Abstract
Background
Fragile X syndrome is the most common genetic disorder of intellectual developmental disorder/mental retardation (IDD/MR). The prevalence of FXS in a Chinese IDD children seeking diagnosis/treatment in mainland China is unknown.
Methods
Patients with unknown moderate to severe IDD were recruited from two children’s hospitals. Informed consent was obtained from the children's parents. The size of the CGG repeat was identified using a commercial TP-PCR assay. The influence of AGG interruptions on the CGG expansion during maternal transmission was analyzed in 24 mother-son pairs (10 pairs with 1 AGG and 14 pairs with 2 AGGs).
Results
553 unrelated patients between six months and eighteen years of age were recruited. Specimens from 540 patients (male:female = 5.2:1) produced high-quality TP-PCR data, resulting in the determination of the FMR1 CGG repeat number for each. The most common repeat numbers were 29 and 30, and the most frequent interruption pattern was 2 or 3 AGGs. Five full mutations were identified (1 familial and 4 sporadic IDD patients), and size mosaicism was apparent in 4 of these FXS patients (4/5 = 80 %). The overall yield of FXS in the IDD cohort was 0.93 % (5/540). Neither the mean size of CGG expansion (0.20 vs. 0.79, p > 0.05) nor the frequency of CGG expansion (2/10 vs. 9/14, p > 0.05) was significantly different between the 1 and 2 AGG groups following maternal transmission.
Conclusions
The FMR1 TP-PCR assay generates reliable and sensitive results across a large number of patient specimens, and is suitable for clinical genetic diagnosis. Using this assay, the prevalence of FXS was 0.93 % in Chinese children with unknown IDD.
Similar content being viewed by others
Background
Intellectual developmental disorder/mental retardation (IDD/MR) encompasses a cluster of symptoms that are characterized by low intelligence and limitations in adaptive behavior and functional capabilities [1, 2]. Generally, IDD occurs in approximately 1-3 % of individuals worldwide, with an incidence of 1 % in high income countries and 2 % in low/middle income countries [3, 4].
Genetic/genomic factors are a major risk factor for IDD, accounting for 85 % of patients with IDD [5]. Among them, fragile X syndrome (FXS, MIM 309550) is the most common form of IDD. The prevalence of FXS is estimated to be 1/4,000 in males and 1/5,000-8000 in females [6, 7]. FXS accounts for approximately 20 % of patients with X-linked IDD [8] and 2–7 % of children with autism [9, 10]. However, the classic facial features of FXS (prominent forehead, a long narrow face, protruding ears, and macroorchidism) are ambiguous until juvenile development, and neurophysical symptoms are also subtle in young children [11]. The lack of a clear phenotype in young children can delay a definitive diagnosis, leading to a diagnostic “odyssey” for families and a delay in the implementation of specific therapies. Indeed, it has been reported that in the USA up to 40 % of families with FXS girls and 25 % with FXS boys have had another child before their first affected child was diagnosed [12]. Additionally, only a 40 % penetrance for mental impairment is reported in affected females [13, 14], making a clear clinical diagnosis even more difficult in girls. Consequently, identifying children, particularly infants and toddlers with FXS, is critically dependent on molecular genetics testing.
FXS is typically caused by an expansion of CGG trinucleotides repeats in the 5′ untranslated region of the FMR1 gene. The most common size of CGG repeats among the general population is 29 and 30 copies [15]. In FXS patients, the CGG number expands to greater than 200 repeats, resulting epigenetic silencing of the FMR1 gene and the absence of the encoded protein, fragile X mental retardation protein (FMRP) [15]. FXS molecular diagnostic tests include region-specific CGG PCR amplification and Southern blot (SB) analysis. Recently, triplet repeat-primed (TP)-PCR methods have been described, which simplify FMR1 genotyping and can detect both full mutation expansions and low-level size mosaicism with high sensitivity [16, 17].
The prevalence of FXS in a Chinese IDD population has been previously reported [18, 19] However, the rate of FXS in IDD children seeking diagnosis/treatment in mainland China is unknown. The availability of such information is expected to help enhance awareness among neurologists in suspicious populations and improve options for intervention and treatment. Herein, we utilized commercial FMR1 TP-PCR reagents to identify the prevalence of FXS in Chinese children with unknown IDD. In addition, the AGG interruption pattern was analyzed in 24 mother-son pairs to investigate the relationship between the AGG structure and characteristics of CGG expansion from mother to child.
Methods
Sample recruitment
Patients with unknown moderate to severe IDD (IQ < 55) were recruited from two children hospitals, namely the affiliated Children's Hospital of Capital Institute of Pediatrics and the Peking University First Hospital. The severity of IDD was scored by the Wechsler Intelligence Scale for Children (WISC) [20] or the Gesell Developmental Schedules [21]. DSM-IV criteria were used to indicate a diagnosis of ASD [22]. The recruited patients met at least one of the following requirements:
-
Male patient with unknown moderate to severe IDD
-
In addition to IDD, the presence of other familial medical problems in the patient’s three-generation pedigree, such as tremor, ataxia, or premature ovarian insufficiency
-
Evidence of familial IDD. In addition to the proband, the presence of at least another person with IDD or other neurodevelopmental disorders in the three-generation pedigree, including ASD, developmental delay (e.g., delayed milestones for sitting, walking, or talking), social or behavior problems, learning difficulty or language delay, or ADHD
-
Suggested facial dysmorphism, such as a long face, prominent nose and jaw, big ears, thick lip, or other distinctive physical features such as enlarged testicles
Urine screening (GC-MS) was performed on enrolled patients to exclude IDD-related metabolic diseases. Any acquired IDD was also excluded. Of note, this Chinese IDD cohort was previously profiled using aCGH/multiplex ligation-dependent probe amplification (MLPA) and some targeted sequencing [23–26]. Consequently, patients with any IDD-related genomic copy number variants or genomic mutation including subtelomeric aberrations, 16p11.2 microdeletion, 15p11-13 microdeletion or 22q11 microdeletion, or SHANK3 deletion were excluded. Female patients with Rett Syndrome were also excluded.
Informed consent was obtained from the children's parents in accordance with the publication of any associated clinical information and images. This study was approved by the Capital Institute of Pediatrics and the Peking University First Hospital Review Board.
DNA extraction and sex identification
DNA was isolated from peripheral blood using the Blood and Tissue kit (Qiagen, Valencia, CA,USA) and quantified with a NanoDrop spectrophotometer (Thermo Scientific, Erembodegem-Aalst, Belgium). The sex of each sample was confirmed after evaluation using a sex-specific PCR assay that targeted the AMEL and SRY alleles (Additional file 1: Table S1).
FMR1 Region-specific CGG PCR
Primers covering the FMR1 promoter region (Additional file 1: Table S1) were designed to amplify the CGG repeat segment. A modified protocol containing PCR enhancer solution (Life Technology, Grand Island, NY, USA) was used to amplify this GC-rich region. The PCR products were purified (Exonuclease I, New England Biolabs, Ipswich, MA, USA) and sequenced using the standard protocol (BigDye, Applied Biosystems, Foster City, CA, USA). Raw sequences were visualized by Mutation Surveyor V3.30 (SoftGenetics, State College, PA, USA) and blasted to the human reference sequence (http://genome.ucsc.edu/, hg19) to determine the CGG repeat number.
FMR1 triplet repeat-primed (TP)–PCR
The diluted DNA sample (40 ng/ul) was amplified using AmplideX® FMR1 PCR reagents (Asuragen, Austin, TX, USA). The PCR product was stored at −20 °C and protected from light before fragment sizing. A 3730xl Genetic Analyzer (Applied Biosystems, Vernon Hills, Illinois, USA) running POP-7 polymer on a 36 cm capillary was used to analyze the amplicon size. A total of 2 ul of unpurified PCR product was mixed with 11 ul Hi-Di Formamide and 2 ul ROX 1000 Size Ladder prior to injection. The raw sequence data was uploaded to GeneMapper 4.0 software (Applied Biosystems, Vernon Hills, Illinois, USA). The size of the PCR product was converted to the repeat number using an MS Excel-based data analysis macro. A mixed internal standard DNA sample was tested in the same plate for each experiment to provide a process control.
Samples were categorized by repeat size as follows. 13–44 CGG repeats were indicated as normal, 45–54 repeats as intermediate, and 55–200 CGG repeats as premutation. Greater than 200 CGG repeats was flagged as full mutation [6]. Six male samples with known CGG repeats (13, 29, 31, 32, 43), and two samples with premutation or full mutation (a gift from Prof. Kun Xia, Central South University, Changsha, China) were assessed as independently genotyped controls. These controls were analyzed by both the FMR1 region-specific CGG PCR and TP-PCR.
AGG interruption status, and assessments of maternal transmission to the next generation
Maternal transmissions of CGG repeat alleles were determined in 24 mother-son pairs with different AGG structures. The AGG interruption pattern was deduced from the electropherogram pattern as previously described [16], using both parent and child data to reconcile any ambiguities in interpretation. Any difference in the overall CGG repeat (i.e., >0 repeat) between mother and son indicated CGG expansion following maternal transmission.
Results
FMR1 Region-specific CGG PCR and TP–PCR produce concordant CGG sizing results that are in agreement with known genotypes
We performed both the Region-specific CGG PCR and TP–PCR assay for seven samples with known CGG sizes. For samples with CGG repeats in the normal size range (13–43 CGG), single well-defined amplification bands were observed on a 2 % agarose gel (Fig. 1a). The sequencing data from region-specific CGG PCR was compared with the predicted sizing from TP–PCR; a deviation of none or one CGG repeat was observed in each case, indicating quantitative consistency in repeat sizing between the two assays. Although the region-specific CGG PCR failed to amplify the sample with >60 repeats (a female premutation, Fig. 1a), the TP–PCR accurately sized this sample (CGG = 31, 69 and 91, Fig. 1b). For the sample with an FMR1 full mutation, only a very faint amplicon band was observed from the region-specific CGG PCR (white arrow in Fig. 1a). However, the TP–PCR reported a full mutation as well as indications of size mosaicism (CGG = 30 and >200, Fig. 1b). Of note, the size mosaicism detected by TP-PCR was not evident by SB analysis. This is not surprising given the superior sensitivity of TP-PCR compared to SB analysis [16].
FMR1 Region-specific CGG PCR and TP-PCR for seven samples with known CGG repeat lengths. a The CGG repeat size of seven samples with known genotype were analyzed on agarose gel after FMR1 region-specific CGG PCR. CGG sizes as determined by the TP-PCR assay or SB analysis is shown at the top of the image. For the premutation (PM) female sample, the gel image reveals the normal FMR1 allele on another chromosome. For the male sample with a full mutation (FM), the weak band (white arrow) indicates size mosaicism. M: DNA maker; NC: negative control, no DNA for PCR reaction. PCR amplicons from samples with a premutation (b) and full mutation (c) were also analyzed by capillary electrophoresis following the FMR1 TP-PCR assay. TP–PCR confirmed size mosaicism in the PM sample (69 and 91 repeats) and the FM sample (30, >200 repeats). This mosaicism was undetected by SB analysis. The black arrow indicates the normal allele, and red arrow indicates the PM allele. The blue arrow indicates the FM allele. The predicted size of the CGG repeats from TP-PCR is labeled
The yield of FMR1 full mutation in Chinese children with unknown IDD
Samples from 553 unrelated children (male:female = 5.2:1) with unexplained IDD were analyzed using the FMR1 TP–PCR assay. Samples from 13 children were excluded from data analysis due to unsatisfactory electropherogram traces, leaving 540 patient samples for genotyping. The ratio of isolated IDD and non-isolated IDD was 2.46 (384:156, Table 1); ASD and learning difficulties were common comorbid phenotypes (28.9 %). The normal CGG range was 13–45 repeats, and 29 and 30 repeats represented the most common alleles (62.6 %), consistent with previous Chinese reports [18, 27–29]. Five full mutations were identified from male cases (one case with familial IDD, four cases with sporadic IDD). As a result, the diagnostic yield of FMR1 full mutation in unknown IDD children was 0.93 % (5/540).
FXS size mosaicism and phenotypic heterogeneity
Size mosaicism was detected in 4 of 5 FXS patients (80 %), suggesting that CGG size mosaicism is a common phenomenon in FXS patients. Further, intellectual phenotypes were distinct among the 5 FXS individuals. Two patients showed moderate IDD, and whereas 3 demonstrated mild IDD. Three patients also presented other cognitive impairments (eg, learning difficulty/language delay, social dysfunctions). For the sporadic IDD case, facial features characteristic of FXS were not apparent, but they did have large testes.
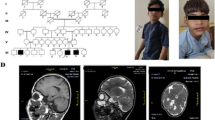
FXS size mosaicism and phenotypic heterogeneity was particularly prominent in one of the families with a history of IDD (Fig. 2a). The proband in this case study is a sixteen-year-old boy (III:1) with moderate IDD and learning difficulties. His mother (II:9) and younger sister (III:4) showed isolated IDD and his maternal uncles (II:8, II:12) manifested social and cognitive deficits. Of note, one uncle (II:8) presented severe social and cognitive dysfunctions. The predominant facial characteristics of FXS (large nose, big ears and thick lip, and large testes) were seen only in the proband and his uncle (II:8). An FMR1 full mutation was detected in the proband, his mother, his younger sister and two maternal uncles. Size mosaicism was identified in family members carrying premutation or full mutation.
The genotypes and phenotypes for a familial FXS. a The pedigree of a classic familial FXS patient (squares indicate males and circles indicate females). IDD-affected individuals are presented as black symbols while normal individuals are presented as white symbols. The proband is indicated with an arrow. The facial features of classic FXS, including long face, predominant nose and large jaw, is seen in the proband (III:1) and his uncle (II:8). b Size mosaicism in affected IDD individuals (II:9 and III:1) identified by TP-PCR. The size of CGG repeats is labeled underneath the peak
The maternal expansion of the CGG repeat was further analyzed in II:9 and III:1 (Fig. 2b). The proband’s mother carries one normal FMR1 allele (CGG = 30, high blue peak), and three mosaic sizes on her affected allele (CGG = 32, black arrow; CGG = 150, red arrow; full mutation, blue arrow). Meanwhile, the proband presented at least two mosaic sizes (CGG = 32, and full mutation). We surmise that the full mutation in the proband was inherited and expanded from the maternal premutation. Alternatively, the mother’s full mutation may be inherited and continued to expand. Any assessment of further expansion, however, would require slab gel analysis of the PCR products using a 2-primer configuration of the AmplideX FMR1 PCR reagents since these full-mutation amplicons are too large for resolution by CE using the POP-7 polymer.
Maternal transmissions of CGG repeat in sample with reduced AGG interruption
AGG sequence interruptions in the CGG repeat segment of the FMR1 promotor region are known to reduce the risk of repeat expansion, possibly by stabilizing strand slippage during DNA replication [30]. For this IDD child cohort, only 21 non-FXS patients had less than two AGG interruptions (21/540 = 3.9 %). This value is similar to previous reports in Chinese populations [29, 31]. Following clinical review and maternal consent, 24 mother-son DNA pairs were available to explore the relationship between AGG interruption and maternal CGG expansion. We subgrouped these 24 samples by AGG interruption status (ten pairs with AGG = 1, fourteen pairs with AGG = 2; see Table 2). Following statistical analysis, the mean maternal CGG repeat was not significantly different between the two groups (29 vs. 30.2, p > 0.05). During the maternal transmission, neither the mean size of CGG expansion (0.20 vs. 0.79, p > 0.05) nor the frequency of CGG expansion (2/10 vs. 9/14, p > 0.05) was significantly different in two subgroups. Consequently, our results indicate that there is no difference between 1 and 2 AGGs with respect to repeat length in the next generation for mothers with normal FMR1 CGG sizes. This result is consistent with previous findings [32].
Discussion
FMR1 TP-PCR is a rapid and accurate method to diagnose FXS in IDD children
Historically, SB analysis has been the standard approach in many clinical laboratories for the molecular diagnosis of FXS patients. However, this process is both time- and labor-intensive (with a typical turn-around time of 2 weeks), requires a large input of DNA, and offers poor resolution to size CGG repeat [16, 33]. Furthermore, SB has a relatively poor analytical sensitivity to detect expansions in samples with low-level size mosaicism [34]. For FMR1 region-specific CGG PCR, the high GC content of the 5′ untranslated segment is refractory to standard PCR amplification, and usually only <50-100 repeats can be reliably amplified and detected. This inability to reliably identify mid- to large-size expanded alleles can produce false negatives, particularly for female samples where the more likely interpretation of a single amplicon product after electrophoresis is that the FMR1 allele is homozygous. A further complication is that full-mutation size mosaicism, seen in 4 of 5 FXS patients in our study, can present an additional product peak after PCR that can confound interpretation. Consequently, a more convenient, sensitive, high resolution and accurate assay with a shorter turn-around time is needed to improve the molecular diagnosis of patients that are suspicious for FXS or premutation phenotypes.
The sensitivity and uniformity of the FMR1 TP-PCR has been previously reported [35]. Our study provides further proof that this single-tube PCR amplification can accurately size CGG repeats from low-input DNA. The total turn-around time from DNA dilution to report acquisition was 48 h, which is considerably less than SB analysis. Moreover, the TP–PCR assay is sensitive enough to detect low-level size mosaicism. We conclude that this FMR1 TP-PCR is appropriate for FXS molecular diagnosis.
The prevalence of FXS in Chinese children with unknown IDD
The prevalence of FXS in IDD populations is diverse because both the test method and recruited target populations are diverse. Recently, Peprah reviewed approximately 45 publications that addressed the FXS prevalence in IDD populations [15]. The results revealed a 0.5-9.7 % diagnostic yield of FXS, with Canadian, Estonian, Japanese, and Taiwanese groups having the lowest prevalence of FXS. In addition, countries that don’t routinely perform FMR1 molecular testing appear to have a significantly lower prevalence than western countries that do [15]. In this study, we determined that the prevalence of FXS in Chinese IDD children is 0.93 % overall, and 1.1 % in male patients and 0 % in female patients. This FXS prevalence is lower than that reported from studies in western counties. A study of 119,232 samples in one large US reference laboratory revealed that the rate of FMR1 full mutation was 1.3 %, with 1.4 % for males and 0.61 % for females [36]. Another study comprised of 1755 children with non-specific mental retardation reported that the overall yield of FXS was about 3.5 % in a Greek MR cohort [37]. Our study confirmed ethnic differences in FXS prevalence.
We identified a high prevalence of size mosaicism in Chinese FXS boys (80 %). Size mosaicism was reported to be common in FXS patient [38–40]. Such mosaicism can arise de novo or be passed on by phenotypically-normal mosaic parents [41, 42]. Recently, an FMR1 mosaic deletion was reported in a Chinese FXS boy, which was initially absent in his phenotypically-normal mother’s blood. However, an in-depth study of his mother’s FMR1 profile using qPCR and breakpoint mapping-PCR confirmed low-level mosaicism in different maternal tissues (eg, blood, skin, eyebrow, urine sediment and menstrual discharge) [40]. The maternal inheritance pattern of FMR1 size mosaicism was also analyzed in the familial FXS pedigree. Evidence of size mosaicism in the proband, his mother, his sister and two uncles suggested that his FMR1 size mosaicism was inherited from his grandfather.
Factors affecting the FXS prevalence in Chinese patient populations
The FXS yield from the patients in this study is also different from that reported in previous Chinese IDD/MR populations [18, 19]. Zhong et al. performed multi-institutional screening of 1127 adult/child individuals with mild-moderate IDD, and found that 2.8 % of the Chinese IDD patients carry a full mutation. In this work, both PCR and SB was performed to exclude full mutation and abnormal methylation [18]. In contrast, only a 0.6 % FXS yield was reported from 324 Hong Kong patients with mild mental retardation [19]. For this cohort, electrophoresis of the fragment from the region-specific CGG PCR followed by hybridization using a CGG probe was analyzed to screen FXS. We suspect that both the screening method used and the severity of IDD can affect the determination of FXS prevalence. Further, previous studies have confirmed that FXS appears to be more prevalent among patients with mild MR than severe MR [15, 43]. In this study, severe and moderate IDD accounted for 50 % of recruited patients, and all FXS patients presented moderate IDD.
Both clinical knowledge and complex comorbidities for FXS can also affect the assessment of FXS yield. In China, it is the pediatric neurologist or physician, rather than the clinical geneticist, that examines patients referred for a suspected genetic disorder. However, most Chinese pediatricians, with the exception of some pediatric specialists, are uninformed about FXS. FXS presents a challenging clinical diagnosis based on the fact that the classic facial features of FXS are ambiguous until juvenile development, and that the cognitive presentation of FXS can be subtle in young children [11]. Recently, Li et al. performed a study surveying the FXS knowledge and attitudes of Chinese medical college students [44]. He found that less than one-third of the participants were aware of FXS. This investigation highlights both the challenges and opportunities of genetic education in China. Additionally, many neuro-developmental disorders can occur as major symptoms or comorbid phenotypes in FXS patients. Since a detailed score checklist for FXS testing from GeneReviewers (http://www.ncbi.nlm.nih.gov/books/NBK1384/) was not provided, it would be difficult for pediatrician to distinguish FXS from other possible neuro-developmental disorders. Also, other genomic common copy number variants or rare single nucleotide variants can contribute to variable neuro-developmental traits in FXS [7, 45]. In the future, further background on FXS should be provided to the clinicians. These actions may increase the yield of FXS, perhaps up to 4 % as recently reported [46].
The role of AGG interruption on maternal FMR1 CGG expansion
Generally, the normal CGG repeat harbors 2 or 3 AGGs, whereas premutations and full mutations present 0 or 1 AGG [47]. The number of AGGs in the 5’ region was correlated with repeat instability during maternal transmission [32, 47, 48]. Recent studies have demonstrated that women with greater than 54 repeats and no AGG have an elevated risk for expansion to a full mutation in the next generation [32]; However, maternal alleles with <45 repeats rarely expanded, even when they had no or only 1 AGG interruption [32]. In this study, 24 mothers had CGG repeats of <54, and thus our finding that none of these alleles significantly expanded after transmission is consistent with previous reports.
Limitations of FMR1 TP-PCR for FXS molecular diagnosis
Over 98 % of FXS is caused by CGG expansions. The remaining cases are attributed to deletions or point variants in the FMR1 region [49–54]. The ACMG recommends that FMR1 copy number variants and sequencing of FMR1 coding regions be performed to exclude possible FMR1 deletions or point mutations [6] for suspicious cases without repeat expansions. In addition, the abnormal methylation status of the FMR1 may be associated with FXS [36]. Both deletion/point variants and methylation status of FMR1 may evade detection by TP-PCR, but methylation analysis can be performed using a separate PCR-based method [34]. Consequently, the above testing is necessary for patients with negative TP-PCR results that are highly suspect for FXS.
Conclusions
In summary, the FMR1 TP-PCR assay can accurately and sensitively quantify and classify CGG repeats with a rapid turn-around time. Using this methodology, we established an incidence of 0.93 % FXS in a Chinese children with unknown IDD, and found that size mosaicism was common (80 %) in the 5 patients fragile X full mutations.
References
Reed G. Toward ICD-11: improving the clinical utility of WHO’s International Classification of mental disorders. Professional Psychology: Research and Practice. 2010;41:457–64.
Salvador-Carulla L, Reed GM, Vaez-Azizi LM, Cooper SA, Martinez-Leal R, Bertelli M, et al. Intellectual developmental disorders: towards a new name, definition and framework for "mental retardation/intellectual disability" in ICD-11. World Psychiatry. 2011;10(3):175–80.
Durkin M. The epidemiology of developmental disabilities in low-income countries. Ment Retard Dev Disabil Res Rev. 2002;8(3):206–11.
Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. 2011;32(2):419–36.
Curry CJ, Stevenson RE, Aughton D, Byrne J, Carey JC, Cassidy S, et al. Evaluation of mental retardation: recommendations of a Consensus Conference: American College of Medical Genetics. Am J Med Genet. 1997;72(4):468–77.
Monaghan KG, Lyon E, Spector EB. ACMG Standards and Guidelines for fragile X testing: a revision to the disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics. Genet Med. 2013;15(7):575–86.
Rousseau F, Labelle Y, Bussieres J, Lindsay C. The fragile x mental retardation syndrome 20 years after the FMR1 gene discovery: an expanding universe of knowledge. Clin Biochem Rev. 2011;32(3):135–62.
Rousseau F, Heitz D, Biancalana V, Blumenfeld S, Kretz C, Boue J, et al. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991;325(24):1673–81.
Reddy KS. Cytogenetic abnormalities and fragile-X syndrome in Autism Spectrum Disorder. BMC Med Genet. 2005;6:3.
Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A. 2004;129A(3):225–34.
Hagerman RJ: Physical and behavioral phenotype. In Hagerman RJ & Hagerman PJ (Eds), Fragile X Syndrome: Diagnosis, Treatment, and Research. 2Ed. Baltimore: The Johns Hopkins University Press 2002;3–109.
Bailey Jr DB, Raspa M, Bishop E, Holiday D. No change in the age of diagnosis for fragile x syndrome: findings from a national parent survey. Pediatrics. 2009;124(2):527–33.
Loesch DZ, Hay DA. Clinical features and reproductive patterns in fragile X female heterozygotes. J Med Genet. 1988;25(6):407–14.
Hull C, Hagerman RJ. A study of the physical, behavioral, and medical phenotype, including anthropometric measures, of females with fragile X syndrome. Am J Dis Child. 1993;147(11):1236–41.
Peprah E. Fragile X syndrome: the FMR1 CGG repeat distribution among world populations. Ann Hum Genet. 2012;76(2):178–91.
Chen L, Hadd A, Sah S, Filipovic-Sadic S, Krosting J, Sekinger E, et al. An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. J Mol Diagn. 2010;12(5):589–600.
Lyon E, Laver T, Yu P, Jama M, Young K, Zoccoli M, et al. A simple, high-throughput assay for Fragile X expanded alleles using triple repeat primed PCR and capillary electrophoresis. J Mol Diagn. 2010;12(4):505–11.
Zhong N, Ju W, Xu W, Ye L, Shen Y, Wu G, et al. Frequency of the fragile X syndrome in Chinese mentally retarded populations is similar to that in Caucasians. Am J Med Genet. 1999;84(3):191–4.
Pang CP, Poon PM, Chen QL, Lai KY, Yin CH, Zhao Z, et al. Trinucleotide CGG repeat in the FMR1 gene in Chinese mentally retarded patients. Am J Med Genet. 1999;84(3):179–83.
Wechsler D. Wechsler Intelligence Scale for Children. Psychological Corporation. 3rd ed. TX: San Antonio; 1991.
Ball RS. The Gesell Developmental Schedules: Arnold Gesell (1880–1961). J Abnorm Child Psychol. 1977;5(3):233–9.
Association A. Diagnostic and Statistical Manual of Mental Disorder. Washington, DC: American Psychiatric Association; 1994.
Shen Y, Chen X, Wang L, Guo J, Shen J, An Y, et al. Intra-family phenotypic heterogeneity of 16p11.2 deletion carriers in a three-generation Chinese family. Am J Med Genet B Neuropsychiatr Genet. 2011;156(2):225–32.
Wu Y, Ji T, Wang J, Xiao J, Wang H, Li J, et al. Submicroscopic subtelomeric aberrations in Chinese patients with unexplained developmental delay/mental retardation. BMC Med Genet. 2010;11:72.
Gong X, Jiang YW, Zhang X, An Y, Zhang J, Wu Y, et al. High proportion of 22q13 deletions and SHANK3 mutations in Chinese patients with intellectual disability. PLoS One. 2012;7(4):e34739.
Ji T, Wu Y, Wang H, Wang J, Jiang Y. Diagnosis and fine mapping of a deletion in distal 11q in two Chinese patients with developmental delay. J Hum Genet. 2010;55(8):486–9.
Tzeng CC, Cho WC, Kuo PL, Chen RM. Pilot fragile X screening in normal population of Taiwan. Diagn Mol Pathol. 1999;8(3):152–6.
Zhong N, Liu X, Gou S, Houck Jr GE, Li S, Dobkin C, et al. Distribution of FMR-1 and associated microsatellite alleles in a normal Chinese population. Am J Med Genet. 1994;51(4):417–22.
Chiu HH, Tseng YT, Hsiao HP, Hsiao HH. The AGG interruption pattern within the CGG repeat of the FMR1 gene among Taiwanese population. J Genet. 2008;87(3):275–7.
Eichler EE, Holden JJ, Popovich BW, Reiss AL, Snow K, Thibodeau SN, et al. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994;8(1):88–94.
Zhong N, Yang W, Dobkin C, Brown WT. Fragile X gene instability: anchoring AGGs and linked microsatellites. Am J Hum Genet. 1995;57(2):351–61.
Nolin SL, Sah S, Glicksman A, Sherman SL, Allen E, Berry-Kravis E, et al. Fragile X AGG analysis provides new risk predictions for 45–69 repeat alleles. Am J Med Genet A. 2013;161A(4):771–8.
Sofocleous C, Kolialexi A, Mavrou A. Molecular diagnosis of Fragile X syndrome. Expert Rev Mol Diagn. 2009;9(1):23–30.
Chen L, Hadd AG, Sah S, Houghton JF, Filipovic-Sadic S, Zhang W, et al. High-resolution methylation polymerase chain reaction for fragile X analysis: evidence for novel FMR1 methylation patterns undetected in Southern blot analyses. Genet Med. 2011;13(6):528–38.
Filipovic-Sadic S, Sah S, Chen L, Krosting J, Sekinger E, Zhang W, et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56(3):399–408.
Strom CM, Crossley B, Redman JB, Buller A, Quan F, Peng M, et al. Molecular testing for Fragile X Syndrome: lessons learned from 119,232 tests performed in a clinical laboratory. Genet Med. 2007;9(1):46–51.
Sofocleous C, Kitsiou S, Fryssira H, Kolialexi A, Kalaitzidaki M, Roma E, et al. 10 years' experience in fragile X testing among mentally retarded individuals in Greece: a molecular and epidemiological approach. In Vivo. 2008;22(4):451–5.
Coffee B, Ikeda M, Budimirovic DB, Hjelm LN, Kaufmann WE, Warren ST. Mosaic FMR1 deletion causes fragile X syndrome and can lead to molecular misdiagnosis: a case report and review of the literature. Am J Med Genet A. 2008;146A(10):1358–67.
Nolin SL, Glicksman A, Ersalesi N, Dobkin C, Brown WT, Cao R, et al. Latham GJ. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet Med: Hadd AG; 2014.
Luo S, Huang W, Xia Q, Xia Y, Du Q, Wu L, et al. Cryptic FMR1 mosaic deletion in a phenotypically normal mother of a boy with Fragile X Syndrome: case report. BMC Med Genet. 2014;15(1):125.
Kousoulidou L, Tanteles G, Moutafi M, Sismani C, Patsalis PC, Anastasiadou V. 263.4 kb deletion within the TCF4 gene consistent with Pitt-Hopkins syndrome, inherited from a mosaic parent with normal phenotype. Eur J Med Genet. 2013;56(6):314–8.
Veltman JA, Brunner HG. De novo mutations in human genetic disease. Nat Rev Genet. 2012;13(8):565–75.
Tzeng CC, Tzeng PY, Sun HS, Chen RM, Lin SJ. Implication of screening for FMR1 and FMR2 gene mutation in individuals with nonspecific mental retardation in Taiwan. Diagn Mol Pathol. 2000;9(2):75–80.
Li J, Huang W, Luo S, Lin Y, Duan R. Attitude of Medical School Students in China Towards Genetic Testing and Counseling Issues in FXS. J Genet Couns. 2013;22(6):733–40.
Battaglia A, Doccini V, Bernardini L, Novelli A, Loddo S, Capalbo A, et al. Confirmation of chromosomal microarray as a first-tier clinical diagnostic test for individuals with developmental delay, intellectual disability, autism spectrum disorders and dysmorphic features. Eur J Paediatr Neurol. 2013;17(6):589–99.
van Karnebeek CD, Jansweijer MC, Leenders AG, Offringa M, Hennekam RC. Diagnostic investigations in individuals with mental retardation: a systematic literature review of their usefulness. Eur J Hum Genet. 2005;13(1):6–25.
Yrigollen CM, Durbin-Johnson B, Gane L, Nelson DL, Hagerman R, Hagerman PJ, et al. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet Med. 2012;14(8):729–36.
Yrigollen CM, Mendoza-Morales G, Hagerman R, Tassone F. Transmission of an FMR1 premutation allele in a large family identified through newborn screening: the role of AGG interruptions. J Hum Genet. 2013;58(8):553–9.
Gedeon AK, Baker E, Robinson H, Partington MW, Gross B, Manca A, et al. Fragile X syndrome without CCG amplification has an FMR1 deletion. Nat Genet. 1992;1(5):341–4.
Hirst M, Grewal P, Flannery A, Slatter R, Maher E, Barton D, et al. Two new cases of FMR1 deletion associated with mental impairment. Am J Hum Genet. 1995;56(1):67–74.
Meijer H, de Graaff E, Merckx DM, Jongbloed RJ, de Die-Smulders CE, Engelen JJ, et al. A deletion of 1.6 kb proximal to the CGG repeat of the FMR1 gene causes the clinical phenotype of the fragile X syndrome. Hum Mol Genet. 1994;3(4):615–20.
De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, et al. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3(1):31–5.
Lugenbeel KA, Peier AM, Carson NL, Chudley AE, Nelson DL. Intragenic loss of function mutations demonstrate the primary role of FMR1 in fragile X syndrome. Nat Genet. 1995;10(4):483–5.
Collins SC, Coffee B, Benke PJ, Berry-Kravis E, Gilbert F, Oostra B, et al. Array-based FMR1 sequencing and deletion analysis in patients with a fragile X syndrome-like phenotype. PLoS One. 2010;5(3):e9476.
Acknowledgments
We thank the patients and their families for their participation. We also extend our appreciation to the neurologists who recruited IDD patient in local hospitals, and Dr. Kun Xia for the samples with known FMR1 premutation and full mutation expansions. This work was funded by the National Nature Science Fund (81100841), Natural Science Foundation of Beijing (7081004) and Special Research Foundation of Ministry of Health, P.R.C (201002006), Beijing Municipal Science & Technology Commission (Z131107002213159), The capital health research and development of special (2014-2-1131) and the Chinese Returned Oversea Scientist Fund to XLC from Beijing Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XC and YJ designed the study. XC wrote the manuscript and GL helped writing and editing the manuscript. WZ and JW performed the FMR1 TP-PCR, and HX analyzed the data. JQ, YZ and ZX contributed to DNA extraction and the Region-specific CGG PCR. XC, JG completed the array-CGH and JW did the MLPA analysis. YW, JW, QG, XZ, TJ and LW, XW contributed to patient recruitment. All authors read and approved the final manuscript.
Xiaoli Chen and Jingmin Wang contributed equally to this work.
Additional file
Additional file 1:
Supplementary Table S1.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Chen, X., Wang, J., Xie, H. et al. Fragile X syndrome screening in Chinese children with unknown intellectual developmental disorder. BMC Pediatr 15, 77 (2015). https://doi.org/10.1186/s12887-015-0394-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-015-0394-8