Abstract
Background
This study aimed to compare the corneal high-order aberrations and surgically induced astigmatism between the clear corneal incision and limbus tunnel incision for posterior chamber implantable collamer lens (ICL/TICL) implantation.
Methods
A total of 127 eyes from 73 myopic patients underwent ICL V4c implantation, with 70 eyes receiving clear corneal incisions and 57 eyes receiving limbus tunnel incisions. The anterior and back corneal surfaces were measured and the Root Mean Square of all activated aberrations (TRMS) was calculated, including higher-order aberration (HOA RMS), spherical aberration Z40, coma coefficients (Coma RMS) Z3−1 Z31, and surgically induced astigmatism (SIA). The measurements were taken preoperatively and postoperatively at 1 day, 1 week, and 1, 3, and 6 months. In this study, the corneal higher-order aberration was estimated as the Zernike coefficient calculated up to 5th order. The measurements were taken at a maximum diameter of 6.5 mm using Pentacam.
Results
One week after the operation, the corneal back Z31 of the clear corneal incision group was 0.06 ± 0.06, while the limbus tunnel incision group showed a measurement of 0.05 ± 0.06 (p = 0.031). The corneal back Z40 of the clear corneal incision group was -0.02 ± 0.25, compared to -0.04 ± 0.21 in the limbus tunnel incision group (p = 0.01). One month after the operation, the corneal back SIA of the clear corneal incision group was 0.11 ± 0.11, compared to 0.08 ± 0.11of the limbus tunnel incision group (p = 0.013), the corneal total SIA of the clear corneal incision group was 0.33 ± 0.30, compared to 0.15 ± 0.16 in the limbus tunnel incision group (p = 0.004); the clear corneal incision group exhibited higher levels of back astigmatism and total SIA than the limbus tunnel incision in the post-operation one month period. During the 6- month post-operative follow-up period, no significant difference in Z31, Z40, and other HOA RMS data was observed between the two groups. The total SIA of the corneal incision group and the limbus tunnel incision group were 0.24 ± 0.14 and 0.33 ± 0.32, respectively (p = 0.393), showing no significant difference between the two groups 6 months after the operation.
Conclusion
Our data showed no significant difference in the high-order aberration and SIA between clear corneal incision and limbus tunnel incision up to 6 months after ICL-V4c implantation.
Similar content being viewed by others
Background
The posterior chamber implantable collamer lens (ICL/TICL) is used for the correction of moderate to high myopia. This intraocular refractive surgery has been approved by the U.S. Food and Drug Administration since 2005 [1]. Comparative studies have reported that ICL provides superior spectacle-corrected visual acuity and refractive predictability and stability compared to keratoconus surgery. Additionally, ICL has a lower risk of retinal detachment compared to refractive lens exchange [2]. This technique results in stable visual quality and safety after 3 to 10 years of follow-up [3,4,5]. Stability is maintained due to minimal corneal damage and a structure that closely resembles the normal corneal structure in patients with high-risk factors for keratoconus [6, 7].
Corneal refractive surgery is one of the most popular surgical methods for correcting myopia, including RPR LASIK and Smile [8]. However, ocular aberration and changes in visual quality have been reported in both surgical approaches. Corneal aberration influences optics quality and is the primary cause of intraocular refractive aberration [9].
Precise measurements and a carefully planned procedure are essential to optimize visual quality. The corneal incision may be a factor that needs to be considered for more accurate calculations of the refractive index. IOL calculations for cataract surgery take into account the position of the surgical incision, its width, the potential astigmatism of the surgical site, and the effect of aberration changes on the patient's visual quality [10]. Nevertheless, current ICL surgery calculations do not consider these parameters, and the methods have been based on cataract surgery. Corneal incisions flatten the perpendicular corneal meridian, which may lead to surgically induced astigmatism (SIA). The magnitude of SIA depends on factors such as the size, location, and number of the incision [11,12,13]. Multiple factors, such as corneal distortion, turbulence in the irrigation solution, mechanical trauma from instruments, contact with nuclear fragments and intraocular lens, and the presence of free oxygen radicals, all contribute to corneal damage during cataract surgery [14]. Unlike cataract surgery, ICL surgery involves fewer incisions and a shorter operative time, while the cornea is not exposed to super-generated energy. These differences may potentially result in varied aberrations and changes in astigmatism. Therefore, this study aimed to compare the corneal high-order aberrations and surgically induced astigmatism (SIA) between the clear corneal incision and limbus tunnel incision for posterior chamber implantable collamer lens (ICL/TICL) implantation at different time points.
Methods
Patients
A total of 127 eyes from 73 myopic patients (aged 29.3 ± 6.6 years, 16 males and 57 females) who underwent ICL V4c implantation at the Myopic Center, Zunyi Medical University Affiliated Hospital from December 2017 to January 2021 were enrolled retrospectively. A clear incision was performed on 70 eyes, and a limbus tunnel incision was carried out on 57 eyes. Informed consent was obtained from all of the patients prior to the study, and the protocol adhered to the principles of the Declaration of Helsinki. This retrospective study was approved by Zunyi Medical University’s Institutional Ethics Committee. A corneal endothelial cell count of more than 2000/mm2 was indicative of the absence of viral or bacterial infection. The exclusion criteria comprised a history of previous ocular surgery, surgical complications, any corneal or macular pathology, refractive instability, poor fixation, etc. Cases involving severe dry eyes were also excluded.
Surgical techniques
All surgeries were performed by the same experienced surgeon, Dr. LTX. The patients were randomly selected and divided into two groups based on the corneal incision performed. Group A received a clear corneal incision of approximately 0.5 mm in front of the corneal scleral rim. Group B received a limbus tunnel incision of 1.0 mm posterior to the corneal limbus. All ICL surgery was performed through a 3.0 mm corneal incision at 11:00 o’clock (Fig. 1). The corneal incision was hydrated at the end of the surgery without any auxiliary incision.
Pupils were sufficiently dilated with tropicamide (Santen Pharmaceutical Co., Ltd., Osaka, Japan) before the surgery. Topical anesthesia was achieved with proparacaine (proparacaine hydrochloride, Alcon), and the foldable V4-ICL was inserted into the posterior chamber through a 2.8 mm corneal or limbus incision using a specialized injector. The ICL was placed in the ciliary sulcus and adjusted to a suitable angle by using a gauge. Afterward, the remaining viscoelastic agent in the anterior chamber was completely removed using irrigation/aspiration (I/A) with a balanced saline solution. Subsequently, the patients were administered a steroidal agent (0.1% fluorometholone, Santen, Osaka, Japan), an antibiotic (0.3% levofloxacin, Santen, Osaka, Japan), an NSAID (Pranoprofen, Senju Pharmaceutical, Japan), and sodium hyaluronate (1.7% sodium hyaluronate; Bausch & Lomb, China), which were gradually reduced over the course a month.
Ophthalmologic measurements
Ophthalmologic measurements were evaluated preoperatively and postoperatively at 1 day, 1 week, and 1, 3, and 6 months. The following measurements were taken: uncorrected distance visual acuity (UDVA), manifest refraction, best corrected visual acuity manifest refraction (CDVA), endothelial cell count (ECC; Topcon SP-2000P; Topcon, Tokyo, Japan), intraocular pressure (IOP) using a TX-10 non-contact tonometer (Canon, Japan), axial length, and anterior chamber depth using IOLMaster-500 (Carl Zeiss Meditec AG, Germany). The corneal higher-order aberration Zernike coefficients were measured before and after the procedure using the Scheimpflug analysis system Pentacam HR (Oculus®, Wetzlar, Germany). The Zernike coefficient was calculated up to the 5th order with a maximum diameter of 6.5 mm (Fig. 2). The wavefront aberration included the root mean square (RMS) of all HOA (HOA RMS), the total trefoil coefficients (TRMS), horizontal coma Z3−1, vertical coma Z31, and the fourth-order spherical aberration Z40 from the anterior aorneal surface, back aorneal surface, and total corneal.
Statistical analysis
Data were expressed as mean ± SD. All the data were analyzed using SPSS Statistics 19.0 (SPSS Inc., Chicago, US), and the Kolmogorov–Smirnov test was used to confirm data normality. Independent t-tests were used for continuous variables, and Wilcoxon tests were used to compare continuous variables that did not follow a normal distribution.
Result
In this study, 127 eyes from 73 myopic patients were included (Table 1). The clear corneal incision group included 70 eyes and the limbus tunnel incision group included 57 eyes. The groups were appropriately matched in terms of age, sex, ocular axis length (AL), uncorrected distance visual acuity (UDVA), corrected visual acuity (CDVA), and spherical equivalent degree (SE). No significant difference was observed between the two groups in terms of subjection (Table 1).
HOA of anterior corneal surface
The data collected one week after the operation showed no significant differences in terms of TMAS (p = 0.559); HOA (p = 0.328), Z3−1 (p = 0.328), Z31 ( p = 0.786), and Z40 (p = 0.175) between the clear corneal incision group and the limbus tunnel incision group. However, significant differences in TMAS (p = 0.001) and Z3−1 (p = 0.031) were found between the two groups 6 months after the operation; there was no significant difference between the two groups in terms of HOA (p = 0.716), Z31 (p = 0.908), and Z40(p = 0.954). Moreover, no significant differences were found between the two groups at other time points (all p > 0.05) (Table 2).
HOA of back corneal surface
A week after the operation, the clear corneal incision group had a significantly higher Z31 value than the limbus tunnel incision group (0.06 ± 0.06 vs. 0.05 ± 0.06, p = 0.031), as displayed in Fig. 3. Furthermore, the corneal back Z40 also showed a significant difference between the clear corneal incision group and the limbus tunnel incision group (-0.02 ± 0.25 vs. -0.04 ± 0.21, p = 0.01), as shown in Fig. 4. Other statistics, including TMAS, HOA, Z3−1, Z31, Z40 revealed no significant difference between the two groups at different times (Table 3).
HOA of total corneal
One week after the operation, the total corneal Z40 of the clear corneal incision group was 0.27 ± 0.19, compared to 0.17 ± 0.19 in the limbus tunnel incision group (p = 0.32). The limbus tunnel incision group had a lower total corneal Z40 value than the clear corneal incision group (Fig. 4). Other statistics, including TMAS, HOA, and Z3−1 showed no significant difference between the two groups at different times (Table 4).
SIA
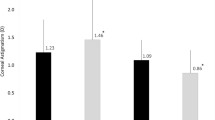
One week after the operation, the corneal back corneal average K value (Km) and astigmatism (CA) of the corneal incision group and the limbus tunnel incision group were (-6.42 ± 0.25, -6.39 ± 0.21, p = 0.02) and (0.46 ± 0.25, 0.44 ± 0.22, p = 0.03), respectively. The limbus tunnel incision group had higher back Km and CA values compared to the corneal incision group. One month after the operation, the corneal back SIA of the clear corneal incision group was 0.11 ± 0.11 compared to 0.08 ± 0.11 of the limbus tunnel incision group (p = 0.013). The clear corneal incision had a higher back SIA than the limbus tunnel incision at the one-month post-operation mark. In addition, the clear corneal incision group also had a significantly higher corneal total SIA than the limbus tunnel incision group (0.33 ± 0.30 vs. 0.15 ± 0.16, p = 0.004), as shown in Fig. 5.
Six months after the operation, the corneal front astigmatism and back astigmatism of the corneal incision group and the limbus tunnel incision group were (1.21 ± 0.16 vs. 1.57 ± 1.05, p = 0.04) and (0.38 ± 0.16 vs. 0.52 ± 0.16, p = 0.007), respectively. However, the total corneal astigmatism was (0.140 ± 0.52 vs. 0.83 ± 1.63, p = 0.410). The total SIA of the corneal incision group and the limbus tunnel incision group were (0.24 ± 0.14, 0.33 ± 0.32, p = 0.393). The results showed no significant difference between the two groups 6 months after the operation (Table 5).
Discussion
ICL is generally restricted to patients with a high refractive error that is beyond the range of corneal procedures due to the risk of corneal ectasia. Precise measurements and a carefully planned procedure are essential to optimize visual quality. In recent years, the number of incisions were reduced from two to one, thereby limiting the negative impact. The limbus tunnel incision and the clear corneal incision are commonly performed. The corneal incision may be a factor to be considered for more precise calculations of the refractive index. In this study, the HOA and SIA after ICL-V4c implantation were analyzed to compare the limbus and the clear cornea incision.
Previous aberration studies revealed that the cornea is the primary source of intraocular refractive aberration. Many IOL calculations consider the impact of surgical source astigmatism SIA. Additionally, the suitability and extent of ICL surgery should also be considered. Astigmatism aberration, coma aberration, and trefoil aberration are the three main factors that affect the quality of optics. High-order aberrations include spherical aberration, coma, cloverleaf, etc.; most originate from the anterior surface of the cornea. LASIK was found to lead to more than twice the corneal spherical aberration than PIOL [15]. Phakic IOLs also induce changes in HOA due to their optic properties and the corneal incision made during the surgery [16]. CariPrez Vives’ study [17] on the effects of ICL on HOA demonstrated a negative impact on spherical aberration. Furthermore, the study conducted by Sun Woong Kim [18] examined the combined effects of ICL and corneal incision on higher-order aberrations (HOA). The results revealed that both the ICL and different large corneal incisions led to changes in spherical aberration. Torii H's research [19] demonstrated that the use of Artisan lenses resulted in a greater increase in positive spherical aberration in 6-mm pupils compared to Article lenses. As opposed to cataract surgery, small changes in the cornea can be accurately detected in ICL surgery using wavefront aberration, without the error of supergenerated energy levels. However, no study has compared the clear corneal incision and limbus tunnel incision for posterior chamber implantable collamer lens placement. The higher-order aberration of the cornea is a crucial indicator of the changes in the surgical incision following ICL surgery.
Our research reveals some differences in the corneal higher-order aberrations at different follow-up times. The main effect following ICL is the spherical aberration Z40, showing a small change in the coma, which is in accordance with Seyed’s results [20]. However, the corneal total Z40 of 0.27 ± 0.19 obtained in this study was higher than in Salmon and Seyed’s study [21]. Some differences in the corneal higher-order aberrations were observed at different times between the clear corneal incision group and the limbus tunnel incision group. One week after the operation, the limbus tunnel incision group demonstrated a lower corneal back Z31, Z40, and Km compared to the clear corneal incision group. These findings may be related to the size of the tunnel incision in the posterior cornea in relation to the central apex of the cornea. These changes gradually return to preoperative levels over 6 months by corneal self-repair. The magnitude of the phase difference was correlated with the diameter. Previous studies on ICL aberrations have shown no effect on corneal higher-order phase differences when measuring diameters of 3 mm, but the higher-order aberrations changed when measuring diameters of 6 mm. To better observe corneal changes, the diameter was adjusted to 6.5 mm. Therefore, our phase contrast results were higher than other previously published articles, and the differences were more pronounced [21].
In Phaco + IOL cataract surgery, corneal incisions flatten the perpendicular corneal meridian, resulting in surgically induced astigmatism (SIA). The magnitude of SIA depends on the size, location, and design of the incision. Previous studies have compared the value of SIA with various incision sizes, and some have also assessed its long-term evolution [22].
The limbal relaxing incisions may result in a median surgically induced astigmatism (SIA) of 1.8D-2.2D [23]. The astigmatism derived from clear keratotomy after cataract surgery starts to stabilize 2 weeks after the procedure and shows no significant change for up to 1 year or even 10 years [24, 25]. The current study found that astigmatism and aberration changes are a dynamic process, with the magnitude of change stabilizing at 6 months after the surgery. Six months after the operation, the limbus tunnel incision group had more back astigmatism and less front astigmatism, but there was no difference in total astigmatism. The total SIA of the clear corneal incision group and the limbus tunnel incision group after the operation 6 months was 0.24 ± 0.14 and 0.33 ± 0.32, respectively (p = 0.393); these statistics showed no significant differences between the two groups across different times, with less corneal surgery astigmatism and HOA changes after the operation 6 months.
In cataract surgery, a 3.0 mm incision may result in 0.6 D SIA [26]. In our study, both incisions caused astigmatism of less than 0.35D, and the magnitude of the changes stabilized 6 months after the procedure. The mean corneal power of total keratometry reduced slightly after cataract surgery (-0.05 dpt), which was mostly attributed to a decrease in back surface power (-0.04 dpt) [27]. The incision width and axial direction caused various changes in astigmatism of surgical origin [28]. In this study, the location of the incision was fixed in both axial direction (110) and width (3.0 mm), and the main difference between the two kinds of incision groups was the distance from the incision’s inner mouth to the central point of the cornea. The Pentacam Scheimpfug principle can directly measure both anterior and posterior corneal curvatures [29]. A gentle tunnel incision produces a longer and wider corneal section, whereas a steeper corneal incision is closer to the pupil area and scar healing leads to corneal aberrations. These changes plateau six months after the operation and have little impact on the patient’s visual outcome.
Conclusion
In ICL-V4c implantation, the clear corneal incision and the limbus tunnel incision both affect the corneal back Z31 and the back and total corneal Z40 within a week after surgery. Moreover, the back and total corneal SIA are affected within a month after surgery. However, the high-order aberrations recovered to the preoperative levels 6 months after the operation in both incision groups, and the total SIA was less than 0.35D. Six months after ICL-V4c implantation, no significant difference in high-order aberration and SIA was found between the clear corneal incision and the limbus tunnel incision.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICL/TICL:
-
Implantable Contact Lens / Toric Implantable Contact Lens
- RMS:
-
Root Mean Square of aberrations
- HOA RMS:
-
Higher-order aberration
- Coma RMS:
-
Coma coefficients
- SIA:
-
Surgically induced astigmatism
- CCI:
-
Clear corneal incision group
- LTI:
-
Limbus tunnel incision group
- AL:
-
Axis length
- UDVA:
-
Uncorrected distance visual acuity
- CDVA:
-
Corrected distance visual acuity
- TMAS:
-
Total Zernike root mean Square of aberrations
- HOA:
-
Higher order Zernike root mean square
- Z3 − 1 :
-
Horizontal coma
- Z3 1 :
-
Vertical coma
- Z4 0 :
-
The 4th order spherical aberration
References
Sanders DR, Doney K, Poco M, ICL in Treatment of Myopia Study Group. United States Food and Drug Administration clinical trial of the implantable collamer lens (ICL) for moderate to high myopia: three-year follow-up. Ophthalmology. 2004;111:1683–92.
Ieong A, Rubin GS, Allan BD. Quality of life in high myopia: implantable Collamer lens implantation versus contact lens wear. Ophthalmology. 2009;116(2):275–80. https://doi.org/10.1016/j.ophtha.2008.09.020. Epub 2008 Dec 16 PMID: 19091407.
Huang D, Schallhorn SC, Sugar A, Farjo AA, Majmudar PA, Trattler WB, Tanzer DJ. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(11):2244–58. https://doi.org/10.1016/j.ophtha.2009.08.018. PMID: 19883852.
Kocová H, Vlková E, Michalcová L, Rybárová N, Motyka O. Incidence of cataract following implantation of a posterior-chamber phakic lens ICL (Implantable Collamer Lens) - long-term results. Cesk Slov Oftalmol. 2017;73(3):87–93 English. PMID: 29394074.
Kamiya K, Shimizu K, Kobashi H, Igarashi A, Komatsu M. Three-year follow-up of posterior chamber toric phakic intraocular lens implantation for moderate to high myopic astigmatism. PLoS One. 2013;8(2):e56453. https://doi.org/10.1371/journal.pone.0056453. Epub 2013 Feb 8. PMID: 23409187; PMCID: PMC3568037.
Zhao J, Yang W, Zhao J, Shen Y, Sun L, Han T, Wang X, Yao P, Zhou X. A four-year observation of corneal densitometry after implantable collamer lens V4c implantation. Ann Transl Med. 2021;9(7):536. https://doi.org/10.21037/atm-20-6628. PMID:33987234;PMCID:PMC8105832.
Li K, Wang Z, Zhang D, Wang S, Song X, Li Y, Wang MX. Visual outcomes and corneal biomechanics after V4c implantable collamer lens implantation in subclinical keratoconus. J Cataract Refract Surg. 2020;46(10):1339–45. https://doi.org/10.1097/j.jcrs.0000000000000262. PMID: 32483075.
D’Oria F, Fernández-Buenaga R, Casanova L, García-Corral M, Vega A, Alio JL. Surface ablation outcomes in high myopia with different epithelium removal techniques. J Cataract Refract Surg. 2021;47(9):1175–82.
Tian H, Gao W, Xu C, Wang Y. Clinical outcomes and higher order aberrations of wavefront-guided LASIK versus SMILE for correction of myopia: A systemic review and meta-analysis. Acta Ophthalmol. 2023;101(6):606–18. https://doi.org/10.1111/aos.15638.
Eom Y, Ryu D, Kim DW, Yang SK, Song JS, Kim SW, Kim HM. Development of a program for toric intraocular lens calculation considering posterior corneal astigmatism, incision-induced posterior corneal astigmatism, and effective lens position. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):1977–86. https://doi.org/10.1007/s00417-016-3446-3. Epub 2016 Aug 19 PMID: 27541160.
Dick B, Jacobi KW. Comparison of the induced astigmatism after temporal clear corneal tunnel incisions of different sizes. J Cataract Refract Surg. 1995;21:417–24. https://doi.org/10.1016/s0886-3350(13)80532-9. PMID: 8523286.
Kohnen S, Neuber R, Kohnen T. Effect of temporal and nasal unsutured limbal tunnel incisions on induced astigmatism after phaco emulsification. J Cataract Refract Surg. 2002;28:821–5. https://doi.org/10.1016/s0886-3350(01)01215-9. PMID: 11978462.
Nikose AS, Saha D, Laddha PM, Patil M. Surgically induced astigmatism after phacoemulsification by temporal clear corneal and superior clear corneal approach: a comparison. Clin Ophthalmol. 2018;12:65–70. https://doi.org/10.2147/OPTH.S149709. PMID: 29379266.
Singh M, Mishra D, Sinha BP, Anand A, Singhal S. Corneal endothelial protection during manual small-incision cataract surgery: A narrative review. Indian J Ophthalmol. 2022;70(11):3791–6. https://doi.org/10.4103/ijo.IJO_1048_22. PMID:36308098;PMCID:PMC9907305.
Gatinel D, Adam PA, Chaabouni S, et al. Comparison of corneal and total ocular aberration before and after myopic LASIK. J Refract Surg. 2010;26(5):333e340.
Sarver FJ, Sanders DR, Vukich JA. Image quality in myopic eyes corrected with laser in situ keratomileusis and phakic intraocular lens. J Refract Surg. 2003;19(4):397e404.
Pérez-Vives C, Ferrer-Blasco T, Madrid-Costa D, et al. Optical quality comparison of conventional and hole-visian implantable collamerr lens at different degrees of decentering. Am J Ophthalmol. 2013;156(1):69–76.
Sun WK, Yang H, Yoon G, et al. Higher-order aberration changes after implantable collamerr lens implantation for myopia - sciencedirect. Am J Ophthalmol. 2011;151(4):653-662.e1.
Torii H, Negishi K, Watanabe K, et al. Changes in Higher-Order Aberrations After Iris-Fixated Phakic Intraocular Lens Implantation[J]. J Refract Surg. 2013;29(10):693–700.
Hashemian SJ, Farrokhi H. Ocular higher-order aberrations changes after implantable collamerr lens implantation for high myopic astigmatism. J Curr Ophthalmo. 2018;30:136e141.
Salmon TO, Vand PC. Normal-eye Zernike coefficients and root-mean-square wavefront errors. J Cataract Refract Surg. 2006;32(12):2064–74.
Jauhari N, Chopra D, Chaurasia RK, Agarwal A. Comparison of surgically induced astigmatism in various incisions in manual small incision cataract surgery. Int J Ophthalmol. 2014;7:1001–4. https://doi.org/10.3980/j.issn.2222-3959.2014.06.16. PMID: 25540754.
Lim R, Borasio E, Ilari L. Long-term stability of keratometric astigmatism after limbal relaxing incisions. J Cataract Refract Surg. 2014;40:1676–81. https://doi.org/10.1016/j.jcrs.2014.01.045. PMID: 25155388.
Pfleger T, Skorpik C, Menapace R, Scholz U, Weghaupt H, Zehetmayer M. Long-term course of induced astigmatism after clear corneal incision cataract surgery. J Cataract Refract Surg. 1996;22:72–7. https://doi.org/10.1016/s0886-3350(96)80273-2. PMID: 8656367.
Kim H, Whang WJ, Joo CK. Corneal astigmatism in patients after cataract surgery: a 10-year followup study. J Refract Surg. 2016;32:404–9. https://doi.org/10.3928/1081597X-20160303-01. PMID: 27304604.
Cataract and IOL Group, Chinese Medical Association Ophthalmology Branch. Expert consensus on the clinical application of astigmatism-correcting IOLs in China (2017). Chin J Ophthalmol. 2017;53(1):4.
Langenbucher A, Szentmáry N, Cayless A, Casaza M, Weisensee J, Hoffmann P, Wendelstein J. Surgically induced astigmatism after cataract surgery - a vector analysis. Curr Eye Res. 2022;47(9):1279–87. https://doi.org/10.1080/02713683.2022.2052108. Epub 2022 Jul 25 PMID: 35380484.
Piao J, Joo CK. Site of clear corneal incision in cataract surgery and its effects on surgically induced astigmatism. Sci Rep. 2020;10(1):3955.
Tonn B, Klaproth OK, Kohnen T. Anterior surface-based keratometry compared with Scheimpflug tomography-based total corneal astigmatism. Invest Ophthalmol Vis Sci. 2014;56(1):291.
Funding
Supported by The National Natural Science Foundation of China, No. 81960183; and The Science and Technology Fund of the Health and Health Commission of Guizhou Province, gzwkj2022-158.
Author information
Authors and Affiliations
Contributions
T-T D, H-Y L, L-L L, and Z-Z L collected the patient's clinical information and analyzed and interpreted the clinical data; T-T D wrote the draft of this manuscript; T-X L reviewed and edited the manuscript; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol adhered to the tenets of the Declaration of Helsinki, and Zunyi Medical University’s institutional ethics committee approved the study (Approval (20212)-524). A written informed consent regarding surgery was obtained from each patient before surgery. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dan, TT., Liu, TX., Luo, Hy. et al. The comparison of corneal higher-order aberration and surgically induced astigmatism between the clear corneal incision and the limbus tunnel incision of posterior chamber implantable collamer lens implantation. BMC Ophthalmol 24, 40 (2024). https://doi.org/10.1186/s12886-024-03311-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03311-1