Abstract
Background
Visual quality after corneal refractive surgery is linked to the postoperative effective optical zone (EOZ). This study aims to compare long-term changes in the EOZ following small incision lenticule extraction (SMILE) and femtosecond laser-assisted in-situ keratomileusis (FS-LASIK) for moderate and high myopia.
Methods
This study included 42 patients (72 eyes) who underwent either SMILE (36 eyes) or FS-LASIK (36 eyes). A custom software program based on the tangential curvature difference map of the Pentacam HR (Oculus Optikgeräte GmbH) was used to define the EOZ at 3 and 7 years postoperatively. The EOZ, its chronological changes compared to the programmed optical zone (POZ), and the corneal wavefront aberrations following SMILE and FS-LASIK were analyzed. Correlations between the EOZ changes and relevant parameters were evaluated.
Results
Three years postoperatively, EOZ following SMILE and FS-LASIK were 5.13 ± 0.27 mm and 4.70 ± 0.24 mm (P < 0.001), respectively. Seven years postoperatively, EOZ following SMILE and FS-LASIK decreased to 5.03 ± 0.28 mm and 4.63 ± 0.23 mm (P < 0.001), respectively. At postoperative 7 years, the percentages of EOZ/POZ were negatively correlated with Q-value changes (β = -5.120, P = 0.009) following SMILE and positively correlated with the cylinder correction (β = 1.184, P = 0.004) following FS-LASIK. The induced spherical aberrations in the SMILE group were less than those in the FS-LASIK group (P < 0.05) and were negatively correlated with the EOZ/POZ (β = -16.653, P < 0.001).
Conclusions
The EOZ following SMILE was larger than that following FS-LASIK in the long postoperative term for moderate and high myopia. Furthermore, a continual reduction in the EOZ was noted after both surgical modalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Keratorefractive surgeries correct myopia by diminishing corneal thickness and altering its curvature [1]. Despite being lauded for their safety, efficacy, predictability, and stability in correcting myopia and myopic astigmatism, [2, 3] these surgeries may still engender postoperative night vision disturbances such as glare, halos, ghosting, and blur, [4, 5] especially when the corrected corneal diameter is smaller than the resting pupil [6, 7]. The corneal area that achieves the intended correction postoperatively, also known as the effective optical zone (EOZ), or functional optical zone, [8] indicates the visual quality following keratorefractive surgeries, making it clinically pivotal to understand the changing patterns and influencing factors of the EOZ. In addition, a greater degree of myopia would necessitate a more substantial removal of the corneal tissue, potentially leading to a constricted EOZ and posing a formidable challenge for the algorithmic design within the constraints of limited corneal thickness.
In recent years, small incision lenticule extraction (SMILE) and femtosecond laser-assisted laser in situ keratomileuses (FS-LASIK) have emerged as the predominant keratorefractive surgical modalities globally [1, 9]. Previous studies have demonstrated that the EOZ following SMILE is comparatively larger than that following FS-LASIK, which may be attributed to divergent biomechanical responses and wound healing processes between the two procedures [10,11,12]. However, these studies only compared the EOZ during a short postoperative period, with potential long-term changes in the EOZ remaining an enigma. Furthermore, the majority of studies did not include patients with high myopia. Given that myopic regression predominantly occurs in the context of high myopia correction and over the long term after both surgical interventions, [13,14,15] we postulate that the wound healing processes and biomechanical responses, purportedly culpable for regression, [16] persist beyond the short postoperative period. Consequently, the EOZ is anticipated to undergo corresponding alterations. The longitudinal examination of EOZ changes is thus poised to deepen our understanding of the enduring corneal modifications post keratorefractive surgery.
The current study aimed to compare the long-term changes in the EOZ following SMILE and FS-LASIK for moderate and high myopia using a new calculation method that we published recently [17] and investigate factors affecting the EOZ changes. It is anticipated that our findings will yield valuable insights, propelling the refinement of surgical algorithms, the discernment of optimal surgical approaches, and, ultimately, the enhancement of postoperative visual acuity in the realm of myopia correction via corneal refractive surgeries.
Methods
Ethics approval
This prospective study was carried out following the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Eye & ENT Hospital of Fudan University in 2010 (KJ2010–18). Written consent was obtained from all enrolled patients for inclusion in this study.
Patients
Patients who visited the Eye & ENT Hospital of Fudan University from January 2013 to December 2013 and met the selection criteria were enrolled in this study. The inclusion criteria are as follows: (1) age over 18 years; (2) spheres between − 4.00 diopters (D) and − 9.50 D with cylinders up to -5.00 D; (3) stable refractive status for at least 2 years; and (4) corrected distance visual acuity (CDVA) better or equal to 20/20. Exclusion criteria include (1) a history of other ocular diseases or ocular surgery or trauma, and (2) a history of the systemic disease including autoimmune disease, atopic disease, and other diseases that may affect the visual outcomes of the surgery.
Examinations including uncorrected distance visual acuity (UDVA), CDVA, manifest refraction, slit lamp biomicroscopy, intraocular pressure, axial length, and corneal tomography (Pentacam HR, Optikgeräte, Wetzlar, Germany, version 6.10r59) were conducted preoperatively, 3 years postoperatively, and 7 years postoperatively.
Surgical techniques
All surgeries were performed by the same surgeon (XZ) between January 2013 and December 2013. The SMILE procedures were performed using a 500 kHz VisuMax femtosecond laser system (Carl Zeiss Meditec, Jena, Germany) with a pulse energy of 130 nJ. The thickness of the cap was 120 μm and the lenticule diameter ranged from 6.10 to 6.70 mm. For FS-LASIK procedures, a flap with a diameter of 8.5 mm and thickness of 100 μm was created using the same femtosecond laser system. Then a MEL 80 excimer laser (Carl Zeiss Meditec) was used for stromal ablation, with a pulse energy of 185 nJ and a planned ablation zone from 6.25 to 6.70 mm. Other surgical details have been described in our previous study [18].
EOZ evaluation
A custom software program that could accurately distinguish the borderline of the area of corneal curvature change in the tangential curvature difference map of Pentacam HR was utilized to objectively evaluate the postoperative EOZ (Fig. 1) [17]. The calculated results would be presented as the total area of the EOZ and then converted into diameters using the following formula: diameters of EOZ = 2 × (area of EOZ/ 𝛑 )1/2.
Assessment of the effective optical zone (EOZ). The difference map of tangential corneal curvature (left); and the EOZ of the tangential curvature difference map marked as the area within the black dashed borderline showing the borderline between yellow and green (right). This figure is taken from the article of the same author team [17]
The programmed optical zone (POZ) was defined as the diameter of the extracted lenticule for SMILE and the planned excimer ablation zone for FS-LASIK. Changes in the EOZ were calculated as the percentage of (POZ-EOZ)/POZ.
Corneal wavefront aberrations measurements
Corneal wavefront aberrations were also measured using the Pentacam HR for a central zone of 6 mm. The root mean square (RMS) of coma (Z31 and Z3 − 1), RMS of spherical aberration (Z40), and RMS of total higher order aberrations (HOAs, from third up to sixth order) were evaluated.
Statistical analysis
R version 4.1.0 (http://cran.r-project.org) was used for all statistical analyses. Continuous variables were expressed as mean ± standard deviation (SD). The normal distribution of the variables was analyzed using Shapiro-Wilk tests. The difference between EOZ and POZ was analyzed using a paired t test, and comparisons between post-LASIK and post-SMILE EOZ changes were conducted using a student t test. The relationship between EOZ changes and relevant parameters including the ablation ratio (ablated thickness/ central corneal thickness) was investigated using Pearson correlation analysis and simple linear regression. Statistical significance was set as a P value of less than 0.05.
Results
Forty-two patients (72 eyes) with complete follow-ups were included in this study. Among them, 36 eyes (mean spherical equivalent (SE): -7.29 ± 1.15 D) underwent SMILE, and the other 36 eyes (mean SE: -7.49 ± 1.70 D) underwent FS-LASIK. Preoperative characteristics and surgical parameters of the patients are summarized in Table 1. No adverse events or complications were observed intraoperatively or postoperatively.
Refractive outcomes
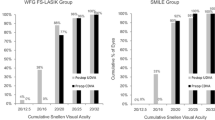
Seven years postoperatively, the safety index (mean postoperative CDVA/mean preoperative CDVA) was 1.09 ± 0.15, and the efficacy index (mean postoperative UDVA/mean preoperative CDVA) was 0.94 ± 0.22 for the SMILE group. For the FS-LASIK group, the corresponding values were 1.12 ± 0.16 and 0.99 ± 0.24, respectively. The 7-year postoperative UDVA was 20/20 or better in 69.4% of SMILE-treated eyes and 61.1% of FS-LASIK-treated eyes, respectively. In addition, 94.4% and 97.2% of the eyes in the SMILE and FS-LASIK groups achieved CDVA equal to or better than the preoperative level, respectively at the 7-year follow-up. At the same follow-up time, the postoperative SE was − 0.47 ± 0.61 D and − 0.55 ± 0.55 D in the SMILE and FS-LASIK groups, respectively. In the SMILE and FS-LASIK groups, the percentage of eyes that obtained within ± 1.00 D of the attempted SE was 77.8% and 80.6%, respectively. The average refractive regression was − 0.31 ± 0.35 D and − 0.35 ± 0.34 D in the SMILE (P < 0.001) and FS-LASIK (P < 0.001) groups from 3 to 7 years of follow-up. Detailed refractive outcomes are summarized in Fig. 2. No significant differences in the safety index, efficacy index, postoperative UDVA, postoperative CDVA, postoperative SE, or refractive regression were found between the SMILE and FS-LASIK groups (all P > 0.05).
Long-term refractive outcomes of the enrolled eyes. Refractive outcomes in the SMILE group (A) and the FS-LASIK group (B). In both panels: (a) uncorrected distance visual acuity (UDVA); (b) change in corrected distance visual acuity (CDVA); (c) predictability of surgery; (d) accuracy of surgery; (e) refractive astigmatism of surgery; (f) stability of refractive outcome over the 7-year follow-up period. D = diopters
Postoperative EOZ
Postoperative EOZ changes are shown in Fig. 3; Table 2. Three years postoperatively, the EOZ diameter was 5.13 ± 0.27 mm and 4.70 ± 0.24 mm in the SMILE and FS-LASIK groups (P < 0.001), respectively. The corresponding EOZ reduction percentages were 20.49% ± 3.89% and 26.64% ± 2.97% in the SMILE and FS-LASIK groups (P < 0.001). Seven years postoperatively, the EOZ in the SMILE group decreased to 5.03 ± 0.28 mm (P = 0.019), which is still significantly larger than that in the FS-LASIK group (4.63 ± 0.23 mm, P < 0.001). The EOZ also significantly decreased from 3 to 7 years postoperatively (P = 0.029) in the FS-LASIK group. The corresponding reduction percentages were 20.49% ± 3.89% and 26.64% ± 2.97% in the SMILE and FS-LASIK groups (P < 0.001), respectively.
Corneal wavefront aberrations
The inductions of corneal aberrations for the total cornea are presented in Supplementary Table 1. Significantly more spherical aberrations were induced by FS-LASIK than SMILE at both postoperative 3 years (FS-LASIK: 0.40 ± 0.22 μm, SMILE: 0.24 ± 0.17 μm, P = 0.002) and postoperative 7 years (FS-LASIK: 0.38 ± 0.19 μm, SMILE: 0.22 ± 0.16 μm, P = < 0.001). No significant differences in the induction of coma and HOAs were observed between post-SMILE and post-FS-LASIK (all P > 0.05) at both postoperative 3 and 7 years. There was no significant changes in the coma, spherical aberrations, or HOAs from postoperative 3 to 7 years following both SMILE and FS-LASIK (all P > 0.05).
Correlation analyses
The results of the correlation analyses are summarized in Table 3. Seven years postoperatively, no significant correlations between the EOZ percentage and the surgical parameters, including the sphere correction and POZ, were found in both groups (all P > 0.05). A significant positive correlation was found between the EOZ percentage and the cylinder correction in the FS-LASIK group (β = 1.184, P = 0.004), indicating a higher degree of astigmatism corrected would lead to a larger EOZ in the long postoperative term following FS-LASIK. However, such a relationship was not found in the SMILE group (β = -1.452, P = 0.142). Among the corneal topographic parameters, only changes in the Q-value (∆Q-value) were found to be negatively correlated with the EOZ percentage (β = -5.120, P = 0.009) in the SMILE group. In addition, the EOZ percentage was negatively correlated with the induced spherical aberrations in the SMILE group (β = -16.653, P < 0.001). No significant correlations were found between the EOZ percentage, the topographic parameters, and the wavefront aberrations in the FS-LASIK group (all P > 0.05).
Since it was found that the EOZ in both groups decreased from 3 to 7 years postoperatively, we further performed correlation analyses between the changes in the EOZ percentage from postoperative 3 to 7 years and the relevant parameters (Table 4). In the SMILE group, the sphere correction (β = 0.597, P = 0.024), cylinder correction (β = -1.115, P = 0.025), and ∆Q-value (β = 6.908, P = 0.002) were found to be significantly correlated with the EOZ reduction. In the FS-LASIK group, the cylinder correction (β = -1.447, P < 0.001) and ∆Q-value (β = 6.576, P = 0.012) were also found to be correlated with the EOZ reduction. Moreover, the ablation ratio (β = -33.360, P < 0.001) and corneal wavefront aberrations (all β > 0, P < 0.05) were also significantly correlated with the EOZ reduction in the FS-LASIK group.
Multivariant analyses were also performed to investigate the effect of the surgical parameters on the EOZ changes from 3 to 7 years postoperatively. For the SMILE group, neither the sphere correction (β = 0.444, P = 0.127) nor the cylinders (β = -0.729, P = 0.189) were significantly correlated with the EOZ change. For the FS-LASIK group, after adjusting the effect of the ablation ratio, the cylinder correction (β = 1.144, P = 0.002) was independently correlated with the EOZ changes.
Discussion
Long-term visual outcome evaluation has always been essential for refractive surgeries, particularly for the correction of moderate and high myopia. The EOZ serves as a critical safety parameter and provides an objective measure of postoperative visual acuity. In the current study, we specifically compared the long-term changes in the EOZ following two trending corneal refractive surgeries, SMILE and FS-LASIK. This endeavor aims to enhance our comprehension of the enduring postoperative corneal alterations and to offer clinical guidance for decision-making in the context of moderate to high myopia correction.
The current study found that the long-term efficacy and safety of the two surgeries for moderate and high myopia were comparable, corroborating results from prior studies with shorter follow-up periods [10, 12, 19,20,21]. Liu et al. [22]. found that 7 years postoperatively, 94.2% of eyes had a UDVA of 20/20 or better following LASIK for high myopia, and the refractions of 90.4% of eyes were within ± 1.00 D of the target refraction. Ikeda et al. [23]. reported that 12 years after LASIK for moderate and high myopia, 75% of the eyes were within ± 1.0 D. For long-term results of SMILE for moderate and high myopia, Damgaard et al. [24]. demonstrated that the UDVA was 20/20 or better in 51.9% of eyes after 7 years, and 81.2% were within ± 1.00 D of the target refraction. Our results are generally in agreement with previous studies with similar outcomes, [14, 25] thereby substantiating that SMILE and FS-LASIK are both effective, safe, and predictable in the long postoperative period for moderate and high myopia. However, both surgeries can result in small long-term regression for high myopia affecting the UDVA, which was suspected to be caused by long near-work time after the surgery [26].
The results of the study showed that the FS-LASIK group had significantly smaller EOZ diameter and EOZ/POZ in comparison to the SMILE group at both the 3-year and 7-year follow-up. Specifically, the EOZ diameter was 4.70 ± 0.24 mm (73.36%) and 4.63 ± 0.23 mm (72.30%) for the FS-LASIK group at the 3-year and 7-year follow-up, respectively, contrasting with the SMILE group’s EOZ diameter of 5.13 ± 0.27 mm (79.51%) and 5.03 ± 0.28 mm (77.95%) at the same follow-up. These outcomes are in concordance with a substantial body of prior research that also found a larger EOZ following SMILE than FS-LASIK [11, 12, 27,28,29,30]. In regards to high myopia, He et al. [10]. demonstrated that the EOZ following SMILE and FS-LASIK 6 months postoperatively were 5.62 ± 0.31 mm and 5.35 ± 0.28 mm, respectively; and the corresponding values described by Liu et al. [12]. were 5.24 ± 0.27 mm and 5.03 ± 0.31 mm, respectively. The values in our study tended to be smaller than those in the literature, a discrepancy potentially ascribed to variations in follow-up durations, methodologies for EOZ assessment, and the characteristics of the patient population enrolled. The difference in the EOZ following the two surgeries is likely a consequence of the divergent healing processes and biomechanical responses resulting from distinct methods of corneal tissue removal, [31], particularly in the peripheral corneal regions. [32,33,34] Additionally, a larger EOZ was observed following hyperopic SMILE than hyperopic LASIK was also found [35]. Our long-term study underscores the superiority of SMILE over FS-LASIK concerning objective visual quality, reinforcing previous short-term studies.
An intriguing observation from our study is the gradual reduction in the effective optical zone (EOZ) from the 3-year to the 7-year postoperative mark in both the SMILE and FS-LASIK groups. Following SMILE and FS-LASIK, the EOZ diameter decreased by 0.10 ± 0.12 mm (1.57%) and 0.07 ± 0.14 mm (1.15%), respectively, with no statistically significant inter-group disparity. This result contradicts previous findings that the EOZ remained stable between 1 and 6 months after the surgery [10, 27, 28]. The discrepancy may be elucidated by the utilization of software in our study, enabling precise and objective EOZ quantification, thereby detecting subtle variations in EOZ measurements [17]. In addition, the four-year interval between the two follow-up assessments in our research is markedly extended compared to prior studies, allowing gradual postoperative corneal reshape. Several studies have reported significant alterations in corneal refractive power after SMILE and LASIK over the years, [15, 24, 36] which is consistent with this observation. It should be noted that the changes in the EOZ diameter in our study are very small, and the percentages are also less than 2% over 4 years. It is imperative to ascertain whether such a reduction could clinically impact patients’ postoperative visual performance, and the etiology of this gradual decline merits exploration in future studies.
The current study primarily investigated the surgical and topographic parameters that may be associated with the EOZ and its reduction over time. Only the cylinder correction was significantly correlated with both the EOZ/POZ at 7 years postoperatively and changes in the EOZ from 3 to 7 years in the FS-LASIK group, indicating that more astigmatism correction would result in a larger EOZ and less decrease over time. Despite not being statistically significant, a similar relationship was also observed in the SMILE group. Liu et al. [37]. compared the EOZ following SMILE between no astigmatism and high astigmatism groups, suggesting that the EOZ was substantially larger in the high astigmatism group, consistent with our findings. Ding et al. [38]. and Wang et al. [39]. also reported that eyes with greater astigmatism gained a larger EOZ in the short-term postoperative period. They found that the EOZ of eyes with high astigmatism was oval compared with others. Based on the evidence, we proposed that similarly to SMILE, eyes with high astigmatism led to a larger EOZ and less decrease in EOZ as a result of better corneal contour preservation [40]. EOZ/POZ and its decrease from 3 to 7 years postoperatively were found to have a significant correlation with Q-value changes in the SMILE group. A similar trend was demonstrated in the FS-LASIK group, which is in accordance with previous short-term observations [27, 38, 40]. As fewer changes in the Q-value indicate better preservation of corneal asphericity, [40, 41] these results also imply that eyes better keeping the corneal contour could gain a larger EOZ over time and experience less decrease. The present study did not find significant correlations between the EOZ and other parameters including the sphere correction and POZ. Concerning these parameters, there is no clear consensus in previous studies [17] and further large-scale studies are required.
Corneal wavefront aberration is also a crucial parameter of objective visual quality. Our study found that postoperative changes in spherical aberrations are substantially greater in the FS-LASIK group than in the SMILE group, which is in line with previous studies [12, 18]. This is also consistent with our observations that the EOZ was substantially larger after SMILE than after FS-LASIK. In line with the literature, we also discovered a significant negative correlation between the EOZ and spherical aberration after SMILE [11, 12]. Surprisingly, significant correlations between the aberrations and the reduction of EOZ from postoperative 3 to 7 years were revealed in the FS-LASIK group but not in the SMILE group. This finding intimates that the reduction of the EOZ in the long term after FS-LASIK may have a clinical influence on the visual quality of patients with moderate and high myopia. However, we did not encompass other visual quality parameters in this study, which warrants investigation in the future.
Our study has limitations. The sample size of our study is small, limited by the completion of a 7-year follow-up. In addition, the subjective visual quality of the patients is not examined, thereby limiting the exploration of the clinical implications of EOZ alterations. Further studies that take these issues into account will need to be undertaken.
Conclusions
The study determined that in cases of moderate to high myopia, the EOZ was larger, and the induction of spherical aberration was less pronounced following SMILE as opposed to FS-LASIK at both the 3-year and 7-year postoperative evaluations. Additionally, a gradual decline in the EOZ was observed from the 3-year to the 7-year postoperative period following both surgeries. Changes in the EOZ might be correlated with the cylinder correction and the Q-value changes, but the quantified relationships warrant further large-scale research. These long-term data would serve as references for the individualization of surgical algorithms tailored to moderate and high myopia.
Data availability
The datasets during and/or analyzed in the current study are available upon request from the co-first authors Yangyi Huang and Tian Han.
Abbreviations
- CDVA:
-
corrected distance visual acuity
- EOZ:
-
effective optical zone
- FS-LASIK:
-
femtosecond laser-assisted laser in situ keratomileuses
- HOAs:
-
higher-order aberrations
- Km:
-
average corneal power
- POZ:
-
programmed optical zone
- RMS:
-
root mean square
- SD:
-
standard deviation
- SE:
-
spherical equivalent
- SMILE:
-
small incision lenticule extraction
- UDVA:
-
uncorrected distance visual acuity
References
Kim T-I, Del Alió JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. 2019;393(10185):2085–98.
Wen D, McAlinden C, Flitcroft I, Tu R, Wang Q, Alió J, Marshall J, Huang Y, Song B, Hu L, et al. Postoperative efficacy, predictability, Safety, and visual quality of laser corneal refractive surgery: a Network Meta-analysis. Am J Ophthalmol. 2017;178:65–78.
Ang M, Farook M, Htoon HM, Mehta JS. Randomized clinical trial comparing Femtosecond LASIK and Small-Incision Lenticule extraction. Ophthalmology. 2020;127(6):724–30.
Han T, Xu Y, Han X, Shang J, Zeng L, Zhou X. Quality of life impact of refractive correction (QIRC) results three years after SMILE and FS-LASIK. Health Qual Life Outcomes. 2020;18(1):107.
Fan-Paul NI, Li J, Miller JS, Florakis GJ. Night vision disturbances after corneal refractive surgery. Surv Ophthalmol. 2002;47(6):533–46.
Alarcón A, Rubiño M, Pééérez-Ocón F, Jiménez JR. Theoretical analysis of the effect of pupil size, initial myopic level, and optical zone on quality of vision after corneal refractive surgery. J Refract Surg. 2012;28(12):901–6.
Freedman KA, Brown SM, Mathews SM, Young RSL. Pupil size and the ablation zone in laser refractive surgery: considerations based on geometric optics. J Cataract Refract Surg. 2003;29(10):1924–31.
Tabernero J, Klyce SD, Sarver EJ, Artal P. Functional optical zone of the cornea. Invest Ophthalmol Vis Sci. 2007;48(3):1053–60.
Ang M, Gatinel D, Reinstein DZ, Mertens E, Alió D, Barrio JL, Alió JL. Refractive surgery beyond 2020. Eye (Lond). 2021;35(2):362–82.
He S, Luo Y, Chen P, Ye Y, Zheng H, Lan M, Zhuang J, Yu K. Prospective, randomized, Contralateral Eye comparison of functional Optical Zone, and visual quality after SMILE and FS-LASIK for high myopia. Transl Vis Sci Technol. 2022;11(2):13.
Damgaard IB, Ang M, Mahmoud AM, Farook M, Roberts CJ, Mehta JS. Functional Optical Zone and Centration following SMILE and LASIK: a prospective, randomized, Contralateral Eye Study. J Refract Surg. 2019;35(4):230–7.
Liu S, Zhang X, Niu L, Yu Z, Zhou X, Zhao J. Comparison of the functional optical zone in eyes with High Myopia with High Astigmatism after SMILE and FS-LASIK. J Refract Surg. 2022;38(9):595–601.
Yan MK, Chang JS, Chan TC. Refractive regression after laser in situ keratomileusis. Clin Exp Ophthalmol. 2018;46(8):934–44.
Blum M, Lauer AS, Kunert KS, Sekundo W. 10-Year results of small incision Lenticule extraction. J Refract Surg. 2019;35(10):618–23.
Li M, Li M, Chen Y, Miao H, Yang D, Ni K, Zhou X. Five-year results of small incision lenticule extraction (SMILE) and femtosecond laser LASIK (FS-LASIK) for myopia. Acta Ophthalmol. 2019;97(3):e373–80.
Moshirfar M, Desautels JD, Walker BD, Murri MS, Birdsong OC, Hoopes PC. Mechanisms of Optical Regression following corneal laser refractive surgery: epithelial and stromal responses. Med Hypothesis Discov Innov Ophthalmol. 2018;7(1):1–9.
Huang Y, Ding X, Han T, Fu D, Yu Z, Zhou X. Effective Optical Zone following small incision Lenticule extraction for myopia calculated with two novel methods. J Refract Surg. 2022;38(7):414–21.
Han T, Xu Y, Han X, Zeng L, Shang J, Chen X, Zhou X. Three-year outcomes of small incision lenticule extraction (SMILE) and femtosecond laser-assisted laser in situ keratomileusis (FS-LASIK) for myopia and myopic astigmatism. Br J Ophthalmol. 2019;103(4):565–8.
Yang W, Liu S, Li M, Shen Y, Zhou X. Visual outcomes after small incision Lenticule extraction and Femtosecond Laser-assisted LASIK for high myopia. Ophthalmic Res. 2020;63(4):427–33.
Han T, Shang J, Zhou X, Xu Y, Ang M, Zhou X. Refractive outcomes comparing small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for high myopia. J Cataract Refract Surg. 2020;46(3):419–27.
Tülü Aygün B, Çankaya Kİ, Ağca A, Yıldırım Y, Yıldız BK, Sucu ME, Kandemir Beşek N, Demirok A. Five-year outcomes of small-incision lenticule extraction vs femtosecond laser-assisted laser in situ keratomileusis: a contralateral eye study. J Cataract Refract Surg. 2020;46(3):403–9.
Liu Z, Li Y, Cheng Z, Zhou F, Jiang H, Li J. Seven-year follow-up of LASIK for moderate to severe myopia. J Refract Surg. 2008;24(9):935–40.
Ikeda T, Shimizu K, Igarashi A, Kasahara S, Kamiya K. Twelve-year Follow-Up of laser in situ Keratomileusis for Moderate to High Myopia. Biomed Res Int. 2017;2017:9391436.
Damgaard IB, Sejersen H, Ivarsen A, Hjortdal J. 7-Year results of SMILE for high myopia: visual and refractive outcomes and aberrations. J Refract Surg. 2021;37(10):654–61.
Xia F, Shen Y, Han T, Zhao J, Xu H, Zhou X. Small incision Lenticule extraction (SMILE) for moderate and high myopia: seven-year outcomes of refraction, corneal tomography, and Wavefront aberrations. J Ophthalmol. 2020;2020:3825864.
Xu Y, Han Y, Lv X, Li J, Zhai C, Zhang F. Associations of Near Work, Time Outdoors, and Sleep Duration with myopic regression 5 years after SMILE and FS-LASIK: a cross-sectional study. J Refract Surg. 2024;40(1):e10–9.
Hou J, Wang Y, Lei Y, Zheng X. Comparison of effective optical zone after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2018;44(10):1179–85.
Li H, Peng Y, Chen M, Tian L, Li D, Zhang F. Six modes of corneal topography for evaluation of ablation zones after small-incision lenticule extraction and femtosecond laser-assisted in situ keratomileusis. Graefes Arch Clin Exp Ophthalmol. 2020;258(7):1555–63.
Qian Y, Chen X, Naidu RK, Zhou X. Comparison of efficacy and visual outcomes after SMILE and FS-LASIK for the correction of high myopia with the sum of myopia and astigmatism from – 10.00 to -14.00 dioptres. Acta Ophthalmol. 2020;98(2):e161–72.
He S, Luo Y, Ye Y, Chen P, Liu C, Lei L, Zhuang J, Yu K. A comparative and prospective study of corneal biomechanics after SMILE and FS-LASIK performed on the contralateral eyes of high myopia patients. Ann Transl Med. 2022;10(13):730.
Luft N, Schumann RG, Dirisamer M, Kook D, Siedlecki J, Wertheimer C, Priglinger SG, Mayer WJ. Wound Healing, inflammation, and corneal ultrastructure after SMILE and Femtosecond Laser-assisted LASIK: a human Ex vivo study. J Refract Surg. 2018;34(6):393–9.
Dupps WJ, Roberts C. Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg. 2001;17(6):658–69.
Spiru B, Kling S, Hafezi F, Sekundo W. Biomechanical properties of Human Cornea tested by two-Dimensional Extensiometry Ex vivo in fellow eyes: Femtosecond Laser-assisted LASIK Versus SMILE. J Refract Surg. 2018;34(6):419–23.
Spiru B, Kling S, Hafezi F, Sekundo W. Biomechanical differences between Femtosecond Lenticule extraction (FLEx) and small incision Lenticule extraction (SmILE) tested by 2D-Extensometry in Ex vivo porcine eyes. Invest Ophthalmol Vis Sci. 2017;58(5):2591–5.
Reinstein DZ, Pradhan KR, Carp GI, Archer TJ, Gobbe M, Sekundo W, Khan R, Dhungana P. Small incision Lenticule extraction (SMILE) for Hyperopia: Optical Zone Diameter and spherical aberration induction. J Refract Surg. 2017;33(6):370–6.
Ivarsen A, Hjortdal J. Seven-year changes in corneal power and aberrations after PRK or LASIK. Invest Ophthalmol Vis Sci. 2012;53(10):6011–6.
Liu S, Gu X, Zhang X, Zhao J, Zhou X. Achieved and functional Optical Zone in myopic eyes with high Astigmatism after Small Incision Lenticule extraction. J Refract Surg. 2022;38(4):243–9.
Ding X, Fu D, Wang L, Zhou X, Yu Z. Functional Optical Zone and Visual Quality after Small-Incision Lenticule extraction for high myopic astigmatism. Ophthalmol Ther. 2021;10(2):273–88.
Wang X, Xia L. Evaluation of the effects of myopic astigmatism correction and anterior corneal curvature on functional Optical Zone after SMILE. J Refract Surg. 2023;39(2):135–41.
Holladay JT, Janes JA. Topographic changes in corneal asphericity and effective optical zone after laser in situ keratomileusis. J Cataract Refract Surg. 2002;28(6):942–7.
Ying J, Zhang J, Cai J, Pan F. Comparative change in anterior corneal asphericity after FS-LASIK and SMILE. J Refract Surg. 2021;37(3):158–65.
Acknowledgements
The authors thank the workmates of EYE&ENT Hospital of Fudan University for help with the conduction of the study.
Funding
This study was supported by the National Natural Science Foundation of China for Young Scholars (Grant No. 82000929, No. 82401319), National Natural Science Foundation of China (Grant No. 82371096), Shanghai Sailing Program (Grant No. 20YF1405000), Joint research project of new frontier technology in municipal hospitals (SHDC12018103), Clinical Research Plan of SHDC (SHDC2020CR1043B), Project of Shanghai Science and Technology (Grant No.20410710100), Project of Shanghai Xuhui District Science and Technology (XHLHGG202104), and Construction of a 3D digital intelligent prevention and control platform for the whole life cycle of highly myopic patients in the Yangtze River Delta (21002411600).
Author information
Authors and Affiliations
Contributions
YH, YW, and TH: study design, manuscript drafting, and statistical analysis; YH, XP, XP, and XZ: study execution and manuscript revision; TH, XZ: conception of the study. All authors read and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This prospective study was carried out in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Eye & ENT Hospital of Fudan University in 2010 (KJ2010–18). Written informed consent was obtained from all enrolled patients for inclusion in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Y., Han, T., Wang, Y. et al. Comparison of long-term changes in the effective optical zone following SMILE and FS-LASIK for moderate and high myopia. BMC Ophthalmol 24, 388 (2024). https://doi.org/10.1186/s12886-024-03662-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03662-9