Abstract
Background
Among the histiocytic disorders, anaplastic lymphoma kinase (ALK)-positive histiocytosis emerged in 2008. As more and more cases of the novel entity are reported, our understanding of it is deepened. However, only a few cases with central nervous system (CNS) involvement have been reported. Furthermore, the lesion in the suprasellar region has not been documented.
Case presentation
We presented a case of ALK-positive histiocytosis involving the suprasellar region of a one-year-and-four-month-old boy. Through clinical, neuropathological, and genomic analyses, the patient was diagnosed with ALK-positive histiocytosis. After lesions were resected he started treatment with a combination of the three compounds vincristine, prednisolone, and crizotinib, but they did not work. Cytarabine was then added as an additional chemotherapy drug for him, and the lesions in the brain and lungs were shrunk by combining treatment of crizotinib, dexamethasone, vincristine, and cytarabine according to the RECIST (esponse Evaluation Criteria In Solid Tumours).
Conclusions
Additional adjuvant chemotherapy drugs are needed when ALK-inhibitor treatment is ineffective.
Similar content being viewed by others
Background
In 2008, JK Chan et al. [1] first described the anaplastic lymphoma kinase (ALK)-positive histiocytosis, which is a novel proliferation of morphologically distinctive histiocytes with a chromosomal translocation involving ALK. Since then, they have reported 10 cases of ALK-positive histiocytosis, including one affecting the cavernous sinus [2]. ALK-positive histiocytosis in the central nervous system (CNS) has been rarely reported. It is either part of multiple disseminated lesions or the only manifestation of localized disease [2,3,4,5,6,7,8,9,10,11,12,13]. In particular, the lesion in the suprasellar region has never been described.
In this study, we reported the case of a one-year-and-four-month-old boy who was diagnosed with ALK-positive histiocytosis involving the suprasellar region following clinicopathological, molecular, and next-generation sequencing (NGS) examinations. Further, the literature review was performed to identify the clinical characteristics of the entity involving CNS.
Case presentation
The timeline of the treatment is shown in Fig. 1.
The patient was a one-year-and-four-month-old boy who was admitted for rapid weight loss and difficulty in walking for three months. A magnetic resonance imaging (MRI) of the head revealed a lesion in the suprasellar region. The result of the neurological examination showed that the fontanelle was slightly prominent. The head circumference was within normal limits. The patient could walk slowly with the help of his parents but not walk independently.
During pregnancy, the imaging examination of the mother of the patient showed no abnormalities. The patient and his family members did not have a similar illness or any other abnormal medical history. No abnormal phenomena were observed at birth. Indicators in routine child health care were normal.
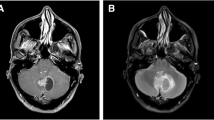
The laboratory examination results, including blood routine and blood biochemistry, were normal. The biological biomarker test revealed that the values of AFP and β-HCG were normal. The contrast-enhanced MRI of the head revealed inhomogeneous lesions with obvious enhancement in the suprasellar region and left middle cranial fossa, ventricular dilation, and peripheral brain edema (Fig. 2A-C). Multiple pulmonary nodules were visible in computed tomography (CT) before the start of systemic treatment (Fig. 2D-E).
Imaging and pathological results of the case. The MRI of the head shows contrast-enhancing lesions involving the suprasellar region (A-C, blue arrow) and the left temporal lobe (A, red arrow). The lung CT reveals multiple nodes in the right lung (D, green arrow) and the left lung (E, brown arrow). Hematoxylin and eosin staining show an infiltrative proliferation of spindle cells (F). Immunostaining for ALK (G), CD68 (H), and CD163 (I) is positive. ALK rearrangement (green and red signals) is detected in the FISH test (J). The microscopy images was captured by multispectral automatic tissue quantitative scanning and analysis system and ZEISS Fully Automatic Upright Fluorescence Microscope.The measured resolution for images was 360 ppi
Due to severe bleeding, we performed partial tumor resection. The pathological diagnosis of the lesions in the brain was confirmed through immunohistochemistry, high-throughput sequencing, and fluorescence in situ hybridization (FISH). The immunostaining result revealed that the positive terms were CD163, CD30, ALK-1, CD4, Cyclin D1, Ki67 (+ , 5%), and CD68/PGM1, while the negative terms were CXCL13, Langerin, EGFR, SSTR2, SALL4, and PLAP () (Fig. 2F-I). The result of the NGS genetic test revealed KIF5B-ALK gene rearrangement (fusion) (K24:A20) (Abundance: 21.59%). The capture-based high-throughput sequencing analysis did not identify any variation in gene copy numbers. Anaplastic lymphoma kinase gene translocation was confirmed by FISH (Fig. 2J).
Additionally, the head CT confirmed hydrocephalus and subdural effusion (SDE) on the right side of the patient. A burr hole drainage procedure was performed (Fig. 3A), but SDE did not relieve (Fig. 3B). After the drainage tube was removed, the consciousness of the patient deteriorated again. Moreover, both the glucose level and the number of white blood cells in cerebrospinal fluid (CSF) were higher than normal. For further treatment, the Ommaya reservoir was implanted in the left lateral ventricle (Fig. 3C), and CSF was aspirated daily through the Ommaya reservoir. The effusion on the right side decreased (Fig. 3D). Anti-infection medication ceftriaxone sodium was prescribed. Half a month later, the patient had a fever, and bacillus cereus was found in the CSF culture. In this case, meropenem was prescribed. Because the fever was not controlled and the bacillus cereus was positive in the CSF, we added vancomycin. Despite the relief of fever, CSF culture showed the presence of bacillus cereus. Bacillus cereus was suspected to be colonized in the Ommaya reservoir. Consequently, external ventricular drainage was performed first, followed by the removal of the Ommaya reservoir (Fig. 3E). The effusion on the right side also disappeared (Fig. 3F). Three consecutive CSF cultures were negative, and the body temperature was normal. We discontinued antibiotic therapy at this point.
The management of CSF and the lung CT during chemotherapy. The SDE did not relieve on day 1 (A) and day 7 (B) after burr hole drainage. SE gradually relieved on day 1 (C) and day 7 (D) after implanting the Ommaya reservoir. SE gradually relieved on day 1 (E) and day 7 (F) after implanting a long-distance open ventricular drainage operation. The nodes (red arrow) on the lungs did not shrink one month (G and H) and two months (I and J) after the management of treatment with crizotinib and vincristine. After two months of cytarabine, crizotinib, and vincristine treatment, lesion remission was observed (K and L). The measured resolution for images was 360 ppi
Approximately two months after the resection of lesions, the patient was given prednisolone acetate, oral crizotinib (80 mg per day, twice a day), and intravenous vincristine (0.55 mg per week). However, the lung CT showed that the pulmonary nodules did not shrink significantly after two months of chemotherapy (Fig. 3G-I). Additionally, there was no significant change in the size of the lesions in the brain (Fig. 4A-F).
The MRI of the head during chemotherapy with the measurement of RECIST (The Response Evaluation Criteria In Solid Tumors). Compared with the lesions (A-C) after resection (2.4 × 3.1x2.6 cm), the lesion on MRI was stable two months after chemotherapy management with crizotinib and vincristine (D-F) (2.6 × 3.3x2.8 cm). After two months of treatment with cytarabine, crizotinib, and vincristine, tthe effect of the treatment was partial response (G-I) (2.4 × 2.3x2.1 cm). The measured resolution for images was 360 ppi
In addition, the PET-CT scan showed that the patient had a new lesion in the right humerus, in addition to for existing lesions in the suprasellar, lungs, and left middle cranial fossa (Fig. 5A-F). We suspected that the lesion might be a possibility of progression of disease. Multiple organs were affected by the ALK-positive histiocytosis. Then, the patient was given cytarabine (40 mg per day, five times in a row, two weeks apart) except for crizotinib and vincristine. The dosage of the crizotinib was increased from 80 mg per day to 100 mg per day, and prednisolone acetate was changed to dexamethasone. Imaging confirmed that the therapeutic regimen was effective (Figs. 3J-L and 4G-I) after application of the cytarabine for 40 days.Unfortunately, the patient passed away due to multiple ALK‑positive histiocytosis, hydrocephalus, subdural effusion, serious intracranial infection, deep vein thrombosis of the lower extremity, cachexia, and pneumonia after the application of crizotinib, dexamethasone, vincristine, and cytarabine for approximately two months.
Literature review
According to the literature review, only KIF5B-ALK fusions found in the CNS,the effectiveness of gross total resection alone, localized or disseminated lesions, more common in Asians, and ALK inhibitors are the characteristics of lesions which involved the CNS (Table 1). Only eight cases of CNS involvement have been reported in the literature: three localized cases and five disseminated cases. These characteristics are not consistent with those of infants with systemic but self-limited disease and older children and adults with localized disease [6].
Discussion and conclusions
To our knowledge, lesions involving mesentery, breast, appendix, extremity, peripheral blood, kidney, bone marrow, lung, brain, and lymph nodes have been reported since the advent of ALK-positive histiocytosis in 2008. Some are local lesions, and some are part of systemic lesions [2,3,4,5,6,7,8,9,10,11,12,13]. However, no lesions in the suprasellar region have been reported. In this study, we first reported the case of a one-year-and-four-month-old boy with ALK-positive histiocytosis in the suprasellar region. After combining the high-quality results of large international collaboration on ALK-positive histiocytosis with our case [14], we believe that this case may provide a reference for patients involving CNS.
Confirmative diagnosis is primarily based on neuropathological screening, which includes the determination of tissue features, immunohistochemical assay, and genetic mutation testing for ALK translocation. Moreover, an accurate pathological diagnosis of ALK-positive histiocytosis can guide treatment. Due to its rarity and the overlapping morphological features with Erdheim-Chester disease (ECD), juvenile xanthogranuloma, Rosai-Dorfman disease (RDD), and Langerhans cell histiocytosis (LCH), the pathological differential diagnosis of this disease is extremely challenging [15]. The features of these entities are shown in Table 2. The presentation, morphology, and immune profile of each disease are helpful in the differential diagnosis. Microscopically, ALK-positive histiocytosis is characterized by large epithelioid cells, Touton-like giant cells, absence of substantial atypia [6], and focal emperipolesis. The immunohistochemical assay shows ALK, CD68, and CD163, but not CD1a, BRAFV 600E, and GFAP. In our case, CD68, CD163, and ALK were positive, so we suspected that the patient had ALK-positive histiocytosis. ALK-positive histiocytosis accompanied by diffuse cytoplasmic positivity of S-100 protein may be mistaken for RDD [9]. However, mutations in the RAS pathway are only found in RDD [13]. Moreover, plasma cells are rare in ALK-positive histiocytosis. In the absence of a BRAF mutation, it is difficult to distinguish ECD from ALK-positive histiocytosis. KIF5B-ALK fusion has also been reported in three adult cases of ECD with disseminated disease [16, 17]. A lack of skeletal involvement and xanthomatous foamy histiocytes may rule out ECD in this case. When KIF5B-ALK fusion is present in juvenile xanthogranuloma (JXG) [18], foamy histiocytes with S-100 protein can be different from ALK-positive histiocytes. A CD1a immunostain can rule out LCH. Thus, high-throughput sequencing and FISH were performed to confirm that the disease was ALK-positive histiocytosis.
Mutations of ALK-positive histiocytosis genes include KIF5B-ALK, TPM3-ALK, COL1A2-ALK, TRIM33-ALK, and EML4-ALK [2,3,4,5,6,7,8,9,10,11,12,13]. However, the only documented fusion of ALK-positive histiocytosis in CNS is KIF5B-ALK [2, 3, 7, 12, 15]. There appears to be no relationship between localization or dissemination of ALK-positive histiocytosis in the CNS and KIF5B-ALK fusion. Therefore, identifying the ALK mutation is vital. KIF5B and ALK encode the ubiquitous isoform of the heavy chain of kinesin-1 and a receptor tyrosine kinase, respectively [19]. In ALK-positive histiocytosis, the KIF5B-ALK fusion may lead to targetable kinase alterations as oncogenic drivers [16].
The treatment strategies for ALK-positive histiocytosis involving the CNS should be specific. Lesion resection can relieve the symptom. The biopsy has low risk and yields substantial information for the confirmative diagnosis. To treat a local primary CNS lesion, only gross total resection may be needed without an ALK inhibitor [2, 3, 15]. An ALK inhibitor may be necessary to control the disease involving the CNS of the disseminated lesion [2, 7, 12, 15]. Even though lesion decompression was used to relieve symptoms and ALK inhibitors were prescribed for adjuvant therapy, the lesion size did not change significantly. In addition, the lesions on the right humerus might be possibility of progression of disease. The poor effect might be caused by the poor penetration of the brain-blood barrier, the big size of the lesions in the brain, multiple lesions, and the weak constitution and intracranial infection due to the several surgeries. At present, many ALK inhibitors have an excellent ability to penetrate the blood–brain barrier. Crizotinib has a limited CNS passage to penetrate the blood–brain barrier [20]. However, the application of these medications, such as alectinib, ceritinib and lorlatinib, can increase the brain-to-blood exposure ratio [21]. Another ALK inhibitor, alectinib, is effective in treating disseminated ALK-positive histiocytosis in CNS [12].
In addition, managing surgery complications, such as hydrocephalus, SDE, and intracranial infection after surgery, can be challenging. A subdural effusion with hydrocephalus (SDEH) has been reported in cases of foramen magnum decompression and clipping of intracranial aneurysms after surgery [22, 23]. Several successful cases with the ventricle drainage tube implanted have been reported [23,24,25], as in the present case. Finding the site of bacterial colonization is crucial. In our case, symptoms of an intracranial infection were relieved after the drainage tube and Ommaya reservoir were removed.
In general, the present study reported the case of a one-year-and-four-month-old boy with ALK-positive histiocytosis involving the suprasellar region. The adjuvant chemotherapy drugs are needed when ALK-inhibitor treatment is ineffective in treating the lesion. The SDEH may be relieved with the implantation of ventricle drainage. The disease in the CNS is characterized by only KIF5B-ALK fusion, the effectiveness of gross total resection alone, localized or disseminated lesion, more common in Asians, and efficacy of ALK-inhibitor treatment.
Availability of data and materials
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Abbreviations
- ALK:
-
Anaplastic lymphoma kinase
- CNS:
-
Central nervous system
- NGS:
-
Next-generation sequencing
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- FISH:
-
Fluorescence in situ hybridization
- SDE:
-
Subdural effusion
- CSF:
-
Cerebrospinal fluid
- ECD:
-
Erdheim-Chester disease
- LCH:
-
Langerhans cell histiocytosis
- SDEH:
-
Subdural effusion with hydrocephalus
References
Chan JK, Lamant L, Algar E, Delsol G, Tsang WY, Lee KC, et al. ALK+ histiocytosis: a novel type of systemic histiocytic proliferative disorder of early infancy. Blood. 2008;112(7):2965–8.
Chang KTE, Tay AZE, Kuick CH, Chen H, Algar E, Taubenheim N, et al. ALK-positive histiocytosis: an expanded clinicopathologic spectrum and frequent presence of KIF5B-ALK fusion. Mod Pathol. 2019;32(5):598–608.
Lucas CG, Gilani A, Solomon DA, Liang X, Maher OM, Chamyan G, et al. ALK-positive histiocytosis with KIF5B-ALK fusion in the central nervous system. Acta Neuropathol. 2019;138(2):335–7.
Tran TAN, Chang KTE, Kuick CH, Goh JY, Chang CC. Local ALK-Positive Histiocytosis With Unusual Morphology and Novel TRIM33-ALK Gene Fusion. Int J Surg Pathol. 2021;29(5):543–9.
Kashima J, Yoshida M, Jimbo K, Izutsu K, Ushiku T, Yonemori K, et al. ALK-positive Histiocytosis of the Breast: A Clinicopathologic Study Highlighting Spindle Cell Histology. Am J Surg Pathol. 2021;45(3):347–55.
Gupta GK, Xi L, Pack SD, Jones JB, Pittaluga S, Raffeld M, et al. ALK-positive histiocytosis with KIF5B-ALK fusion in an adult female. Haematologica. 2019;104(11):e534–6.
Qiu L, Weitzman SP, Nastoupil LJ, Williams MD, Medeiros LJ, Vega F. Disseminated ALK-positive histiocytosis with KIF5B-ALK fusion in an adult. Leuk Lymphoma. 2021;62(5):1234–8.
Swain F, Williams B, Barbaro P. ALK-Positive Histiocytosis with Peripheral Blood Histiocytes: A Case Report. Acta Haematol. 2021;144(2):218–21.
Huang H, Gheorghe G, North PE, Suchi M. Expanding the Phenotype of ALK-positive Histiocytosis: A Report of 2 Cases. Pediatr Dev Pathol. 2018;21(5):449–55.
Jaber OI, Jarrah DA, Hiasat M, Hussaini MA. ALK-Positive Histiocytosis: A Case Report and Literature Review. Turk Patoloji Derg. 2021;37(2):172–7.
Osako T, Kurisaki-Arakawa A, Dobashi A, Togashi Y, Baba S, Shiozawa S, et al. Distinct Clinicopathologic Features and Possible Pathogenesis of Localized ALK-positive Histiocytosis of the Breast. Am J Surg Pathol. 2021.
Tian Y, Li J, Liu B, Xie H, Zheng M, Yao W. ALK-positive histiocytosis with disseminated disease responded to alectinib: a case report. Ann Palliat Med. 2021;10(9):10095–101.
Bai Y, Sun W, Niu D, Yang X, Diao X, Yu Y, et al. Localized ALK-positive histiocytosis in a Chinese woman: report of a case in the lung with a novel EML4-ALK rearrangement. Virchows Arch. 2021;479(6):1079–83.
Kemps PG, Picarsic J, Durham BH, Helias-Rodzewicz Z, Hiemcke-Jiwa L, van den Bos C, et al. ALK-positive histiocytosis: a new clinicopathologic spectrum highlighting neurologic involvement and responses to ALK inhibition. Blood. 2022;139(2):256–80.
Rossi S, Gessi M, Barresi S, Tamburrini G, Giovannoni I, Ruggiero A, et al. ALK-rearranged histiocytosis: Report of two cases with involvement of the central nervous system. Neuropathol Appl Neurobiol. 2021;47(6):878–81.
Diamond EL, Durham BH, Haroche J, Yao Z, Ma J, Parikh SA, et al. Diverse and Targetable Kinase Alterations Drive Histiocytic Neoplasms. Cancer Discov. 2016;6(2):154–65.
Durham BH, Lopez Rodrigo E, Picarsic J, Abramson D, Rotemberg V, De Munck S, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25(12):1839–42.
Ross JS, Ali SM, Fasan O, Block J, Pal S, Elvin JA, et al. ALK Fusions in a Wide Variety of Tumor Types Respond to Anti-ALK Targeted Therapy. Oncologist. 2017;22(12):1444–50.
Wilson MH, Holzbaur EL. Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development. 2015;142(1):218–28.
Frampton JE. Crizotinib: a review of its use in the treatment of anaplastic lymphoma kinase-positive, advanced non-small cell lung cancer. Drugs. 2013;73(18):2031–51.
Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17(4):452–63.
Rossini Z, Milani D, Costa F, Castellani C, Lasio G, Fornari M. Subdural Fluid Collection and Hydrocephalus After Foramen Magnum Decompression for Chiari Malformation Type I: Management Algorithm of a Rare Complication. World Neurosurg. 2017;106(1057):e9–15.
Yoshimoto Y, Wakai S, Hamano M. External hydrocephalus after aneurysm surgery: paradoxical response to ventricular shunting. J Neurosurg. 1998;88(3):485–9.
Tzerakis N, Orphanides G, Antoniou E, Sioutos PJ, Lafazanos S, Seretis A. Subdural effusions with hydrocephalus after severe head injury: successful treatment with ventriculoperitoneal shunt placement: report of 3 adult cases. Case Rep Med. 2010;2010:743784.
Wu R, Ye Y, Ma T, Jia G, Qin H. Management of subdural effusion and hydrocephalus following decompressive craniectomy for posttraumatic cerebral infarction in a patient with traumatic brain injury: a case report. BMC Surg. 2019;19(1):26.
Acknowledgements
All authors appreciate the patient and his family for their consent to publish this report.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
QL and QH contributed to the conception and design of the manuscript. QH and WJZ collected the data and drafted the manuscript. QL reviewed and modified the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. The patient’s family provided written informed consent prior to the investigation.
Consent to publication
Written informed consent for publication was obtained from the parents of the patient. Our study complied with the CARE Checklist.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, Q., Zhang, W. & Li, Q. Failure of crizotinib based systemic treatment in ALK positive histiocytosis involving the central nervous system: a case report and literature review. BMC Pediatr 22, 308 (2022). https://doi.org/10.1186/s12887-022-03368-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03368-1