Abstract
Background
Several autoimmune disorders have been linked to polymorphisms in IL10 and IL6R genes. This research aimed to study whether single nucleotide polymorphisms (SNPs) in the genes of IL10 and IL6R were associated with acute anterior uveitis (AAU) in Han Chinese.
Methods
Genotyping was carried out by the iPLEX Gold Genotyping Assay. Our study comprised 420 patients with AAU and 918 healthy subjects from Han Chinese. Using the chi-square (χ2) test, alleles and genotypes were analyzed between AAU subjects and healthy controls.
Results
All ten SNPs were successfully genotyped and four SNPs (IL10/rs1800871, IL10/rs3021094, IL10/rs2222202, IL6R/rs4845618) exhibited weak associations with AAU, as indicated by their Puncorr values. However, upon applying the Bonferroni correction, there was no significant association between AAU and the control subjects. Additionally, the haplotype analysis of the ten SNPs revealed no association with AAU.
Conclusion
Our findings suggested that polymorphisms of the tested ten SNPs on the IL10 and IL6R genes did not show any association with the risk of developing AAU among the Han Chinese population.
Similar content being viewed by others
Background
Uveitis may result in significant visual impairment. The incidence of this condition differs globally, with a rate of 200 per 100,000 in North America and 111.3 per 100,000 in Taiwan [1, 2]. Acute anterior uveitis (AAU) is a common form of uveitis [3, 4]. It is characterized by sudden eye pain, redness, and photophobia, and is more prevalent in young to middle-aged adults, with a slightly higher incidence in females than males [3, 5]. While the etiology and pathogenesis of AAU is still unclear, previous studies suggested the involvement of immune responses and genetic factors. Recent studies identified the associations of HLA-B27, FOXO1, TRAF5 and TNFSF15 with AAU [6,7,8]. However, the exact genetic susceptibility to the disease is not entirely clear.
Genetic variations in interleukin-10 (IL10) and interleukin-6 receptor protein (IL6R) genes are identified to be associated with autoimmune disorders. Interleukin-10 (IL10) act as an anti-inflammatory cytokine that modulates immune response in autoimmune diseases including psoriasis and uveitis [9, 10]. Several investigations have discovered that IL10 gene polymorphisms played a role in a wide range of autoimmune disorders including inflammatory bowel disease (IBD), Behcet’s disease (BD), systemic sclerosis (SS) and ankylosing Spondylitis (AS) [11,12,13,14]. IL6R is commonly expressed on the cell surface and exists in a soluble form as well. Dysregulation of IL-6/IL6R pathway can increase immune cell activation and the secretion of pro-inflammatory cytokines, resulting in persistent inflammation and damage to tissues [15, 16]. Multiple studies have linked variations in the IL6R gene to several conditions, including multiple sclerosis (MS), rheumatoid arthritis (RA), type I diabetes (T1D), IBD and AS in multiple studies [17,18,19,20]. Furthermore, a GWAS study in a Caucasian population indicated that IL10 and IL6R were associated with the onset of AAU in AS and IBD, reaching a suggestive level of significance [21].
In light of the aforementioned findings, we undertook a study to explore whether SNPs covering the IL10 and IL6R genes influence the susceptibility to AAU in Han Chinese.
Methods
Patients and controls
To identify disease susceptibility loci in the IL10 and IL6R genes, 420 AAU subjects and 918 healthy participants were enrolled in the present research. AAU and healthy subjects were all Han Chinese. All the blood samples were from the patients and healthy controls referred to the first affiliated hospital of Chongqing medical university and the first affiliated hospital of Zhengzhou university from November 2016 to March 2021. AAU subjects were all in acute episodes and had no other autoimmune conditions. Patients with AAU were diagnosed on the basis of symptoms such as blurred vision, red eyes, and ocular pain, as well as clinical examinations that showed ciliary congestion, anterior chamber cells, and keratic precipitates. Our study features a control group of 918 individuals, none of whom have AS, AAU, or any additional autoimmune diseases. our study adhered to the Helsinki Declaration and received approval from the Ethics Research Committees of aforementioned universities. Additionally, all participants provided written informed consent to take part in the study.
SNP selection
According to prior research, ten tag SNPs were considered for the association study: IL10/(rs1800871, rs3790622, rs1800896, rs3021094 and rs2222202) and IL6R/( rs2228145, rs6690230, rs4845618, rs4845374 and rs4129267) and alleles and genotype data of the SNPs were all present in single nucleotide polymorphisms database (dbSNP) of the National Center of Biotechnology Information (NCBI) in Han Chinese. Each of the ten loci had a minor allele frequency (MAF) greater than 0.05. Genotyping data for IL6R and IL10 genes in Han Chinese was obtained from HapMap Website (http://hapmap.ncbi.nlm.nih.gov/Index.html.en).
DNA extraction and genotyping test
The QIAmp DNA Blood Mini Kit (Qiagen Inc., CA, USA) was utilized to extract genomic DNA from peripheral whole blood. Following extraction, DNA samples were kept at -80°C. IL10/(rs1800871, rs3790622, rs1800896, rs3021094 and rs2222202) and IL6R/( rs2228145, rs6690230, rs4845618, rs4845374 and rs4129267) were genotyped by iPlex Gold Genotyping Assay. The MassARRAY Assay design program was utilized to design the primers listed in Table 1.
Statistical analysis
Direct enumeration was applied to calculate the allelic and genotypic frequencies. A Chi-Square (X2) test was utilized to determine HWE. SNP variants were evaluated with the Fisher Precision or SPSS 2 test in SPSS (version 19.0) to identify allele and genotypic differences. Estimation of the linkage disequilibrium (LD) was performed by calculating R2 and D′ values, while haplotype construction was carried out through the utilization of SHEsis (Shi and He, 2005). The Bonferroni correction method was utilized to alter P values, and a corrected P-value (Pc) of less than 0.05 (Pc<0.05) was considered as statistically significant.
Results
Clinical features
The demographics information for cases and controls were displayed in Table 2. The healthy cohort comprised 456 males and 462 females, with a mean age of 39.73 ± 10.06 years. While the AAU patient group included 211 males and 209 females, with a mean age of 40.94 ± 11.86 years. Among the 353 AAU patients who underwent HLA-B27 assessment, a total of 241 individuals tested positive (Table 2). The statistical analysis revealed no appreciable difference in age or gender was found between the cases and controls (P > 0.05).
Genotype results
Ten SNPs covering IL10 and IL6R genes were successfully genotyped, with each SNP exhibiting a genotyping success rate exceeding 95%. None of them deviated from the HWE in controls (P > 0.05). Puncorr values showed weak associations between AAU and four SNPs. In IL6R/rs4845618, the frequency of TT genotype (P = 0.034, OR = 1.329, 95%CI = 1.021–1.729) was increased in AAU subjects versus healthy participants while the frequency of GT phenotype (P = 0.028, OR = 0.772, 95%CI = 0.612–0.973) was decreased (Table 3). In the IL10 gene, a preliminary association between rs1800871 and AAU was also observed with the allele frequency of G being lower (P = 0.035, OR = 0.824, 95%CI = 0.688–0.987) in AAU subjects as compared with controls. Additionally, the GG genotype in IL10/rs3021094 and the AA genotype in IL10/rs2222202 showed a minor increased trends in AAU patients (P = 0.011, OR = 1.417, 95%CI = 1.083–1.853; P = 0.012, OR = 1.204, 95%CI = 0.955–1.518, respectively). However, after the Bonferroni corrections were performed, all of the positive results that were mentioned earlier disappeared (Table 4). The remaining 6 SNPs had no significant correlation with AAU (see supplemental Table 1).
Given the significant association between AAU and HLA-B27, we have performed a further stratified analysis on the genotyping results of ten loci based on the AAU patients’ HLA-B27 outcomes. Unfortunately, after the Bonferroni corrections, we did not find any evidence that the ten SNPs of IL10/(rs1800871, rs3790622, rs1800896, rs3021094 and rs2222202) and IL6R/( rs2228145, rs6690230, rs4845618, rs4845374 and rs4129267) were associated with the HLA-B27+ AAU risk (Pc > 0.05) (see supplemental Table 2).
The haplotype analysis
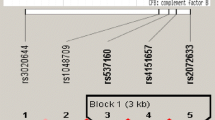
The association between haplotypes of the ten SNPs and AAU was investigated by means of analysis. After applying the correction, there was no association observed. The comprehensive information regarding haplotype assessment was displayed in Table 5.
Discussion
In our study, genotyping analysis of 420 AAU subjects and 918 healthy individuals were conducted for ten SNPs in IL10 and IL6R genes, in a Chinese Han population. Although four SNPs, IL6R/rs4845618, IL10/rs1800871, IL10/rs3021094 and IL10/rs2222202 showed preliminary associations with AAU, the significance was no longer present after applying the Bonferroni correction. Furthermore, analysis was carried out to study the correlation of LD block of the ten SNPs with AAU, and no association was observed.
AAU is considered as an inflammatory ocular disease, and its recurrent attacks can cause severe visual impairment or even blindness. While the precise cause of the condition remains unknown, extensive research suggests that immune and genetic components may both contribute to its development [22, 23]. Although HLA-B27 has been linked to AAU strongly, not all HLA-B27 positive subjects will develop the disease, suggesting the involvement of additional genes [23, 24]. Recent association studies have uncovered numerous susceptibility genes outside of the MHC region, such as the IL23R, FOXO1, TRAF5 and TNFSF15 genes [6,7,8, 21]. However, further investigation is necessary to gain a greater understanding of the genetic foundation of the disease.
IL10 is a vital immunoregulatory cytokine for inflammatory and immune responses [11]. Low IL10 expression has been linked to the etiology of inflammatory and autoimmune illnesses [12] including Behcet’s disease (BD), and polymorphisms in multiple immunoregulatory genes have been linked to an increased risk of developing BD [25]. IL6R is a key member of IL-6 signal pathway and has been linked to various autoimmune and inflammatory conditions (including IBM, AS and RA). A humanized antibody against the IL6 receptor called tocilizumab has displayed remarkable clinical effectiveness in the treatment of RA and JIA [10, 25]. IL6R gene may be involved in the development and treatment of autoimmune diseases including uveitis [10, 26]. Taking into account of aforementioned discoveries, we have developed a significant interest in the genetic roles of IL6R and IL10 in AAU.
As is well-known, selecting candidate SNPs is critical for gene variation study. In our research, ten SNPs covering IL10 and IL6R genes were chosen based on previous association studies of autoimmune disorders, such as IBD, RA, MS and AS. IL10/rs1800896, rs1800871, rs2222202 and rs3790622 have been found to be associated with CD in several studies [11, 12]. Moreover, rs3021094 and rs1800871 have both been demonstrated to be associated with BD in the Chinese population [13]. According to a GWAS investigation within the Caucasian demographic, IL6R/rs6690230 C allele has been identified to be a protective element against AS and AAU [21]. Multiple analyses have demonstrated a notable link between IL6R/rs4845618 and IL6R/rs4845374 and RA [27]. Various research has established a connection between IL6R/rs2228145 and an array of autoimmune conditions, including IBD and RA [19]. A study conducted on genetic polymorphisms revealed a significant correlation between IL6R/rs4129267 and AS [20]. In our study, IL10/rs1800871, rs3021094, rs2222202 and IL6R/rs4845618 showed a weak association with AAU, but unfortunately, this association disappeared after correction.
To ensure robustness of data, a number of efforts have been made. Firstly, the diagnosis of AAU patients was strictly based on their symptoms (such as ocular pain, red eyes, and vision loss), clinical examinations (such as keratic precipitates, ciliary congestion, and anterior chamber cells) as well as instrumental tests (ultrasound biomicroscopy and anterior segment image). Patients with any uncertainty in their diagnoses were not included in this study. Secondly, all patients were all in the acute phase of the disorder and none of the patients had a history of any other autoimmune disorders. Thirdly, all AAU patients come from a Chinese Han population in order to prevent racial bias in the outcomes. Age and gender were matched between the patients and controls.
All the above-mentioned efforts have made the results reliable. According to the current investigation, none of the tested ten SNPs of IL10 and IL6R genes were connected to AAU. However, it is worthwhile to point out that our study does not rule out the possibility of an association between these SNPs and AAU in other ethnic. Additionally, it is unknown whether there is an association between other SNPs on IL10 and IL6R genes and AAU, which should be investigated in future research.
Conclusions
Our findings suggested that polymorphisms of the tested ten SNPs of IL10 and IL6R genes did not have any correlation with the susceptibility to AAU among the Han Chinese population.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article (tables and supplementary table), Further request about the data of this study are available from the corresponding author on reasonable request (dulplab@live.cn).
Abbreviations
- AAU:
-
Acute anterior uveitis
- IL6R:
-
Interleukin-6 receptor
- IL10:
-
Interleukin-10
- MS:
-
Multiple sclerosis
- IBD:
-
Inflammatory bowel disease
- T1D:
-
Type 1 diabetes
- RA :
-
Rheumatoid arthritis
- BD :
-
Behcet’s disease
- AS:
-
Ankylosing spondylitis
- SS:
-
Systemic sclerosis
- LD:
-
Linkage disequilibrium
References
Darrell RW WH KL. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol 1962(68:502–14.).
Hwang DK, Chou YJ, Pu CY, Chou P. Epidemiology of uveitis among the Chinese population in Taiwan: a population-based study. Ophthalmology. 2012;119(11):2371–6.
Pan J, Kapur M, McCallum R. Noninfectious immune-mediated uveitis and ocular inflammation. Curr Allergy Asthma Rep. 2014;14(1):409.
Yang P, Zhang Z, Zhou H, Li B, Huang X, Gao Y, Zhu L, Ren Y, Klooster J, Kijlstra A. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2005;30(11):943–8.
Parthasarathy R, Santiago F, McCluskey P, Kaakoush NO, Tedla N, Wakefield D. The microbiome in HLA-B27-associated disease: implications for acute anterior uveitis and recommendations for future studies. Trends Microbiol. 2023;31(2):142–58.
Hongsong Yu YL, Zhang L, Wu L, Zheng M, Cheng L, Luo L. Aize Kijlstra, Peizeng Yang: FoxO1 gene confers genetic predisposition to acute anterior uveitis with ankylosing spondylitis. Invest Ophthalmol Vis Sci. 2014;55(12):7970–4.
Li H, Hou S, Yu H, Zheng M, Zhang L, Zhang J, Zhang Q, Cao Q, Yuan G, Kijlstra A, et al. Association of Genetic Variations in TNFSF15 with Acute Anterior Uveitis in Chinese Han. Invest Ophthalmol Vis Sci. 2015;56(8):4605–10.
Qin Xiang1 LC JF. Shengping Hou1, Lin Wei1, Lin Bai1, Yunjia Liu1, Yan Zhou1, Aize Kijlstra2 and peizeng Yang1*: TNF receptor-associated factor 5 gene confers genetic predisposition to acute anterior uveitis and pediatric uveitis. Invest Ophthalmol Vis Sci. 2013;15:R113.
van Laar JAM, PhD M, van Hagen PM. MD, PhD: cytokines in Uveitis. Clin Med Res. 2018;44:248–9.
Hausmann JS. Targeting cytokines to treat autoinflammatory diseases. Clin Immunol. 2019;206:23–32.
Sun A, Li W, Shang S. Association of polymorphisms in the IL-10 promoter region with Crohn’s disease. J Clin Lab Anal. 2022;36(12):e24780.
Amre DK, Mack DR, Morgan K, Israel D, Lambrette P, Costea I, Krupoves A, Fegury H, Dong J, Grimard G, et al. Interleukin 10 (IL-10) gene variants and susceptibility for paediatric onset Crohn’s disease. Aliment Pharmacol Ther. 2009;29(9):1025–31.
Wu Z, Zheng W, Xu J, Sun F, Chen H, Li P, Chen S, Shen M, Zhang W, You X, et al. IL10 polymorphisms associated with Behcet’s disease in Chinese Han. Hum Immunol. 2014;75(3):271–6.
Peng WJ, Wang BX, Pan HF, Tao JH, Zhang JQ, He Q, Xiao CC, Wang J. Association of the interleukin-10 1082G/A, 819 C/T and 3575T/A gene polymorphisms with systemic sclerosis: a meta-analysis. Mol Biol Rep. 2012;39(6):6851–5.
Baran P, Hansen S, Waetzig GH, Akbarzadeh M, Lamertz L, Huber HJ, Ahmadian MR, Moll JM, Scheller J. The balance of interleukin (IL)-6, IL-6.Soluble IL-6 receptor (sIL-6R), and IL-6.sIL-6R.sgp130 complexes allows simultaneous classic and trans-signaling. J Biol Chem. 2018;293(18):6762–75.
Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20.
Marinou I, Walters K, Winfield J, Bax DE, Wilson AG. A gain of function polymorphism in the interleukin 6 receptor influences RA susceptibility. Ann Rheum Dis. 2010;69(6):1191–4.
Bank S, Skytt Andersen P, Burisch J, Pedersen N, Roug S, Galsgaard J, Ydegaard Turino S, Brodersen JB, Rashid S, Kaiser Rasmussen B, et al. Polymorphisms in the inflammatory pathway genes TLR2, TLR4, TLR9, LY96, NFKBIA, NFKB1, TNFA, TNFRSF1A, IL6R, IL10, IL23R, PTPN22, and PPARG are associated with susceptibility of inflammatory bowel disease in a Danish cohort. PLoS ONE. 2014;9(6):e98815.
Zhang M, Bai Y, Wang Y, Cui H, Tang M, Wang L, Wang X, Gu D. Cumulative evidence for associations between genetic variants in interleukin 6 receptor gene and human diseases and phenotypes. Front Immunol. 2022;13:860703.
Ruan WF, Xie JT, Jin Q, Wang WD, Ping AS. The Diagnostic and Prognostic Role of Interleukin 12B and interleukin 6R gene polymorphism in patients with Ankylosing Spondylitis. J Clin Rheumatol. 2018;24(1):18–24.
Robinson PC, Claushuis TA, Cortes A, Martin TM, Evans DM, Leo P, Mukhopadhyay P, Bradbury LA, Cremin K, Harris J, et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol. 2015;67(1):140–51.
Wang Q, Su G, Tan X, Deng J, Du L, Huang X, Lv M, Yi S, Hou S, Kijlstra A, et al. UVEOGENE: an SNP database for investigations on genetic factors associated with uveitis and their relationship with other systemic autoimmune diseases. Hum Mutat. 2019;40(3):258–66.
Yang P, Wan W, Du L, Zhou Q, Qi J, Liang L, Wang C, Wu L, Kijlstra A. Clinical features of HLA-B27-positive acute anterior uveitis with or without ankylosing spondylitis in a Chinese cohort. Br J Ophthalmol. 2018;102(2):215–9.
Rosenbaum JT. Uveitis in spondyloarthritis including psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease. Clin Rheumatol. 2015;34(6):999–1002.
Gao Y, Zhong Z, Yang P. Genetics in Behcet’s Disease: an Update Review. Front Ophthalmol 2022, 2.
Karkhur S, Hasanreisoglu M, Vigil E, Halim MS, Hassan M, Plaza C, Nguyen NV, Afridi R, Tran AT, Do DV, et al. Interleukin-6 inhibition in the management of non-infectious uveitis and beyond. J Ophthalmic Inflamm Infect. 2019;9(1):17.
Lopez-Lasanta M, Julia A, Maymo J, Fernandez-Gutierrez B, Urena-Garnica I, Blanco FJ, Canete JD, Alperi-Lopez M, Olive A, Corominas H, et al. Variation at interleukin-6 receptor gene is associated to joint damage in rheumatoid arthritis. Arthritis Res Ther. 2015;17(1):242.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China [82301271, 81970792, 82171040 and 82101108], Medical Science and Technology Project of Health Commission of Henan Province [YXKC2020026], Major Program of Medical Scientific and Technological of Henan Province (XXX), Medical Scientific and Technological Project of Henan Province (SBGJ2020003031).
Author information
Authors and Affiliations
Contributions
The idea and the experiment design were done by L.L., LD and P.Y. The experiments were conducted by L.L., F.L., J.S.and K.X. L.L., H.Z., L.D., X.J. and P.Z. analysed the data. The manuscript was authored by L.L., L.D. and P.Y., L.L. prepared the tables. The manuscript was reviewed by all authors.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Our study adhered to the Helsinki Declaration and received approval from the Ethics Research Committees of the First Affiliated Hospital of Zhengzhou University(2021-KY-0246-001)and the First Affiliated Hospital of Chongqing Medical University (2009- 201008). All participants provided written informed consent to take part in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, L., Li, F., Shan, J. et al. Genetic variations of IL10 and IL6R genes in acute anterior uveitis in Han Chinese. BMC Ophthalmol 24, 228 (2024). https://doi.org/10.1186/s12886-024-03495-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03495-6