Abstract
Background
The study aim was to obtain epidemiological data on vitamin D levels for the pediatric population in Japan. We assessed the prevalence of vitamin D deficiency and insufficiency in 2-year-old Japanese children using data from a large ongoing birth cohort study.
Methods
Data for analysis was obtained from the Japan Environment and Children’s Study (JECS) and a Sub-Cohort Study (SCS) of JECS. We evaluated the children’s serum 25(OH) D levels by 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles, and the rates of vitamin D deficiency or insufficiency. We also presented a weighted prevalence rate for vitamin D deficiency or insufficiency among all children in JECS.
Results
After excluding children with missing 25(OH)D2 or 25(OH)D3 data, we analyzed 4655 remaining children, of whom 24.7% (95% CI, 23.5–26.0%) had vitamin D deficiency (< 20 ng/mL), and 51.3% (95% CI, 49.8–52.7%) were at risk of vitamin D insufficiency (20–30 ng/mL). The estimated prevalence of vitamin D deficiency and insufficiency among all children in JECS were 25.4% (95% CI, 24.1–26.7%) and 50.9% (95% CI, 49.4–52.4%). Vitamin D deficiency was found in 22.9% of boys and 26.5% of girls. Median serum 25(OH) D concentrations were lower among participants measured during winter and spring than among those measured in summer and autumn. The highest rate of vitamin D deficiency was observed in Hokkaido, the northernmost prefecture of Japan.
Conclusion
We analyzed data on serum 25(OH) D levels from a birth cohort study and found that vitamin D deficiency and insufficiency are very common among 2-year-old Japanese children. Sex, season, and latitude affect serum 25(OH) D concentrations.
Similar content being viewed by others
Background
Vitamin D in its hormonal active form, 1,25-dihydroxyvitamin D, functions in regulating calcium levels and phosphate homoeostasis, and is important for bone growth and metabolism [1]. Vitamin D deficiency contributes to two metabolic bone diseases: rickets in children and osteomalacia in adults [1,2,3,4]. In addition, vitamin D helps regulate the immune system and cell proliferation and differentiation. Vitamin D deficiency is associated with a wide range of chronic diseases and conditions, including infections, asthma, adverse pregnancy outcomes, cancer, autoimmune disease, cardiovascular diseases, and multiple sclerosis [5,6,7,8,9,10,11,12]. Diet, supplement use, age, geographic latitude, culture, lifestyle, avoiding sunlight exposure, skin pigmentation, and individual differences in vitamin metabolism are well-known risk factors for low vitamin D levels [1]. A recent review of vitamin D status implied vitamin D deficiency was widespread, regardless of a country’s economic level and latitude [1]. Around 1 billion people in the world are suspected to have vitamin D deficiency or insufficiency [13]. In Europe, it might affect more than 50% of the population, depending on the season [14].
Serum 25-hydroxyvitamin D (25(OH)D) is a major circulating metabolite of vitamin D, which has a long half-life in the body, relative stability, and plentiful concentration in the blood. Thus, serum 25(OH) D concentration is the best indicator for recent vitamin D input among those without kidney disease [15,16,17]. Published studies suggest that serum 25(OH)D3 levels of ≥30 ng/mL are optimal for bone metabolism; levels between 20 and 29 ng/mL indicate insufficiency; and levels < 20 ng/mL suggests vitamin D deficiency [18]·. However, consensus on the optimal serum concentration of 25(OH) D has not been demonstrated yet [19].
Few studies have evaluated the prevalence of vitamin D deficiency in children in Japan. One study testing serum 25(OH) D levels in 574 3-year-old Japanese children, living at latitude 35°N, found 29.6% children had insufficient vitamin D (< 20 ng/mL) [20]. Another study found that 17% of 132 Japanese infants had serum 25(OH) D levels < 20 ng/mL [21].
The study aim was to obtain epidemiological data on vitamin D levels in the pediatric population in Japan using an accurate and reliable measurement approach. Accordingly, we examined serum 25(OH) D levels to assess the prevalence of vitamin D deficiency and insufficiency in 2-year-old Japanese children using data from a large ongoing birth cohort study.
Method
Study design
Data for analysis was obtained from the Japan Environment and Children’s Study (JECS) and a Sub-Cohort Study (SCS) of JECS. The JECS is a large-scale birth cohort study launched by the Ministry of the Environment, Japan in 2011. The cohort study includes 15 Regional Centres (Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka, and South Kyushu/Okinawa). Participants were pregnant woman and their partners who were recruited at first trimester of pregnancy when the Maternal and Child Health Handbook was issued or at clinic when woman got a diagnosis of pregnancy. The main aim of the JECS was to evaluate associations between environmental factors and children’s health and development. Over 100,000 pregnant women were recruited between January 2011 and March 2014. Children in the cohort will be followed up until they reach 13 years of age. The Main Study uses information from all the recruited participants. JECS protocols are described in detail elsewhere [22,23,24].
In addition to the Main Study, JECS is conducting an SCS with 5017 participants randomly selected from all the participants in the Main Study [25]. The participants of SCS are children in the Main Study who were born after 1 April 2013, and met all of the following requirements: 1) have a response to all the questionnaire surveys conducted during the period from the first trimester of pregnancy to 6 months after delivery, and data of medical records collected during the same period should be available, 2) data of biospecimens (except for umbilical cord blood) collected at the first trimester, second/third trimester, and delivery should be available [26].
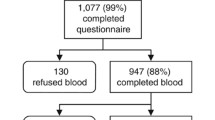
Data on blood test was derived from the SCS. Blood samples of 4695 2-year-old children were collected and analyzed by contract lab in JECS. Lidocaine and prilocaine cream were used to relief pain associated with venipuncture [27] .The total amount of blood collected at 2-year-olds was 4 ml. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) (LSI Medience Corporation; Japan) was used to measure serum 25(OH)D3 and 25(OH)D2 concentrations. The system was calibrated with‘6PLUS1 Multilevel Serum Calibrator Set 25-OH-Vitamin D3/D2’. The sample testing was validated through Accuracy-Based Vitamin D Survey (ABVD). The intra- assay CV for 25(OH)D3 was L: 9.9% (17.2 ng/mL) and H:9.1% (44.2 ng/mL). The inter-assay CV for 25(OH)D3 was L: 4.7% (15.7 ng/mL) and H:5.2% (39.3 ng/mL).
Missing on 25(OH)D2 or 25(OH)D3 were excluded from analysis. Because there were only 40 missing cases (< 5%), excluding these cases will not affect results significantly. Data used for analysis are from the jecs-ta-20,190,930-qsn dataset, and the jecs-ta-20,190,930-mdv dataset, released by the Program Office in October 2019.
The SCS was performed based on the guidelines specified in the Declaration of Helsinki. All procedures involving human participants were approved by the Japan Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (No. 100406001) and the ethics committees of all participating institutions. Written consent to the SCS protocol is signed separately from that of the Main Study and obtained from all participating mothers and fathers [26].
Cut-points for serum 25(OH)D
Values for 25(OH)D2 or 25(OH)D3 concentrations below 4 ng/mL were truncated as 4 ng/mL. Total serum 25(OH) D levels was defined as the sum of serum 25(OH)D3 and 25(OH)D2 concentrations. To clarify rates of vitamin D deficiency and insufficiency in this population, we considered those with serum 25(OH) D levels of < 20 ng/mL at risk of deficiency, 20–29 ng/mL at risk of insufficiency, and ≥ 30 ng/mL to have sufficient levels, according to the assessment criteria published by the Japanese Society for Bone and Mineral Research [28].
Other variables
Data on the participating children’s sex and region of residence were collected from medical record transcripts during the participating mothers’ pregnancy and at the children’s births. With respect to seasons when participants’ blood was drawn, we considered March–May for spring, June–August for summer, September–November for autumn, and December–February for winter.
Statistical analysis
We examined rates of vitamin D deficiency (less than 20 ng/mL) or insufficiency (20–29 ng/mL) by sex, season, and Regional Center. Categorical variables were summarized using proportions (%). Serum 25(OH) D level was also used as a continuous variable to describe the distribution according to the 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles, and to evaluate relationships with sex, season, and latitude. The difference in 25(OH) D levels between boys and girls was evaluated using the Wilcoxon test. The Kruskal–Wallis test was used to compare medians of serum 25(OH) D concentrations between the four season groups. Wilcoxon tests were performed for multiple comparisons after the Kruskal–Wallis test, and P values were adjusted using the Bonferroni correction. The strength and direction of association between latitude and serum 25(OH) D concentrations was assessed using the Spearman correlation coefficient.
To assess vitamin D status among all children in JECS, we also presented a weighted prevalence rate for 25(OH) D deficiency or insufficiency. The weights were the inverse of the predicted probability of participants by fitting a logistic model adjusting for maternal age, parental degrees, family income, birthweight, and child’s sex. Descriptive analysis, models, and bootstrap processes were performed using R version 3.6.1 software (Institute for Statistics and Mathematics, Vienna, Austria; www.r-project.org).
Results
After excluding those with missing data for serum 25(OH)D2 or D3 concentrations, 4655 cases were left for analysis. Rates of vitamin D deficiency or insufficiency are list in Tables 1. The 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles of serum 25(OH)-D levels are listed in Additional file 2. The median serum 25(OH) D concentration was 24.7 ng/mL in SCS. We found 24.7% (95% CI, 23.5–26.0%) of children in SCS had a level below 20 ng/mL, and 51.3% (95% CI, 49.8–52.7%) were at risk of vitamin D insufficiency (20–30 ng/mL). A total of 22.9% (95% CI, 21.2–24.6%) of boys and 26.5% (95%CI, 24.7–28.4%) of girls had 25(OH) D levels less than 20 ng/mL, whereas 26.5% (95% CI, 24.7–28.3%) of boys and 21.6% (95% CI, 19.9–23.3%) of girls had serum 25(OH) D levels greater than 30 ng/mL.
Figure 1 shows serum 25(OH) D status by sex. The median 25(OH) D concentration was 25.4 ng/mL in boys, significantly higher than the 24.0 ng/mL in girls (P Wilcoxon test < 0.001).
Figure 2 shows the distribution of serum 25(OH) D concentrations by season. The distribution was different between the four season groups (PKruskal–Wallis test < 0.001). The median serum 25(OH) D concentrations were lower among participants measured during winter and spring than among those measured in summer and autumn. All the pairwise Wilcoxon tests showed significant between-group differences in distribution (P < 0.0001), except for spring vs autumn.
Figure 3 shows the serum 25(OH) D concentrations by latitude and season. We found a very weak but significant relationship between latitude and serum 25(OH) D concentrations (Spearman’s rank correlation rho = − 0.04, P = 0.004). Higher rates of vitamin D deficiency were found in areas north of 40°N, including Sapporo, the Asahikawa part of Kitami, Oketo, Kunneppu, Tsubetsu, and Bihoro, which are located in Hokkaido Prefecture; whereas lower rates were found in areas south of 30°N, explaining data from Okinawa, the southernmost prefecture of Japan. In winter, 80% of the children living in Hokkaido areas had deficient serum 25(OH) D levels (Additional file 1).
Estimated rates of vitamin D deficiency or insufficiency among 2 years old children in JECS are showed in Table 2. The estimated proportion of serum 25(OH) D levels less than 20 ng/mL and 20 to < 30 ng/mL among all children in JECS were 25.4% (95% CI, 24.1–26.7%) and 50.9% (95% CI, 49.4–52.4%). Back-weighted rates were similar to observed rates from the SCS.
Discussion
This is the first effort to report serum 25(OH) D levels for 2-year-old Japanese children with a large sample. Here, we present descriptive statistics, using data from the JECS and SCS of JECS, which show approximately 20% of children in JECS had serum 25(OH) D levels less than 20 ng/mL, and approximately 70% had levels below 30 ng/mL.
High prevalence rates of vitamin D insufficiency or deficiency among the pediatric population have been observed in many countries. In U.K. population, (mainly white 7–11 yr olds), 29% were vitamin D deficient (< 20 ng/mL) and 75% had concentrations of total 25(OH) D less than 30 ng/mL [29]. Study based on a nationally representative sample of US children aged 1 to 21 years in the National Health and Nutrition Examination Survey 2001–2004 found that 9% of children had 25(OH) D levels less than 15 ng/mL, and 61% of the children had 25(OH) D levels between 15 and 29 ng/mL [30]. A recent study in Finland found almost 20% of the children (6–8 years) had serum 25(OH) D concentrations below 20 ng/mL, and about 70% of the children had serum 25(OH) D concentrations below 30 ng/mL [31]. Prevalence of vitamin D deficiency (≤20 ng/mL) among healthy infants and toddlers (380 primary care US children aged 8–24 months) was 12.1, and 40.0% had levels below 30 ng/mL [32]. A study in Japan mentioned above estimated an insufficient vitamin D (< 20 ng/mL) prevalence of 29.6% in 3-year-old Japanese children [20]. In 2012, Zhu et al. investigated the vitamin D status of children aged 1 month to 16 years in Huangzhou, China. They reported 22% of children aged 2–5 years lacked vitamin D(< 20 ng/mL) [33]. The variations in the prevalence of vitamin D insufficiency and deficiency could be explained by differences in study design, measurement methods, participant age, season of measurement, and resident latitude.
Our study indicates that girls had lower average 25(OH) D than boys, which is similar to findings from other countries. This may reflect differences in time spent playing outdoors rather than biological differences in vitamin D metabolism between boys and girls.
Our study also found regional differences in vitamin D levels among Japanese children. UV exposure may partly explain this geographical difference. People living in high latitudes are prone to vitamin D insufficiencies, especially in winter [34, 35]. The highest vitamin D deficiency rate was found in Hokkaido Prefecture, the northernmost regional Prefecture of Japan, which partly reflects the effects of sunlight exposure. Surprisingly, although located in southern areas, our finding also indicated that the springtime vitamin D deficiency rate was higher in the Fukuoka Regional Centre than in most other areas, even those located in more northern latitudes. We speculate that Aeolian dust blown during spring from semi-arid areas of the Asian continent may lead to the high rate of hypovitaminosis D observed in the Fukuoka Regional Centre. According to the Japan Meteorological Agency, the total days of observed Aeolian dust were nearly 1 week each during March 2013 and May 2014 in cities in Fukuoka, which is much higher than in other areas. Aeolian dust could decrease sunlight exposure, because people are inclined to stay indoors when Aeolian dust arrives [21].
The lack of access to vitamin D-fortified food and vitamin D supplements for children may partly explain the vitamin D deficiency or insufficiencies in Japanese children. Consumption of vitamin D-fortified food is minimal in Japan. In addition, there is no recommendation or guidelines for vitamin D supplementation for Japanese children [20].
One advantage of this study was the large number of participants from a large birth cohort study in Japan. Children in the JECS represented about 45% of live births in the catchment area of all Japanese Regional Centres, and their characteristics were similar to those of children in the Japanese Vital Statistics; thus results from the cohort can be generalized to the Japanese population. In addition, the study covers a wide geographical area from the northernmost prefecture (Hokkaido) to the southernmost prefecture (Okinawa), which allows us to explore the relationship with latitude and vitamin D status. We used LC-MS/MS to test serum 25(OH) D concentrations in current study. LC-MS/MS is widely accepted in clinical laboratories to differentiate between vitamin-D deficiency and sufficiency, because of its excellent analytical specificity and sensitivity [36,37,38,39].
The study has some limitations that should be considered when comparing with other studies. First, more than 60% of children in this cohort were tested during the summer and autumn, and time of year significantly affects serum 25(OH) D levels. As shown in Table 2, the prevalence rates of 25(OH) D less than 20 ng/mL in our study were 52.6% in winter vs 12.2% in summer; thus, the overall vitamin D deficiency rate might be underestimated here. In addition to time of year, when comparing serum 25(OH) D levels between studies, differences in latitude must be taken into account [31]. Second, values for serum 25(OH) D concentration that were lower than 4 ng/mL were truncated to 4 ng/mL in this analysis, which may cause serum 25(OH) D concentrations to be slightly overestimated. Finally, the lack of consensus on optimal vitamin D levels [19] and normal range for 25(OH) D concentration further complicates studies of serum 25(OH) D concentration. Moreover, serum 25(H) D concentration vary depending on the assays used for testing, which make defining vitamin deficiency with serum 25(OH) D concentration more difficult [40] [31]. Some studies suggest 30 ng/mL as the lower limit for sufficient serum 25(OH) D level [41]; others suggest 20 ng/mL [20, 42]. Only around 24% of children in our cohort had serum 25(OH) D levels > 30 ng/mL. Whether rates of vitamin D deficiency or insufficiency were overestimated by using the current cut-off value (> 30 ng/mL) also needs to be further assessed. Developing cut-off points for the definition of vitamin D status for young population is needed.
In conclusion, we analyzed data on serum 25(OH) D levels from a birth cohort study and found that vitamin D deficiency and insufficiency are very common among 2-year-old Japanese children. Sex, season, and latitude affect serum 25(OH) D concentrations. Further analysis of other factors that may affect vitamin D levels, such as supplementation and dietary intake, should be conducted.
Availability of data and materials
The JECS data are not publicly available due to ethical restrictions and the legal framework of Japan. All inquiries about access to the data should be sent to the JECS Programme Office, National Institute for Environmental Studies (jecs-en@nies.go.jp).
Abbreviations
- 25(OH)D:
-
25-hydroxyvitamin D
- JECS:
-
Japan Environment and Children’s Study (JECS)
- CI:
-
Confidence interval
- SCS:
-
Sub-Cohort Study
References
Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. 2018;1430(1):44–79.
Bischoff-Ferrari H. Health effects of vitamin D. Dermatol Ther. 2010;23(1):23–30.
Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634–9.
Gonzalez-Gross M, Valtuena J, Breidenassel C, Moreno LA, Ferrari M, Kersting M, et al. Vitamin D status among adolescents in Europe: the healthy lifestyle in Europe by nutrition in adolescence study. Br J Nutr. 2012;107(5):755–64.
Annweiler C, Allali G, Allain P, Bridenbaugh S, Schott AM, Kressig RW, et al. Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol. 2009;16(10):1083–9.
Stoffman N, Gordon CM. Vitamin D and adolescents: what do we know? Curr Opin Pediatr. 2009;21(4):465–71.
Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
Kim MJ, Kim SN, Lee YW, Choe YB, Ahn KJ: Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8(12).
Feng R, Li Y, Li G, Li Z, Zhang Y, Li Q, et al. Lower serum 25 (OH) D concentrations in type 1 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2015;108(3):e71–5.
Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and Meta-analysis. Inflamm Bowel Dis. 2015;21(11):2708–17.
Huang SJ, Wang XH, Liu ZD, Cao WL, Han Y, Ma AG, et al. Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther. 2017;11:91–102.
Li HB, Tai XH, Sang YH, Jia JP, Xu ZM, Cui XF, et al. Association between vitamin D and development of otitis media: a PRISMA-compliant meta-analysis and systematic review. Medicine (Baltimore). 2016;95(40):e4739.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807–20.
Alshahrani F, Aljohani N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients. 2013;5(9):3605–16.
Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87(4):1087S–91S.
Millen AE, Bodnar LM. Vitamin D assessment in population-based studies: a review of the issues. Am J Clin Nutr. 2008;87(4):1102S–5S.
Muschitz C, Kocijan R, Stutz V, Kaider A, Muschitz GK, Resch H, et al. Vitamin D levels and comorbidities in ambulatory and hospitalized patients in Austria. Wien Klin Wochenschr. 2015;127(17–18):675–84.
Saggese G, Vierucci F, Prodam F, Cardinale F, Cetin I, Chiappini E. De' Angelis GL, Massari M, Miraglia Del Giudice E, Miraglia Del Giudice M et al: vitamin D in pediatric age: consensus of the Italian pediatric society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian federation of pediatricians. Ital J Pediatr. 2018;44(1):51.
Ando E, Morisaki N, Asakura K, Sasaki S, Fujiwara T, Horikawa R. Serum 25-hydroxyvitamin D levels showed strong seasonality but lacked association with vitamin D intake in 3-year-old Japanese children. Br J Nutr. 2018;120(9):1034–44.
Ayabe T, Yamamoto-Hanada K, Mezawa H, Konishi M, Ishitsuka K, Saito M, et al. Regional differences in infant 25-Hydroxyvitamin D: pilot study of the Japan environment and Children's study. Pediatr Int. 2018;60(1):30–4.
Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K, et al. Baseline profile of participants in the Japan environment and Children's study (JECS). J Epidemiol. 2018;28(2):99–104.
Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, et al. Rationale and study design of the Japan environment and children's study (JECS). BMC Public Health. 2014;14:25.
Japan Environment and Children's Study (JECS) Study Protocol. Available from https://www.env.go.jp/chemi/ceh/en/about/advanced/material/jecs-study_protocol_14_en.pdf. [https://www.env.go.jp/chemi/ceh/en/about/advanced/material/jecs-study_protocol_14_en.pdf.]
Sekiyama M, Yamazaki S, Michikawa T, Nakayama SF, Nitta H, Taniguchi Y, et al. Study design and participants' profile in the sub-cohort study in the Japan environment and Children's study (JECS). J Epidemiol. 2020.
Japan Environment and Children's Study (JECS) Sub-Cohort Study Protocol. Available from https://www.env.go.jp/chemi/ceh/en/about/advanced/material/jecs-sub-cohort_study_protocol_101-en.pdf.
Yamamoto-Hanada K, Futamura M, Kitazawa H, Ohya Y, Kobayashi F, Kusuda T, et al. Relieving pain and distress during venipuncture: pilot study of the Japan environment and Children's study (JECS). Pediatr Int. 2015;57(5):1044–7.
Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, et al. Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the research program of intractable diseases, Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society [opinion]. J Bone Miner Metab. 2017;35(1):1–5.
Tolppanen AM, Fraser A, Fraser WD, Lawlor DA. Risk factors for variation in 25-hydroxyvitamin D(3) and D(2) concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab. 2012;97(4):1202–10.
Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124(3):e362–70.
Soininen S, Eloranta AM, Lindi V, Venalainen T, Zaproudina N, Mahonen A, et al. Determinants of serum 25-hydroxyvitamin D concentration in Finnish children: the physical activity and nutrition in children (PANIC) study. Br J Nutr. 2016;115(6):1080–91.
Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162(6):505–12.
Zhu Z, Zhan J, Shao J, Chen W, Chen L, Li W, et al. High prevalence of vitamin D deficiency among children aged 1 month to 16 years in Hangzhou. China BMC Public Health. 2012;12:126.
Rodriguez A, Santa Marina L, Jimenez AM, Esplugues A, Ballester F, Espada M, et al. Vitamin D status in pregnancy and determinants in a southern European cohort study. Paediatr Perinat Epidemiol. 2016;30(3):217–28.
Nikooyeh B, Abdollahi Z, Hajifaraji M, Alavi-Majd H, Salehi F, Yarparvar AH, et al. Vitamin D status, latitude and their associations with some health parameters in children: National Food and nutrition surveillance. J Trop Pediatr. 2017;63(1):57–64.
Galior K, Ketha H, Grebe S, Singh RJ. 10 years of 25-hydroxyvitamin-D testing by LC-MS/MS-trends in vitamin-D deficiency and sufficiency. Bone Rep. 2018;8:268–73.
Kushnir MM, Ray JA, Rockwood AL, Roberts WL, La'ulu SL, Whittington JE, et al. Rapid analysis of 25-hydroxyvitamin D(2) and D(3) by liquid chromatography-tandem mass spectrometry and association of vitamin D and parathyroid hormone concentrations in healthy adults. Am J Clin Pathol. 2010;134(1):148–56.
Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(3):441–8.
Park EJ, Lee HS, Lee SH, Shim KW, Cho C, Yoo BW. The level of vitamin D using the LC-MS/MS method and related factors in healthy Korean postmenopausal women. J Obstet Gynaecol Res. 2018;44(10):1977–84.
Janssen MJ, Wielders JP, Bekker CC, Boesten LS, Buijs MM, Heijboer AC, et al. Multicenter comparison study of current methods to measure 25-hydroxyvitamin D in serum. Steroids. 2012;77(13):1366–72.
Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and nutrition examination survey 2005-2006. J Allergy Clin Immunol. 2011;127(5):1195–202.
Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea--a greater threat to younger generation: the Korea National Health and nutrition examination survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011;96(3):643–51.
Acknowledgements
We thank the children and their families for participating in the JECS and all individuals involved in data collection. We also thank Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript. The findings and conclusions of this article are the sole responsibility of the authors and do not represent the official views of the above government agency. Members of the JECS Group as of 2020: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (Na-tional Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Youichi Kurozawa (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan),and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Funding
This study was funded and supported by the Ministry of the Environment, Japan.
Author information
Authors and Affiliations
Consortia
Contributions
All authors approved the final manuscript. L.Y. initiated the concept and designed the study to which M. S, M.S.-A., M.I., M.N. H.S., M.K., K.I., H.M., K.Y.-H. and Y.O. gave advice. L.Y., M. S, M. S.-A., M.I., M.N. H.S., M.K., K.I., H.M., K.Y.-H. and Y. O collected the data. L.Y. analyzed the data and wrote the manuscript. L.Y., M. S, M. S.-A., M.I., M.N. H.S., M.K., K.I., H.M., K.Y.-H., Y. O and the JECS group reviewed the manuscript and gave critical advice. All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors’ original work, hasn’t received prior publication and isn’t under consideration for publication elsewhere.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The SCS was performed based on the guidelines specified in the Declaration of Helsinki. All procedures involving human participants were approved by the Japan Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (No. 100406001) and the ethics committees of all participating institutions. Written consent to the SCS protocol is signed separately from that of the Main Study and obtained from all participating mothers and fathers.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, L., Sato, M., Saito-Abe, M. et al. 25-Hydroxyvitamin D levels among 2-year-old children: findings from the Japan environment and Children’s study (JECS). BMC Pediatr 21, 539 (2021). https://doi.org/10.1186/s12887-021-03005-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-021-03005-3