guo PL, Xue Y, Gou WL, Tong M, Chen Q. An analysis of the differences between early and late preeclampsia with severe hypertension. Pregnancy Hypertens. 2016;6(1):47–52.
Gray PH, O’Callaghan MJ, Mohay HA, Burns YR, King JF. Maternal hypertension and neurodevelopmental outcome in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;79(2):F88–93.
Schlapbach LJ, Ersch J, Adams M, Bernet V, Bucher HU, Latal B. Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr. 2010;99(10):1504–9.
McCowan LME, Pryor J, Harding JE. Perinatal predictors of neurodevelopmental outcome in small-for-gestational-age children at 18 months of age. Am J Obstet Gynecol. 2002;186(5):1069–75.
Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, Fazzi E. Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev Med Child Neurol. 2009;51(7):518–25.
Degirmencioglu H, Say B, Ustunyurt Z, Oguz SS. Influence of Maternal Preeclampsia on Neurodevelopmental Outcomes of Preterm Infants. Gynecol Obstetric Reprod Med. 2018;24(2):99–103.
Wade M, Jenkins JM. Pregnancy hypertension and the risk for neuropsychological difficulties across early development: A brief report. Child Neuropsychol. 2016;22(2):247–54.
Bharadwaj SK, Vishnu Bhat B, Vickneswaran V, Adhisivam B, Bobby Z, Habeebullah S. Oxidative Stress, Antioxidant Status and Neurodevelopmental Outcome in Neonates Born to Pre-eclamptic Mothers. Indian J Pediatr. 2018;85(5):351–7.
Chen Z, Li R, Liu H, Duan J, Yao C, Yang R, et al. Impact of maternal hypertensive disorders on offspring’s neurodevelopment: a longitudinal prospective cohort study in China. Pediatr Res. 2020;88(4):668–75.
Maher GM, O’Keeffe GW, O’Keeffe LM, Matvienko-Sikar K, Dalman C, Kearney PM, et al. The Association Between Preeclampsia and Childhood Development and Behavioural Outcomes. Matern Child Health J. 2020;24(6):727–38.
Phatak P. Manual on Developmental Assessment Scales for Indian Infants (DASII) – Revised Baroda Norms. Pune: Anand Agencies; 1997: 5.
Frankenburg WK, Dodds JB, Fandal AW, Kazuk E, Cohrs M. The Denver Developmental Screening Test: Reference manual. Denver: University of Colorado Medical Center; 1975.
Gesell A, Amatruda CS. Developmental Diagnosis: Normal and Abnormal Child Development Clinical Methods and Practical Applications. JAMA. 1942;118(3):259.
Beijing Mental Development Cooperative Group. Gesell Developmental Diagnosis Scale. Beijing: Beijing Mental Development Cooperative Group; 1985.
Burns YR, Ensbey RM, Norrie MA. The Neuro-sensory Motor Developmental Assessment part 1: development and administration of the test. Aust J Physiother. 1989;35(3):141–9.
Nahum Sacks K, Friger M, Shoham-Vardi I, Sergienko R, Spiegel E, Landau D, et al. Long-term neuropsychiatric morbidity in children exposed prenatally to preeclampsia. Early Human Dev. 2019;130:96–100.
Acknowledgements
The authors wish to thank the St. George Obstetric Medicine Research Group, UNSW Medicine School of Women and Children’s Health and Lynne Roberts PhD for their support of this review.
Funding
AH and MLG are supported by NHMRC Early Career Fellowships (APP1141570 and APP1158876, respectively), and MEC is supported by a NHMRC Practitioner Fellowship (APP1136735). The funders did not participate in the work.
Author information
Authors and Affiliations
Contributions
PV conducted the literature review, authored this review, and prepared the figure and tables, with editorial input and critical review provided by AH, MEC and MLG. The author(s) read and approved the final manuscript.
Authors’ information
AH is Associate Professor in Obstetrics and Gynaecology, an Obstetrician at St George Public Hospital and the Royal Hospital for Women, Sydney, and Honorary Senior Research Fellow, Global Women’s Health at The George Institute for Global Health. MEC is Professor of Paediatric Endocrinology, The Children’s Hospital at Westmead, a Principal Investigator at Westmead Applied Research Centre, an Academic Co-Director at Charles Perkins Centre Westmead, and Medical Director of the Australasian Diabetes Data Network (ADDN). MLG is a National Health and Medical Research Council (NHMRC) Early Career Fellow, Senior Lecturer and dietitian at The University of Sydney Children’s Hospital Westmead Clinical Schools.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
No individual data presented.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vakil, P., Henry, A., Craig, M.E. et al. A review of infant growth and psychomotor developmental outcomes after intrauterine exposure to preeclampsia. BMC Pediatr 22, 513 (2022). https://doi.org/10.1186/s12887-022-03542-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03542-5