Abstract
Background
Vancomycin has recently gained popularity as an empiric therapy for late onset sepsis in the NICU. Changes in resistance patterns in common organisms has resulted in targeting higher trough concentrations of vancomycin. Consequently, an increase in vancomycin associated nephrotoxicity has been speculated. The objective of this study is to compare the incidence of acute kidney injury (AKI) in neonates with serum vancomycin trough concentrations less than 10 mg/L, 10–15 mg/L, or greater than 15 mg/L.
Methods
A retrospective chart review of patients in the neonatal intensive care unit (NICU) was conducted to determine the incidence of AKI in neonates receiving vancomycin.
Results
The overall incidence of AKI was 2.7%. Comparison of the incidence of AKI in the three groups using Mantel-Haenszel Chi-Square test showed a statistically significant association between increasing vancomycin trough concentration and incidence of AKI.
Conclusion
There is a low incidence of AKI in neonates receiving vancomycin. However, there is a positive correlation between increasing vancomycin trough concentrations and an increasing serum creatinine.
Similar content being viewed by others
Background
Vancomycin is a glycopeptide antibiotic which gained popularity in 1980’s for treatment of coagulase negative Staphylococcus (CONS) and Methicillin resistant staphylococcus aureus (MRSA). Late onset sepsis is a common concern in premature infants in neonatal intensive care unit (NICU). Vancomycin is widely used as an empiric therapy for late onset sepsis, and in confirmed infections with CONS and MRSA. [1, 2] Consequently, vancomycin is used in the NICU even though limited information is available concerning the dosing, monitoring, and adverse effects of this medication in neonates. Additionally, increasing antibiotic resistance among familiar pathogens in the NICU, as evidenced by higher minimum inhibitory concentrations (MICs), has resulted in targeting higher vancomycin trough concentrations. [3, 4] Vancomycin associated nephrotoxicity has not been well studied in the neonatal population and limited data exists on the association between higher vancomycin trough and incidence of acute kidney injury (AKI).
This study aims to measure the association between increasing trough concentrations and AKI in neonates receiving vancomycin therapy. We also look at the effect of co-administration of other nephrotoxic agents on the incidence of AKI.
Methods
A retrospective chart review was performed for patients in NICU at University of Texas Medical Branch (UTMB) at Galveston. The electronic medical record was reviewed between January 2008 and December 2012 to determine the incidence of AKI in neonates receiving vancomycin. The patient population consisted of premature neonates admitted to NICU at UTMB and received at least one course of vancomycin. Each patient may have received several courses of vancomycin during this period. Courses of vancomycin were included in the study group if they met the following inclusion criteria: a) Duration of treatment at least 5 days b) Availability of serum creatinine (SCr) values both prior to and after completing the vancomycin therapy and c) at least one vancomycin trough collected during the duration of treatment. Courses of vancomycin therapy were excluded if: a) evidence of pre-existing renal insufficiency or congenital anomalies including renal agenesis, renal hypoplasia, polycystic kidney disease, or renal dysplasia were present b) extracorporeal membrane oxygenation was required or c) there was incomplete data in the UTMB electronic medical record, Epic. Baseline serum creatinine was defined as the serum creatinine obtained prior to starting vancomycin therapy. Post vancomycin creatinine was defined as the serum creatinine value obtained at the end of vancomycin therapy or after a suspected episode of AKI.
Courses of vancomycin therapy were divided into three groups based on highest achieved vancomycin trough concentrations; less than 10 mg/L, 10–15 mg/L, or greater than 15 mg/L. Standardized vancomycin dosage proposed by Capparelli et al., based on gestational age was used for each patient.[5] The trough was obtained prior to the fourth dose. If the trough was found sub-therapeutic or toxic, changes in dose or frequency of vancomycin administration were accordingly made. The change in dose was subsequently followed by a repeat trough measurement prior to the 4th dose. For each course of vancomycin therapy, multiple vancomycin trough concentrations may have been measured, but the highest achieved trough concentration was used to classify courses of vancomycin therapy into the three study groups. The incidence of AKI was determined in each group. AKI was defined as an increase in SCr of at least 0.5 mg/L or an increase of at least 100% from lowest trough previously available. This definition is based on pRIFLE criteria for renal injury proposed by Akcan-Arikan et al. and the increase in serum creatinine proposed by moghal et al. [6–8] A decrease in urine output was not used as a defining criteria due to lack of this being a universal finding.
Data collected included gestational age; postnatal age; gender; birth weight; APGAR scores; vancomycin dose, frequency, duration, and trough concentrations; date and time of vancomycin doses and trough concentrations; concurrent nephrotoxic medications administered including amphotericin B, acyclovir, amikacin, captopril, dobutamine, dopamine, enalapril, epinephrine, ganciclovir, gentamicin, indomethacin, ibuprofen, naficillin, and tobramycin; blood culture results; type of infection; presence of a patent ductus arteriosus; and final discharge status. Maternal history was also collected and included the presence of pregnancy-induced hypertension, chorioamnionitis, diabetes, renal dysfunction, or urinary tract infection.
Mantel-Haenszel Chi-Square test was used to compare the incidence of AKI between the three groups. A p-value of < 0.05 was considered to be statistically significant. Regression analysis was used to examine the relationship between vancomycin trough concentrations and serum creatinine. All statistical analyses were done using SAS 9.3©. The study was approved by the institutional review board (IRB) at UTMB, Galveston.
Results
Nine hundred and sixty-two patients receiving vancomycin therapy administered between January 2008 and December 2012 were evaluated for study inclusion. Eight hundred and fifty-two patients were excluded due to inability to meet one or more inclusion criteria. The majority of patients were excluded due to vancomycin being administered for less than five days. The second most common reason for exclusion was incomplete data in the electronic medical record. Therefore, 110 patients were included in the analysis. The majority of patients were male. The mean birth weight was 1200 g, and the mean gestational age was 29 weeks. (Table 1) Central line associated blood stream infection (CLABSI), sepsis and necrotizing enterocolitis (NEC) were the most common suspected diagnoses for which patients were started on vancomycin therapy. The most common organism was Coagulase Negative Staphylococcus aureus (32 patients) followed by Enterococcus faecalis (5 patients) and Staphylococcus aureus.
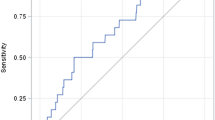
One hundred ten patients were further studied to determine an association with AKI. There were 72 patients with a highest vancomycin trough concentration less than 10 mg/L, 27 patients with a highest vancomycin trough concentration of 10–15 mg/L, and 11 patients with a highest vancomycin trough concentration greater than 15 mg/L. The incidence of AKI was 1.39% (1/72 patients) in the group achieving vancomycin trough concentrations less than 10 mg/L, 0% (0/27 patients) in the group achieving vancomycin trough concentrations between 10–15 mg/L, and 18.18% (2/11 patients) in the group achieving vancomycin trough concentrations greater than 15 mg/L (Table 2). There was a statistically significant association between AKI and vancomycin trough groups (p = 0.04). Regression analysis between increasing vancomycin trough concentrations and post vancomycin serum creatinine values demonstrated a positive correlation value of 0.32 (p < 0.05) (Fig.1).
Each of the patients with AKI were also receiving at least one concurrent nephrotoxic medication including dobutamine, dopamine, and/or gentamicin (Table 3). Gentamicin was the only nephrotoxic drug that a large percentage of the 87 patients received during vancomycin therapy (79%). There was no significant correlation between post vancomycin creatinine values and the total number of days’ gentamicin was received (p = 0.10) or of a change in creatinine of greater than or equal to 0.5 mg/dl from pre- to post-vancomycin administration (p = 0.13). In an ANOVA regression equation with gentamicin days run as a covariate with vancomycin trough group, however, gentamicin days were significantly associated with post vancomycin creatinine values (p = 0.04). The vancomycin trough groups remained significantly associated with post vancomycin creatinine values independent of gentamicin days (p < 0.01).
Discussion
There has been an on-going debate on the dosage, frequency of administration and the most appropriate monitoring of vancomycin therapy. This is the result of multiple factors responsible for clearance of vancomycin including gestational age, post-natal age, weight, renal tubular function and creatinine levels. [9] Historically, area-under-the-curve concentration versus time to the minimum inhibitory concentration (AUC:MIC), was accepted as the pharmacokinetic and pharmacodynamic predictor of adequate treatment with vancomycin. AUC: MIC > 400 has been recommended to achieve desired anti-microbial effect in both adult and pediatric populations. [10] A consensus statement released by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists in 2009 recommended vancomycin as the first choice drug for MRSA with MIC < 2. [10] The consensus statement also recommends assessment of vancomycin clinical effectiveness through serum vancomycin trough concentrations at steady state. Studies have demonstrated that trough between 7–10 mg/L corresponds to AUC:MIC > 400 if the MIC of MRSA is < 1. [11] However, with increasing MIC’s higher trough concentrations are recommended. In serious clinical infections such as endocarditis, meningitis, hospital-acquired pneumonia, bacteremia, and osteomyelitis trough concentrations between 15–20 are recommended.[12] These higher troughs allow for greater vancomycin exposure, a higher AUC and the ability to reach the goal AUC:MIC ≥ 400, or a trough concentration approximately four to five times the MIC of the infecting organism. [12] Concerns for increased nephrotoxicity, an adverse effect traditionally associated with vancomycin, has accompanied the recommendation for more aggressive dosing.
In adults, increasing serum vancomycin trough concentrations have been associated with increasing incidence of AKI. In a study done to evaluate vancomycin-associated nephrotoxicity incidence in adults with MRSA infections with serum trough concentrations of 15–20 mg/L and receiving concomitant nephrotoxic medications, a significantly higher incidence of AKI was noted.[3] A 2011 prospective study also showed similar results. Adults with MRSA infections had a greater risk of AKI with serum vancomycin trough concentration greater than 15 mg/L.[13] Similarly, adults with MRSA pneumonia were shown to be at a 3–5 times greater risk for developing AKI with vancomycin serum trough concentrations of 15 mg/L.[14] On the other hand, there are other studies in adults which did not show increased risk of AKI with elevated serum vancomycin trough > 15 mg/L. In study done by Prabaker et al., an overall incidence of 2.1% was noticed with vancomycin serum trough concentration between 15–20 mg/L [15].
Nephrotoxicity in the neonatal population is poorly defined. Several definitions of acute kidney injury (AKI) exist in the literature; however, there are no standard criteria for diagnosing AKI in neonates. Commonly used definitions of AKI in neonates include oliguria of less than 1 mL/kg/h of urine output that develops 24 h after birth and persists for at least 24 h, an increase in serum creatinine (SCr) to greater than 1.5 mg/L 72 h after birth, or an increase in SCr between 0.5 and 1 mg/L per day.[8, 16] Nephrotoxicity attributed to vancomycin use is not clearly defined in neonates; however, the mechanism of injury to the kidney is believed to be the result of proximal tubule damage. [17, 18] Although causality has not been proven, a higher risk of vancomycin-induced nephrotoxicity in the pediatric population with higher vancomycin trough concentrations has been speculated.
Due to pharmacokinetic differences between adults and neonates, and since neonates have immature renal function compared to adults, the applicability and adverse effects of increased target troughs in premature neonates remain unknown. Lastly, it has been documented in adult populations that concurrent treatment using other known nephrotoxic agents, such as aminoglycosides, is associated with an increased risk for nephrotoxicity with vancomycin. [19] Again, it is unclear whether this is true in the neonatal population.
Previously done studies in children receiving vancomycin alone have reported incidence of AKI from 9%–14%. Linder et al. and Nahata et al. found the incidence of AKI in neonatal population to be low. Their studies did not show statistically significant difference in the incidence of AKI with concomitant administration of gentamicin.[20, 21] However, McKamy and Knoderer et al. reported slightly higher overall incidence of AKI at 14% in children between the ages 1 month and 17 years.[17, 22] Both the studies also reported significantly higher incidence of AKI with vancomycin trough > 15 mg/L at 28% and 18% respectively.
In our study, the overall incidence of AKI was low at 2.71%. A statistically significant association between AKI and vancomycin trough groups was found (p = 0.04). There was also a positive correlation between vancomycin trough concentrations and post vancomycin creatinine values, indicating that vancomycin may have some role in AKI in predisposed individuals. It also indicates that higher vancomycin troughs are associated with rising creatinine values post treatment. Additionally, we looked at several covariates using a linear regression model to assess whether these were significantly associated with higher post vancomycin creatinine values. Highest vancomycin trough value (p < 0.001), total vancomycin days (p ~ 0.0021) and gestational age (p value < 0.001) were found to have an independent association. Gender and APGAR scores were also looked at and were not found to be significantly associated with vancomycin trough and rising creatinine values. Even after controlling for these independent variables, the highest trough value and post vancomycin creatinine values showed a significant association.
Also of interest is the observation that vancomycin trough groups continued to be positively associated with increasing post vancomycin creatinine values independent of the number of days of gentamicin the infants had received. In addition, independent of vancomycin trough groups there was a positive association between the number of days that gentamicin was received and post vancomycin creatinine values. Thus, gentamicin, another potentially nephrotoxic had its effect on post vancomycin creatinine values obscured by the effect of vancomycin. Only after having the effect of vancomycin controlled for in the regression equation was gentamicin’s effect revealed. Because of the small number of courses associated with our definition of acute kidney failure whether or not vancomycin and gentamicin were additive in their nephrotoxic effects cannot be determined in this analysis. Larger studies are needed to confirm these findings.
Our study has limitations. The study was retrospective in nature and had a small sample size. Due to the lack of a standardized definition a hybrid definition of AKI was used in this study. Another limitation is that patients may have received multiple courses of vancomycin therapy during the same admission. However, courses of vancomycin therapy were often separated by weeks. Further, for each course of vancomycin therapy, multiple vancomycin trough concentrations may have been measured, but the highest achieved trough concentration was used to divide the vancomycin courses into the three study groups. Finally, despite the fact that patients were often on concurrent nephrotoxic medications, only three cases of AKI were identified in this study which is different from the previously reported studies.
Conclusion
Based on our study, vancomycin troughs greater than 20 mg/dl may be associated with increased incidence of AKI. We recommend close monitoring of vancomycin trough concentrations during therapy and appropriate alteration in dosage/frequency should be made based on serum trough concentrations. However, this was a single-center retrospective study, and larger prospective studies are required to validate this finding.
Abbreviations
- AKI:
-
Acute kidney injury
- AUC: MIC:
-
Area-under-the-curve concentration versus time to the minimum inhibitory concentration
- CONS:
-
Coagulase negative staphylococcus aureus
- MRSA:
-
Methicillin resistant staphylococcus aureus
- NICU:
-
Neonatal intensive care unit
- SCr:
-
Serum creatinine
- UTMB:
-
University of Texas Medical Branch
References
Klingenberg C, Aarag E, Ronnestad A, Sollid JE, Abrahamsen TG, Kjeldsen G, Flaegstad T. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. 2005;24(9):817–22.
Healy CM, Palazzi DL, Edwards MS, Campbell JR, Baker CJ. Features of invasive staphylococcal disease in neonates. Pediatrics. 2004;114(4):953–61.
Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–44.
Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering Jr RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42(6):2398–402.
Capparelli EV, Lane JR, Romanowski GL, McFeely EJ, Murray W, Sousa P, Kildoo C, Connor JD. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J Clin Pharmacol. 2001;41(9):927–34.
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–35.
Moghal NE, Brocklebank JT, Meadow SR. A review of acute renal failure in children: incidence, etiology and outcome. Clin Nephrol. 1998;49(2):91–5.
Viswanathan S, Manyam B, Azhibekov T, Mhanna MJ. Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr Nephrol. 2012;27(2):303–11.
Pacifici GM, Allegaert K. Clinical pharmacokinetics of vancomycin in the neonate: a review. In: Clinics (Sao Paulo). 67th ed. 2012. p. 831–7.
Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering Jr RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29(11):1275–9.
Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant Staphylococcal infections. Pediatr Infect Dis J. 2013;32(10):1077–9.
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55.
Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, Mauldin PD. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother. 2011;55(12):5475–9.
Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107–15.
Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91–7.
Ringer SA. Acute Renal Failure in the Neonate. 2010.
McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158(3):422–6.
Zappitelli M, Selewski DT, Askenazi DJ. Nephrotoxic Medication Exposure and Acute Kidney Injury in Neonates. 2012.
Fauconneau B, Favreliere S, Pariat C, Genevrier A, Courtois P, Piriou A, Bouquet S. Nephrotoxicity of gentamicin and vancomycin given alone and in combination as determined by enzymuria and cortical antibiotic levels in rats. Ren Fail. 1997;19(1):15–22.
Linder N, Edwards R, MeClead R, Mortensen ME, Walson P, Koren G. Safety of vancomycin with or without gentamicin in neonates. Neonatal Netw. 1993;12(8):27–30.
Nahata MC. Lack of nephrotoxicity in pediatric patients receiving concurrent vancomycin and aminoglycoside therapy. Chemotherapy. 1987;33(4):302–4.
Knoderer CA, Nichols KR, Lyon KC, Veverka MM, Wilson AC. Are Elevated Vancomycin Serum Trough Concentrations Achieved Within the First 7 Days of Therapy Associated With Acute Kidney Injury in Children? J Pediatric Infect Dis Soc. 2014;3(2):127–31.
Acknowledgements
Not applicable
Funding
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files. A separate file containing all the raw data is also provided along with the manuscript (Additional file 1). Permission was obtained from IRB to access electronic medical records and conduct a chart review.
Authors’ contributions
VB was involved in writing the manuscript and collection of data. MM was involved in data interpretation and statistical analysis of the data. He was also involved in critical review of the article and multiple corrections prior to submission. RF is the chief investigator in the study. He was involved in designing the study, reviewing the results and reviewing the manuscript and the whole project at each stage. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the ethics committee of Institutional Review Board (IRB) at UTMB. Consent required to participate in the study was waived by the IRB at UTMB.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
The file contains raw data used for analysis during the study. This includes patient gender, prenatal history, birth history, vancomycin start and end dates for each course of vancomycin administered. It also contains information regarding vancomycin trough and serum creatinine values obtained during each vancomycin course. (XLSX 55 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bhargava, V., Malloy, M. & Fonseca, R. The association between vancomycin trough concentrations and acute kidney injury in the neonatal intensive care unit. BMC Pediatr 17, 50 (2017). https://doi.org/10.1186/s12887-017-0777-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-017-0777-0