Abstract
Background
Type 1 diabetes (T1D) is caused by immune-mediated destruction of the β-cells. After initiation of insulin therapy many patients experience a period of improved residual β-cell function leading to partial disease remission. Cytokines are important immune-modulatory molecules and contribute to β-cell damage in T1D. The patterns of systemic circulating cytokines during T1D remission are not clear but may constitute biomarkers of disease status and progression. In this study, we investigated if the plasma levels of various pro- and anti-inflammatory cytokines around time of diagnosis were predictors of remission and residual β-cell function in children with T1D followed for one year after disease onset.
Methods
In a cohort of 63 newly diagnosed children (33% females) with T1D with a mean age of 11.3 years (3.3–17.7), ten cytokines were measured of which eight were detectable in plasma samples by Mesoscale Discovery multiplex technology at study start and after 6 and 12 months. Linear regression models were used to evaluate association of cytokines with stimulated C-peptide.
Results
Systemic levels of tumor necrosis factor (TNF)-α, interleukin (IL)-2 and IL-6 inversely correlated with stimulated C-peptide levels over the entire study (P < 0.05). The concentrations of TNFα and IL-10 at study start predicted stimulated C-peptide level at 6 months (P = 0.011 and P = 0.043, respectively, adjusted for sex, age, HbA1c and stage of puberty).
Conclusions
In recent-onset T1D, systemic cytokine levels, and in particular that of TNFα, correlate with residual β-cell function and may serve as prognostic biomarkers of disease remission and progression to optimize treatment strategies.
Trial Registration
The study was performed according to the criteria of the Helsinki II Declaration and was approved by the Danish Capital Region Ethics Committee on Biomedical Research Ethics (journal number H-3-2014-052). The parents of all participants gave written consent.
Similar content being viewed by others
Background
Type 1 diabetes (T1D) is the consequence of immune-mediated destruction of the insulin-producing pancreatic β-cells. A transient period of partial disease remission is seen in many T1D patients shortly after initiation of insulin therapy. This ‘honeymoon’ period is characterized by transitory restoration of endogenous insulin production, lower insulin requirement and better glycemic control [1, 2]. Prediction of partial remitters at time of diagnosis may facilitate optimized and individualized patient care.
The pathogenesis of T1D is multifactorial and not fully understood. Cytokines are important regulators of immune and inflammatory processes and specific cytokines such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α released by intra-pancreatic immune cells contribute to T1D by directly causing β-cell impairment [3, 4]. Systemic plasma levels of cytokines in new-onset T1D may reflect the detrimental events occuring in the pancreas and might predict disease remission and/or be biomarkers of disease status. Accordingly, a recent study suggested an association between immune cell subtypes and serum cytokine patterns with metabolic control in children newly diagnosed with T1D [5].
In this study, we investigated the correlation between ten systemic cytokines and metabolic deterioration in children with recent-onset T1D to predict disease course during first year after diagnosis.
Methods
Study subjects
Blood samples obtained from a total of 63 children and adolescents with newly diagnosed type 1 diabetes (T1D) based on the ISPAD (International Society for Pediatric and Adolescent Diabetes) criteria for diagnosis defined as the first visit with high blood glucose levels and initiation of insulin treatment as described by Madsen et al. [6]. The mean age at study start was 11.3 (3.3–17.7). Mean time from diagnosis to first visit and, hence, baseline sample was 2.45 ± 0.65 months. At each visit a physician examined wether the participants had entered puberty according to the Tanner criteria [7, 8]. Pubertal stages were then categorized as prepubertal (Tanner 1) or pubertal (Tanner 2-5). Parents or guardians all gave written informed consent. The study was performed according to the criteria of the Helsinki II Declaration and was approved by the Danish Capital Region Ethics Committee on Biomedical Research Ethics (journal number H-3-2014-05). Plasma samples from first visit after being enrolled in study (baseline), and repeated 6 and 12 months later were used in this study.
Residual β-cell function and HbA1c
The endogenous insulin production in the participants was assesed by measuring stimulated serum C-peptide levels at study start (defined as baseline) and 6 and 12 months after inclusion. Blood was drawn at fasting and 90 minutes after ingestion of a standardized liquid mixed-meal (Boost High Protein, Nestlé Health Science, or Ensure Plus, Abbott Nutrition). Plasma and serum were isolated accordingly and samples were stored at − 80˚C until analysed. C-peptide and HbA1c were measured using an Immulite® 2000 chemiluminescent immunometric assay and high-pressure liquid chromatography (Tosoh Bioscience, South San Francisco, CA, USA), respectively.
Cytokine quantifications
Cytokine concentrations in fasting plasma samples were measured using the V-PLEX Proinflammatory Panel 1 Human Kit (Meso Scale Diagnostics® [MSD], Gaithersburg, MD, USA) quantifying interferon (IFN)-γ, interleukin (IL)-1β, interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin (IL-8), interleukin (IL-10), interleukin-12p70 (IL-12p70), interleukin-13 (IL-13) and tumor necrosis factor (TNF)-α according to the manufacturers instructions using the MESO QuickPlex SQ120 instrument. Analytes below the detection limit of the assay and analytes with a coefficient of variation (CV) > 30% were discarded for further analysis and included IL-1β and IL-13. Plate-to-plate variation was assessed by including identical pooled plasma samples and the CV between plates for each analyte was 8-25.8%.
Statistical analysis
Variance in clinical and anthropometrics data between time points were assessed using ANOVA with a Tukey post hoc test. Associations between cytokine levels and stimulated C-peptide levels were evalutaed using linear regression. Linear regression models adjusted for sex, age, HbA1c and puberty status according to the Tanner criteria were constructed using cytokine levels at baseline as predictors of stimulated C-peptide levels at 6 and 12 months as outcome. We did not include BMI as a covariate because of the strong correlation between age and BMI (Pearson correlation: r = 0.6093, P < 0.0001). C-peptide levels were log transformed before statistical analysis. All statistical tests were performed using GraphPad Prism8 or the free statitistical environment R (https://www.r-project.org/).
Results
Correlation of cytokine levels with stimulated C-peptide
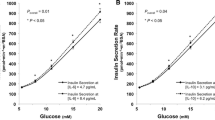
The clinical characteristics of the study participants and the cytokine concentrations measured are shown in Table 1. We first determined the fluctuation of the eight measurable cytokines over time. No significant differences in the concentrations of any of the cytokines between baseline and 6 and 12 months were observed (Table 1). However, the levels of TNFα, IL-2 and IL-6 correlated negatively with stimulated C-peptide levels (Fig. 1).
Cytokine levels at baseline as predictors of stimulated C-peptide
Throughout the study period, most study participants were in partial remission as defined by a stimulated C-peptide level > 300 pmol/L. The highest mean stimulated C-peptide level was at baseline and declinded gradually at 6 and 12 months (Table 1). Baseline levels of IL-10 (intercept − 0.57 (confidence interval (CI): -1; -0.1)) and TNFα (intercept − 0.38 (CI: -0.6; -0.1)) individually predicted lower stimulated C-peptide at 6 months (P = 0.043 and P = 0.011, respectively, adjusted for sex, age, HbA1c and Tanner criteria) (Table 2).
Discussion
The partial disease remission seen in many T1D patients within a few months after diagnosis may be a possible target checkpoint for future therapeutic interventions aiming at preserving residual β-cell mass. It may also be used to identify individuals with a lower or higher risk of developing long-term complications. Identification of specific biomarkers of disease remission are therefore important to facilitate optimized treatment strategies.
We measured the systemic levels of ten cytokines in a cohort of children newly diagnosed with T1D. There were no differences in the mean concentrations of any of the eight measurable cytokines over the entire study period indicating intra-individual stability of cytokines over time. The concentrations of TNFα, IL-2 and IL-6, however, associated inversely with stimulated C-peptide. Further, the levels of IL-10 and TNFα at baseline predicted lower stimulated C-peptide at 6 months. That TNFα turned out significant in both analyses suggest that TNFα may be a particularly important determinant of residual β-cell function in newly diagnosed T1D patients.
TNFα is well-known to exert detrimental effects on β-cells in vitro especially when combined with other cytokines such as IL-1β and/or IFN-γ, and are believed to contribute to β-cell destruction in T1D [3]. A small placebo-controlled clinical trial investigating the effect of a 24-week therapy with etanercept, an anti-TNF agent used to treat rheumatoid arthritis, in children newly diagnosed with T1D reported an increase in C-peptide and a decrease in HbA1c in the etanercept group [9]. These results suggest that targeting TNFα early in T1D can boost and possibly prolong the honeymoon phase in T1D via improved β-cell function. The observed inverse correlation between TNFα plasma levels and stimulated C-peptide during the remission period may help stratify patients for anti-TNF therapy in future studies.
In line with our findings, a previous study of T1D remission in adults found that the levels of circulating cytokines including IL-10 at time of diagnosis are predictive of clinical remission [10]. Another study of T1D remission in children also found an inverse correlation between serum IL-10 and C-peptide [11]. Association between IL-10 and C-peptide in recent-onset T1D therefore seems strong and IL-10 may therefore be a robust inverse biomarker of residual β-cell function.
The pathogenic role of IL-10 in T1D is unclear. IL-10 is an anti-inflammatory cytokine that dampens the activity of antigen-presenting cells and T helper and cytotoxic T cells [12]. Our finding that IL-10 is inversely associated with stimulated C-peptide therefore seems counterintuitive. However, as IL-10 may also possess pro-inflammatory effects depending on the exact biological setting [13], it cannot be excluded that IL-10 has a dual role in T1D. No human trials administering or targeting IL-10 have so far been reported, but studies using the non-obese diabetic (NOD) mouse model of T1D found that IL-10 has protective effects on diabetes development [14, 15]. Irrespective of the precise pathophysiological role of IL-10 in T1D, our results suggest that measurement of plasma IL-10 around time of diagnosis may be used to predict remitters and the speed of decline in residual β-cell function.
A major limitation of our study is the limited number of study participants which hamper the statistical power. Hence, larger studies with more power are warranted and will likely provide more information about the relationship between systemic inflammatory factors and partial remission. Another drawback of our study is that the baseline plasma samples were taken on average 2.45 months after T1D diagnosis. The reason for this is that the samples originally were obtained with the purpose of studying bone markers at the peak of remission. Therefore, at this time point, most of the patients had the highest level of residual β-cell function reflecting maximal partial remission. It would have been preferable to have the baseline samples taken closer to the time of diagnosis to see if the levels of cytokines at that time could predict the peak amplitude of remission.
Conclusions
In summary, our results show an inverse correlation between certain cytokines and residual β-cell function in children and adolescents with newly diagnosed T1D. The level of two of the cytokines, i.e. TNFα and IL-10, at time around diagnosis are predictors of β-cell function after 6 months and may thus be exploited as biomarkers to better monitor and predict clinical remission. As TNFα was both a prognostic marker of residual β-cell function and was negatively associated with C-peptide levels throughout the study period highlight TNFα as the main finding of this study. Whether TNFα (and IL-10) plays a causative role in disease remission or merely associate with remission remains unclear.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- HbA1c:
-
Hemoglobin A1c
- IFN:
-
Interferon
- IL:
-
Interleukin
- MSD:
-
Meso Scale Discovery
- T1D:
-
Type 1 diabetes
- TNF:
-
Tumor necrosis factor
References
Greenbaum CJ, Speake C, Krischer J, Buckner J, Gottlieb PA, Schatz DA, Herold KC, Atkinson MA. Strength in Numbers: Opportunities for Enhancing the Development of Effective Treatments for Type 1 Diabetes-The TrialNet Experience. Diabetes. 2018;67:1216–25.
Madsbad S. Prevalence of residual B cell function and its metabolic consequences in Type 1 (insulin-dependent) diabetes. Diabetologia. 1983;24:141–7.
Berchtold LA, Prause M, Storling J, Mandrup-Poulsen T. Cytokines and Pancreatic beta-Cell Apoptosis. Adv Clin Chem. 2016;75:99–158.
Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–26.
Fitas AL, Martins C, Borrego LM, Lopes L, Jorns A, Lenzen S, Limbert C. Immune cell and cytokine patterns in children with type 1 diabetes mellitus undergoing a remission phase: A longitudinal study. Pediatr Diabetes. 2018;19:963–71.
Madsen JOB, Jorgensen NR, Pociot F, Johannesen J. Bone turnover markers in children and adolescents with type 1 diabetes-A systematic review. Pediatr Diabetes. 2019;20:510–22.
Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303.
Plesser YM, Doljanski F, Polliack A. Alteration in lymphocyte surface morphology and membrane fluidity induced by cholesterol depletion. Cell Mol Biol Incl Cyto Enzymol. 1979;25:203–6.
Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–9.
Schloot NC, Hanifi-Moghaddam P, Aabenhus-Andersen N, Alizadeh BZ, Saha MT, Knip M, Devendra D, Wilkin T, Bonifacio E, Roep BO, Kolb H, Mandrup-Poulsen T. Association of immune mediators at diagnosis of Type 1 diabetes with later clinical remission. Diabet Med. 2007;24:512–20.
Kaas A, Pfleger C, Kharagjitsingh AV, Schloot NC, Hansen L, Buschard K, Koeleman BP, Roep BO, Mortensen HB, Alizadeh BZ. Association between age, IL-10, IFNgamma, stimulated C-peptide and disease progression in children with newly diagnosed Type 1 diabetes. Diabet Med. 2012;29:734–41.
Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7.
Muhl H. Pro-Inflammatory Signaling by IL-10 and IL-22: Bad Habit Stirred Up by Interferons? Front Immunol. 2013;4:18.
Mishra PK, Patel N, Wu W, Bleich D, Gause WC. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 2013;6:297–308.
Xu A, Zhu W, Li T, Li X, Cheng J, Li C, Yi P, Liu L. Interleukin-10 gene transfer into insulin-producing beta cells protects against diabetes in non-obese diabetic mice. Mol Med Rep. 2015;12:3881–9.
Acknowledgements
We are grateful to Anne Jørgensen for excellent technical assistance.
Funding
This work was supported by Aase og Ejnar Danielssens fond (supported analyses), Vissing Fonden (supported salary), Dronning Louises Børnehospitals Fond (supported salary and analyses), Poul og Erna Sehested Hansen Fonden (supported salary and analyses) and the research council at Herlev and Gentofte Hospital (supported salary).
Author information
Authors and Affiliations
Contributions
J.O.B.M. and J.J. recruited participants, conducted the clinical study and collected the samples. A.J.O., F.P. and J.S. concieved and performed the cytokine measurements, analysed the data and wrote the manuscript. All authors reviewed the manuscript and contributed with critical input to data interpretation and discussion. All authors have read and approved the manuscript. The authors declare no conflicts of interest.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Danish Capital Region Ethics Committee on Biomedical Research Ethics (journal number H-3-2014-052). The parents of all participants gave written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Overgaard, A.J., Madsen, J.O.B., Pociot, F. et al. Systemic TNFα correlates with residual β-cell function in children and adolescents newly diagnosed with type 1 diabetes. BMC Pediatr 20, 446 (2020). https://doi.org/10.1186/s12887-020-02339-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-020-02339-8