Abstract
Purpose
Measurement of corneal endothelial cells is critical for postoperative evaluation of phakic intraocular lens (pIOL) surgery. However, inter-instrument differences in corneal endothelial cell density (ECD) after pIOL implantation have not yet been reported. This study aimed to compare automated corneal endothelial cell analysis between CellChek-20 (Konan Medical, Hyogo, Japan) and EM-4000 (Tomey, Nagoya, Japan) in healthy and postoperative eyes with pIOL.
Methods
We retrospectively analyzed 154 healthy and 236 postoperative eyes after pIOL surgery. Endothelial cell measurements were performed using CellChek-20 and EM-4000 with autofocusing and automated image analysis. ECD, percentage of hexagonal cells (HEX), coefficient of variation in cell size (CoV), and central corneal thickness (CCT) were compared between the two devices.
Results
The ECDs of the two devices were highly correlated in both healthy (Spearman’s correlation coefficient [r] = 0.805; p < 0.001) and postoperative (r = 0.901; p < 0.001) groups. ECD from CellChek-20 was higher than EM-4000 in both healthy (mean difference = 228.9 cells/mm2; p < 0.001) and postoperative (mean difference = 115.6 cells/mm2; p < 0.001) groups. The CCT values also showed a strong correlation in healthy eyes (r = 0.974; p < 0.001) and in postoperative eyes (r = 0.936; p < 0.001); however, significant inter-instrument differences were observed. HEX and CV showed significant differences and relatively weak correlations (r < 0.7) between the two devices in both healthy and postoperative groups.
Conclusion
The ECD values between the two instruments were correlated, but that of the CellChek-20 was significantly higher than that of the EM-4000 in both healthy and postoperative eyes after pIOL surgery. Most previous studies have also shown that the Konan software overestimated the ECD compared to other products in automatic measurement mode. The possibility of measurement bias should be considered when replacing equipment used for corneal endothelial cell measurements.
Similar content being viewed by others
Introduction
Corneal endothelial cells, which cover the posterior cornea in a honeycomb pattern, play an important role in regulating corneal hydration and maintaining transparency [1]. If their function in pumping into the cornea is abnormal, the cornea swells and becomes pathologically opaque. When an extreme decrease in corneal endothelial cell density occurs owing to trauma or inflammation, its regulatory function cannot compensate for the passive leakage of corneal hydration. Because corneal endothelial cells do not regenerate, corneal transplantation is required in the event of severe pathological damage [2].
The morphological appearance of corneal endothelial cells is closely related to their functions [3]. The morphology and number of corneal endothelial cells can be determined using a specular microscope. Previously, examiners had to manually read specular microscopic images. Recently, autofocusing specular microscopy and automated image analysis systems have been widely used because they provide fast calculations of morphological factors, including endothelial cell density (ECD), variation in cell size (CoV), and hexagonality (HEX). There are two main algorithms for measuring ECD: the “border method”, which extracts the boundaries of cells, and the “center method”, which estimates cell centers based on the cell boundaries [4]. Both methods have reported accurate and reliable measurement results [5, 6]. Because the “center method” was approved by US FDA through clinical trials [7], most manufacturers have used this method to evaluate ECD in both automated or semi-automated calculation modes. However, ECD measurement results vary from manufacturer to manufacturer depending on the camera, image processing method, and analysis range. Several studies have reported significant variations in ECD measurements among instruments [8, 9]. It has been reported that pathological corneas that had undergone keratoplasty showed larger ECD variation than healthy corneas [10, 11].
The stability of corneal endothelial cells is the most important factor in phakic intraocular lens implantation (pIOL), such as the posterior chamber implantable collamer lens (ICL) or the anterior chamber IOL [12, 13]. Severe endothelial cell loss is a major cause of IOL removal after pIOL [14, 15]. Therefore, regular follow-up of endothelial cells after pIOL implantation has been emphasized. However, no study has investigated the variation in corneal endothelial cell measurements between noncontact specular microscopy instruments for postoperative corneas with a pIOL. Specifically, if endothelial cells are measured using different equipment at each follow-up visit, significant ECD changes due to variations between instruments can lead to incorrect interpretations.
Various specular microscopy devices are available from various manufacturers. EM-4000 (Tomey, Nagoya, Japan) and CellChek-20 (Konan Medical, Hyogo, Japan) are advanced non-contact specular microscopes with automated image analysis systems. A comparison of these two instruments in eyes with pIOL is necessary, because they have been widely used to evaluate corneal endothelial cells in patients with pIOL [12, 16]. In this study, we compared endothelial cell measurements from EM-4000 and CellChek-20 in both healthy and postoperative eyes with pIOL.
Methods
Patient selection
In this cross-sectional study, we retrospectively analyzed 154 healthy eyes of 77 subjects and 236 postoperative eyes of 118 subjects after IOL surgery. We collected data on healthy eyes with no surgical history and postoperative eyes that had undergone pIOL from patients who visited the B&VIIT Eye Center (Seoul, South Korea) between May and July 2023. The inclusion criteria for this study were age between 18 and 40 years and availability of corneal endothelial cell evaluation results using both instruments. The pIOL group included eyes with phakic IOL surgeries using Artisan, Artiflex (Ophtec, Groningen, Netherlands), ICL V4, or EVO-ICL (STAAR, Surgical, Nidau, Switzerland) of patients undergoing follow-up between 6 months and 15 years after surgery. Patients with corneal opacity, inflammatory ocular disease, or angle-closure glaucoma were excluded.
This study was approved by the Institutional Review Board of the Korean National Institute for Bioethics Policy (KNIBP; no. 2023–0860-001), which waived the requirement for informed consent. All research methods were performed in accordance with the Declaration of Helsinki and KNIBP guidelines.
Measurements
All healthy subjects were evaluated during routine preoperative examinations, and all postoperative patients were evaluated during routine postoperative examinations for pIOL. Corneal endothelial cell indices, including central ECD, CoV, HEX, and central corneal thickness (CCT) were evaluated using Tomey EM-4000 and Konan CellChek-20 specular microscopy instruments on the same day. Both Tomey’s EM series and Konan’s CellChek series are representative endothelial calculation tools based on the "center method" [5, 17]. The “center method” and fully automated modes, which were set as default for both instruments, were used [18]. To capture the center of the cornea, the patient was asked to focus on the central fixation target during the examination. The endothelial cell indices were calculated using fully automated methods with each embedded software package. All measurements were performed by a trained examiner. An examiner randomly selected the order of the two measurements.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS; IBM Corp., Armonk, NY, USA). The comparative analysis was conducted separately in each of two groups (healthy and postoperative eyes). Wilcoxon rank-sum and chi-square tests were used to compare demographic data between the healthy and postoperative groups. We performed a nonparametric paired Wilcoxon test to compare the ECD, CoV, HEX, and CCT results of the two instruments. We also calculated the Spearman’s correlation coefficient of the measurements between the two instruments. The comparability of the measurement results for the two instruments was visualized in Bland–Altman plots using MedCalc Statistical Software version 22.009 (MedCalc Software Ltd., Ostend, Belgium). All statistical tests were two-sided, and the significance level was set at p < 0.05.
Literature review
PubMed was searched for updates on previous studies involving comparisons between specular microscopy instruments. The following keywords were applied: “specular,” “corneal endothelial cell,” “endothelial cell count,” or “comparison study.” Two authors (HC and TKY) manually screened abstracts for relevant studies. We excluded the following articles: (1) studies that did not compare two or more specular microscopic instruments and (2) studies that only investigated human corneas. We extracted information on the studied populations and statistical differences in ECD measurements between the calculation methods (automated or manual) and instruments from previous studies.
Results
The basic demographic data of the 154 healthy eyes and 236 eyes that underwent surgery are shown in Table 1. The mean ages (± standard deviation) of the study subjects were 26.51 ± 4.77 years and 29.86 ± 5.85 years in the healthy and postoperative groups, respectively. The healthy group consisted of 25 males (32.5%) and 52 women (67.5%), whereas the postoperative group consisted of 38 males (32.2%) and 80 females (67.8%). The mean keratometry values were 43.40 ± 1.01 D and 42.85 ± 1.27 D in the healthy and postoperative groups, respectively. The mean axial lengths in the healthy and postoperative groups were 25.27 ± 1.48 mm and 26.77 ± 1.95 mm, respectively. Except for sex (p = 0.969), there were significant differences between the two groups in terms of age, mean keratometry, and axial length (p < 0.001). In the postoperative group, 48 eyes underwent iris-claw anterior chamber IOL (Artiflex or Artisan) implantation, and 188 eyes underwent posterior chamber IOL (ICL-V4 or EVO-ICL) implantation.
A comparison of the measurements between the Tomey EM-4000 and Konan CellChek-20 specular microscopy instruments in the healthy eyes is summarized in Table 2. The mean ECD values were 2651.4 ± 20.9.6 cells/mm2 when measured using EM-4000 and 2880.3 ± 248.4 cells/mm2 when measured using CellChek-20. CellChek-20 showed significantly higher values than EM-4000 (p < 0.001). The Spearman’s correlation coefficient for the ECD between the two instruments was 0.805 (p < 0.001). The EM-4000 showed higher CoV and lower HEX measurements than the CellChek-20 (p < 0.001). The correlation coefficients for CoV and HEX were 0.634 (p < 0.001) and 0.616 (p < 0.001), respectively. The mean CCT values were 521.2 ± 35.6 μm when measured using EM-4000 and 540.1 ± 34.0 μm CellChek-20 when measured using CellChek-20. There was a significant difference in CCT measurements between the groups (p < 0.001).
Similar relationships were observed in the post-operative group. Table 3 shows a comparison of the measurements between the EM-4000 and CellChek-20 specular microscopy instruments in the postoperative eyes. The mean ECD values were 2615.0 ± 329.3 cells/mm2 when measured using EM-4000 and 2730.6 ± 372.2 cells/mm2 when measured using CellChek-20. CellChek-20 also showed significantly higher values than EM-4000 (p < 0.001). The Spearman’s correlation coefficient for ECD between the two instruments was 0.901 (p < 0.001). Similarly, EM-4000 showed higher CoV and lower HEX values than CellChek-20 (p < 0.001). The correlation coefficients for the CoV and HEX were 0.479 (p < 0.001) and 0.554 (p < 0.001), respectively. The mean CCT values were 517.2 ± 35.9 μm when measured using EM-4000 and 527.0 ± 34.5 μm when measured using CellChek-20. There was a significant difference in CCT measurements between the groups (p < 0.001).
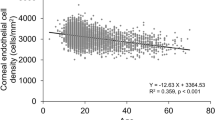
The scatter plots of the ECD measurements are shown in Fig. 1. Conversion equations were derived from the distribution using linear regression. The conversion equation for healthy eyes was as follows: ECDCellChek-20 = 0.995 × ECDEM-4000 + 236.525 (cells/mm2). The conversion equation for the postoperative eyes was as follows: ECDCellChek-20 = 1.046 × ECDEM-4000 + 90.155 (cells/mm2). The Bland–Altman plots are shown in Fig. 2. The mean differences between the two instruments were 228.9 cells/mm2 and 115.6 cells/mm2 in the healthy and postoperative groups, respectively. The standard deviation of the ECD differences were 147.5 cells/mm2 and 161.7 cells/mm2 in the healthy and postoperative groups, respectively.
The Bland–Altman plots for the CoV measurements are shown in Fig. 3. The mean differences between the two instruments (CoVCellChek-20—CoV EM-4000) were -6.3 and -5.3 in the healthy and postoperative groups, respectively. The standard deviation of the CoV differences were 5.7 and 6.2 in the healthy and postoperative groups, respectively.
Figure 4 shows the Bland–Altman plots for the HEX measurements. The mean differences between the two instruments (HEXCellChek-20 and HEXEM-4000) were 17.1 and 17.0 in the healthy and postoperative groups, respectively. The standard deviation of the HEX differences were 7.3 and 7.6 in the healthy and postoperative groups, respectively.
In the present study, CCT was performed using specular microscopy. Figure 5 shows the Bland–Altman plots for the CCT measurements. The mean differences between the two instruments (CCTCellChek-20 and CCTEM-4000) were 18.9 μm and 10.8 μm in the healthy and postoperative groups, respectively. The standard deviation of the CCT differences were 8.2 μm and 12.8 μm in the healthy and postoperative groups, respectively.
Figure 6 shows the results of the analysis of two IOL types in the post-operative group: iris-claw anterior chamber IOL (Artiflex or Artisan) and posterior chamber IOL (ICL-V4 or EVO-ICL). Similar distributions and conversion formulas were derived in the two groups. In both subgroups, CellChek-20 measured higher ECD than EM-4000, but the difference was not significant in the anterior chamber IOL group. The mean differences between the two instruments were 36.8 cells/mm2 (p = 0.082) for the anterior chamber IOL group and 135.8 cells/mm2 (p < 0.001) for the posterior chamber IOL group.
The literature review is presented in Table 4. Eleven studies compared ECD measurements between instruments and calculation algorithms. Four studies [3, 19,20,21] compared manual and automated calculations of ECD measurements. Except for one study [19], the other studies reported that the automated methods overestimated ECD values compared to manual calculations. Two studies compared Konan and Topcon specular microscopy products [8, 22] and reported that ECD values calculated by Konan software were significantly higher than those calculated by Topcon. Three studies had data about ECD comparisons between Konan and Tomey specular microscopy products [10, 11, 23]. Among them, two studies reported that automated calculations using Konan software overestimated the ECD compared to Tomey products [10, 11], and one showed no significant difference [23]. In addition, Konan products showed higher ECD measurements than Nidek products [9, 11].
Discussion
Corneal endothelial cell damage is irreversible due to a lack of cell regeneration. Therefore, accurate ECD measurement is the most important task to protect vision from various pathological factors [25, 26]. This study demonstrated a comparative analysis of corneal endothelial cell measurements between two major non-contact specular microscope instruments in healthy and postoperative eyes after pIOL implantation. The ECD values of the Konan CellChek-20 were significantly higher than those of the Tomey EM-4000 in both healthy and postoperative eyes following pIOL surgery. In this study, healthy corneas without guttae were analyzed, and the pIOL surgery group also exhibited healthy corneal endothelial cell status without guttae. As a result, devices using the two center methods showed some measurement bias, but the measurement tendency in the postoperative state did not change. To the best of our knowledge, this study has made the following contributions: first, this is the first study to compare corneal endothelial cell measurements of the EM-4000 with those of the Konan software. Second, a large sample size was used to clarify the differences between the two instruments. A larger number of subjects (154 healthy eyes and 236 postoperative eyes with pIOL) was studied compared with previous studies that compared the instruments of two or more manufacturers. Third, this is the first study to analyze the variation in ECD measurements in postoperative eyes after pIOL implantation. In our study, there was a significant inter-instrument difference in the ECD measurements in both healthy and postoperative eyes after pIOL implantation. The two instruments also showed significant differences in CoV and ECD, indicating that caution is required when analyzing data from these instruments. The literature review also indicated that Konan's products generally calculate ECD higher than Tomey's products in automatic measurement mode.
For most instruments, the “center method” is currently the standard method for measuring ECD [7]. This method uses software algorithms to identify the centers of endothelial cells, relying heavily on the quality of imaging and the accuracy of the software. There is a potential for errors if the algorithm misidentifies cell centers, especially in cases of irregular or damaged endothelial cells. A previous study showed that when using the center method, more than 30% of cells in the image periphery are excluded from analysis [5]. In this case, there was a possibility that morphometric data would be calculated incorrectly, especially if endothelial cell damage was severe. To overcome these shortcomings of the center method, the flex center method has been developed and is used as an auxiliary method. A study reported that the flex center method was effective in patients with corneal guttae [27]. Because our study did not include patients with guttae, we did not use the flex center method.
Figure 7 shows the cases in which there was a significant difference in the ECD measurements between the two instruments. Even though the same eye was measured, the ECD, CoV (shown as CV in the figure), and HEX (shown as 6A or HEX% in the figure) values showed differences. Both EM-4000 and CellChek-20 instruments used the "center method", but the visualization of corneal endothelial cells was different. When comparing specular corneal endothelial images, the central part of the cornea was studied using each instrument; however, the captured areas and cell counting algorithms were different. Although most instruments have used the “center method” to calculate ECD, detailed image processing and automated evaluation options have not been standardized [28]. Therefore, ECD measurements may differ for each manufacturer. A literature review showed that the automated calculation of the Konan software tends to overestimate the ECD values compared to other product calculations. Our study also showed that the automated calculation of the Konan CellChek-20 overestimated the ECD values compared to the Tomey EM-4000. ECD from CellChek-20 was higher than EM-4000 in both healthy (mean difference = 228.9 cells/mm2; P < 0.001) and postoperative (mean difference = 115.6 cells/mm2; P < 0.001) groups. The CCT values also showed a strong correlation, but significant inter-instrument differences were observed. HEX and CV showed relatively weak correlations between the two devices in both healthy and postoperative groups. This result indicates that the automated measurements of the CellChek-20 and EM-4000 are currently uninterchangeable. When observing endothelial cells after pIOL surgery, clinicians must be aware of the biases that may occur if the specular microscopy instrument is replaced with another company's product.
Example of Tomey EM-4000 and Konan CellChek-20 measurement images that shows between-instrument differences in the corneal endothelial cell density (ECD) values (a) Example where the ECD value is measured low at Tomey EM-4000 (left) and high at Konan CellChek-20 (right) (b) Example where the ECD value is measured high at Tomey EM-4000 (left) and low at Konan CellChek-20 (right)
Damage to endothelial cells can occur during pIOL surgery, and even after surgery, a faster reduction in endothelial cells can occur compared to that in normal eyes [29]. Therefore, it is clinically important to check for differences between specular microscopy instruments in postoperative eyes with pIOL [12]. In our study, the healthy and postoperative groups showed the same pattern of measurement differences between the instruments. When the anterior chamber and posterior chamber IOLs were analyzed separately, there was no difference in endothelial cell measurements between the two groups. Previous studies have reported differences in the ECD between automated and manual methods for damaged corneal endothelial cells. The Konan software’s automated method has shown an overestimation of ECD in glaucomatous eyes with damaged endothelial cells compared to manual calculations [20, 21]. In addition, when the difference between instruments was studied in the damaged and normal cornea groups, Konan's automatic measurement system showed higher ECD values than the Tomey and Nidek instruments in both groups [11]. These results are consistent with our findings. In conclusion, ECD measurement using Konan's software can result in higher values than manual counting and other devices, so caution is required when comparing ECD values measured by other devices.
Our study has several limitations. First, the single-center data collection may limit the generalizability of the study results. However, this study did not include patients with guttata. To confirm the results of this study, endothelial cell data should be collected from various patient groups at diverse institutions. Second, we did not collect ECD values from manual counting data. We focused on a comparison of automatic measurement methods between the CellChek-20 and EM-4000. Several studies have used manual endothelial cell counting as a reference [10, 11]. Therefore, the superiority of one instrument over the other cannot be concluded from this study. Additional limitations include the cross-sectional inter-instrument comparison design, which was unable to evaluate intra- and inter-observer agreements.
Conclusion
The ECD values between the two instruments were correlated, but Konan CellChek-20 showed significantly higher values than the Tomey EM-4000 in both healthy and postoperative eyes after pIOL surgery. HEX and CV showed significant differences and relatively weak correlations between the two devices. Most previous studies have also shown that the Konan software overestimated the ECD compared to other products in automatic measurement mode. Therefore, specular microscopy devices are currently uninterchangeable, and the possibility of measurement bias should be considered when replacing the equipment for corneal endothelial cell measurement.
Availability of data and materials
The data that support the findings of this study are available upon request from the corresponding author.
References
Van den Bogerd B, Zakaria N, Adam B, Matthyssen S, Koppen C, Ní DS. Corneal endothelial cells over the past decade: are we missing the mark(er)?. Transl Vis Sci Technol. 2019;8:13.
Singh S, Chaurasia S. Recent and evolving therapies in the management of endothelial diseases. Semin Ophthalmol. 2023;38:207–15.
Cheung SW, Cho P. Endothelial cells analysis with the TOPCON specular microscope SP-2000P and IMAGEnet system. Curr Eye Res. 2000;21:788–98.
Deb-Joardar N, Thuret G, Zhao M, Acquart S, Péoc’h M, Garraud O, et al. Comparison of Two semiautomated methods for evaluating endothelial cells of eye bank corneas. Invest Ophthalmol Vis Sci. 2007;48:3077–82.
Patel SV, McLaren JW, Bachman LA, Bourne WM. Comparison of flex-center, center, and corner methods of corneal endothelial cell analysis. Cornea. 2010;29:1042.
Miyagi H, Stanley AA, Chokshi TJ, Pasqualino CY, Hoehn AL, Murphy CJ, et al. Comparison of automated versus manual analysis of corneal endothelial cell density and morphology in normal and corneal endothelial dystrophy-affected dogs. Vet Ophthalmol. 2020;23:44–51.
McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27:1–16.
Gasser L, Reinhard T, Böhringer D. Comparison of corneal endothelial cell measurements by two non-contact specular microscopes. BMC Ophthalmol. 2015;15:87.
Karaca I, Yilmaz SG, Palamar M, Ates H. Comparison of central corneal thickness and endothelial cell measurements by Scheimpflug camera system and two noncontact specular microscopes. Int Ophthalmol. 2018;38:1601–9.
Price MO, Fairchild KM, Price FWJ. Comparison of manual and automated endothelial cell density analysis in normal eyes and DSEK eyes. Cornea. 2013;32:567.
Luft N, Hirnschall N, Schuschitz S, Draschl P, Findl O. Comparison of 4 specular microscopes in healthy eyes and eyes with cornea guttata or corneal grafts. Cornea. 2015;34:381.
Choi H, Ryu IH, Lee IS, Kim JK, Yoo TK. Comparison of implantation of posterior chamber phakic IOL implantation and laser vision correction in terms of corneal endothelial cells: 3-year observational paired-eye study. J Cataract Refract Surg. 2023;49:936.
Yoo TK, Lee SM, Lee H, Choi EY, Kim M. Retropupillary iris fixation of an artisan myopia lens for intraocular lens dislocation and aphakia in eyes with extremely high myopia: a case series and a literature review. Ophthalmol Therapy. 2022;11:1251–60.
Sayman Muslubas IB, Kandemir B, Aydin Oral AY, Kugu S, Dastan M. Long-term vision-threatening complications of phakic intraocular lens implantation for high myopia. Int J Ophthalmol. 2014;7:376–80.
Morral M, Güell JL, El Husseiny MA, Elies D, Gris O, Manero F. Paired-eye comparison of corneal endothelial cell counts after unilateral iris-claw phakic intraocular lens implantation. J Cataract Refract Surg. 2016;42:117–26.
Packer KT, Vlasov A, Greenburg DL, Coggin A, Weightman JW, Beltran T, et al. U.S. military implantable collamer lens surgical outcomes: 11-year retrospective review. J Cataract Refract Surg. 2022;48:649.
Tekin K, Sekeroglu MA, Kiziltoprak H, Yilmazbas P. Corneal densitometry in healthy corneas and its correlation with endothelial morphometry. Cornea. 2017;36:1336.
Kösekahya P, Üçgül Atılgan C, Atılgan KG, Koç M, Tekin K, Çağlayan M, et al. Corneal endothelial morphology and thickness changes in patients with gout. Turk J Ophthalmol. 2019;49:178–82.
Doughty MJ, Oblak E. A comparison of two methods for estimating polymegethism in cell areas of the human corneal endothelium. Ophthalmic Physiol Opt. 2008;28:47–56.
Huang J, Maram J, Tepelus TC, Modak C, Marion K, Sadda SR, et al. Comparison of manual & automated analysis methods for corneal endothelial cell density measurements by specular microscopy. J Optom. 2018;11:182–91.
Minami M, Chihara E. Overestimation of corneal endothelial cell density by automated method in glaucomatous eyes with impaired corneal endothelial cells. Int Ophthalmol. 2022;42:133–45.
de Sanctis U, Machetta F, Razzano L, Dalmasso P, Grignolo FM. Corneal endothelium evaluation with 2 noncontact specular microscopes and their semiautomated methods of analysis. Cornea. 2006;25:501–6.
Jbara D, Achiron A, Antman G, Buhbut O, Hecht I, Tuuminen R, et al. Agreement of corneal endothelial cell analysis between konan-noncon robo SP-6000 and tomey EM-3000 specular microscopes in healthy subjects. Eye Contact Lens. 2021;47:191–5.
Szalai E, Németh G, Berta A, Módis L. Evaluation of the corneal endothelium using noncontact and contact specular microscopy. Cornea. 2011;30:567–70.
Ong Tone S, Bruha MJ, Böhm M, Prescott C, Jurkunas U. Regional variability in corneal endothelial cell density between guttae and non-guttae areas in Fuchs endothelial corneal dystrophy. Can J Ophthalmol. 2019;54:570–6.
Fang CEH, Khaw PT, Mathew RG, Henein C. Corneal endothelial cell density loss following glaucoma surgery alone or in combination with cataract surgery: a systematic review protocol. BMJ Open. 2021;11:e050992.
Huang J, Liu X, Tepelus TC, Nazikyan T, Chopra V, Sadda SR, et al. Comparison of the Center and Flex-Center Methods of Corneal Endothelial Cell Analysis in the Presence of Guttae. Cornea. 2017;36:1514.
Qu J-H, Qin X-R, Peng R-M, Xiao G-G, Cheng J, Gu S-F, et al. A fully automated segmentation and morphometric parameter estimation system for assessing corneal endothelial cell images. Am J Ophthalmol. 2022;239:142–53.
Shaaban YM, Badran TAF. Three-year effect of phakic intraocular lenses on the corneal endothelial cell density. Clin Ophthalmol. 2020;14:149–55.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
HC acquired and analyzed data and drafted the manuscript. IHR, JKK, and ISL suggested the original study idea, interpreted the results, contributed to writing. HC and TKY contributed to data interpretation and manuscript editing and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Korean National Institute for Bioethics Policy (KNIBP; no. 2023–0860-001), which waived the requirement for informed consent. All research methods were performed in accordance with the Declaration of Helsinki and KNIBP guidelines. The Institutional Review Board waived the requirement for informed consent because the data were fully de-identified, thereby protecting patient confidentiality.
Consent for publication
Not applicable.
Competing interests
Ik Hee Ryu and Jin Kuk Kim are directors at VISUWORKS and own company stock. Ik Hee Ryu serves on the Advisory Board for Carl Zeiss Meditec AG and Avellino Lab USA/MAB for Avellino Lab Korea. Jin Kuk Kim is an executive of the Korea Intelligent Medical Industry Association (KIMIA). Tae Keun Yoo is an employee of VISUWORKS and receives salary and stock as part of the standard compensation package. The remaining authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, H., Ryu, I.H., Lee, I.S. et al. Comparison of automated corneal endothelial cell analysis in healthy and postoperative eyes with phakic intraocular lens: a cross-sectional study and literature review. BMC Ophthalmol 24, 318 (2024). https://doi.org/10.1186/s12886-024-03590-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03590-8