Abstract
Background
Triamcinolone acetonide (TA) is administered as an intravitreal or posterior sub-Tenon’s capsule injection, as treatment for diabetic macular edema (DME). The intravitreal use of TA is limited because commercially available triamcinolone acetonide contains benzyl alcohol, a neurotoxic preservative. Few studies have compared effects of preservative-free intravitreal TA (IVTA) and posterior sub-Tenon capsule TA (STTA) injections for DME. Thus, herein, we compared the effectiveness of preservative-free IVTA and STTA for treatment of bevacizumab-resistant DME.
Methods
In this retrospective cohort study, bevacizumab-resistant DME was defined as a lack of response to at least three consecutive intravitreal bevacizumab (IVB) injections. Changes in mean central macula thickness (CMT), best-corrected visual acuity (BCVA), and intraocular pressure (IOP) between IVTA and STTA groups were compared at baseline and at 1, 2, and 3 months after treatment.
Results
Forty eyes from 40 patients were included in this study. In the IVTA group, the mean CMT improved significantly from 400.2 ± 144.42 μm at baseline to 288.35 ± 151.74 μm at 3 months after treatment (p = 0.01). Similarly, in the STTA group, the mean CMT improved significantly from 446.65 ± 120.74 μm at baseline to 382.9 ± 113.58 μm at 3 months after treatment (p = 0.009). The mean BCVA of the IVTA group also showed improvement, decreasing from 0.75 ± 0.55 logarithm of the minimum angle of resolution (logMAR) at baseline to 0.625 ± 0.50 logMAR at 3 months after treatment (p = 0.089). Similarly, the mean BCVA of the STTA group improved, from 0.6 ± 0.36 logMAR at baseline to 0.54 ± 0.35 logMAR at 3 months after treatment (p = 0.094).
Conclusion
Given that IVTA and STTA demonstrated statistically equivalent anatomical and functional effects in patients with bevacizumab-resistant DME, the less invasive STTA may be considered the preferred treatment approach for the management of bevacizumab-resistant DME.
Trial registration
Retrospectively registered.
Similar content being viewed by others
Background
Diabetic macular edema (DME) is a well-known cause of long-term visual impairment in patients with diabetes mellitus (DM) [1]. According to the Early Treatment Diabetic Retinopathy Study (ETDRS), DME is defined as retinal thickening at or within 2 disc-diameters of the macular center, with or without accompanying definitive hard exudates in this area [2].
The pathophysiology of DME is multifactorial and involves a complex interplay between various biochemical, cellular, and molecular processes. Persistent hyperglycemia leads to the loss of pericytes, leukostasis, overexpression of vascular endothelial growth factor (VEGF) and angiotensin II, and the accumulation of advanced glycation end-products, all of which induce vascular inflammation. These changes eventually result in breakdown of the blood–retinal barrier and development of DME [3]. With the increasing life expectancy of the population, the number of patients with diabetes is rising, escalating the importance of DME treatment.
DME treatment has evolved rapidly from the era of laser therapy to the era of anti-VEGF pharmacotherapy. Numerous studies have demonstrated the anatomical and functional efficacy of anti-VEGF agents for the treatment of DME [2, 4,5,6,7]. Although monthly injections of anti-VEGF agents have been shown to be effective in previous studies, other strategies, such as pro re nata or treat and extend treatment have been proposed [8].
As steroid therapy can reduce inflammation in DME [9], intravitreal steroids have been used to treat DME [10]. Therefore, when DME persists despite repeated anti-VEGF injections, steroids can be considered an alternative treatment. However, steroids are associated with disadvantages, such as cataracts, increased intraocular pressure (IOP), and glaucoma [11, 12]. Steroids can be administered via three major routes: intravitreal implant, intravitreal injection, and posterior sub-Tenon’s capsule injection. The BEVORDEX trial demonstrated that dexamethasone implants yielded clinical results similar to those of bevacizumab for DME treatment [13]. Triamcinolone acetonide (TA) is used intravitreally [13,14,15] or via the posterior sub-Tenon capsule [16,17,18].
Although various long-acting steroid implants have been approved for the treatment of DME, TA is still used intravitreally or as a posterior sub-Tenon’s capsule injection because of its low cost and convenience of injection. The intravitreal use of TA is limited because commercially available triamcinolone acetonide contains benzyl alcohol, a neurotoxic preservative [19]. Few studies have compared the effects of preservative-free intravitreal TA (IVTA) and posterior sub-Tenon’s capsule TA (STTA) injections for DME [20, 21].
In this study, we aimed to compare the effectiveness of preservative-free IVTA and STTA in the treatment of bevacizumab-resistant DME.
Methods
We reviewed 108 patients who previously underwent IVTA or STTA for bevacizumab-resistant DME between April 2020 and May 2022. In this study, “refractory” DME was defined by the absence of response to three consecutive bevacizumab injections in patients with DME. This retrospective study adhered to the tenets of the Declaration of Helsinki, and the collection of data was carried out in accordance with the approval of the Institutional Review Board of Incheon St. Mary’s Hospital, Korea. All patients provided written informed consent after receiving an explanation of the potential risks associated with IVTA or STTA.
The criteria for participant inclusion in the study were as follows: [1] presence of DME involving the fovea that did not respond to at least three consecutive IVB injections over a period of more than 3–6 months (we defined bevacizumab-resistant DME as a lack of response to IVB if the central macula thickness (CMT) did not decrease after IVB); [2] CMT > 300 μm; [3] absence of steroid use (eye drops or injections); [4] availability of optical coherence tomography (OCT) images for a period of ≥ 3 months after treatment; [5] glycemic levels, indicated by a hemoglobin A1c (HbA1c) of < 8.0%; and [6] no conventional laser treatment in the last 1 year. The criteria for participant exclusion in the study were as follows: [1] presence of other retinal diseases, including age-related macular degeneration, retinal vascular occlusion, polypoidal choroidal vasculopathy, glaucoma, and pathologic myopia; [2] history of vitrectomy; [3] previous ocular surgery, including cataract surgery, within the last 6 months; [4] history of focal laser or panretinal photocoagulation conducted for < 1 year; and [5] previous IVTA or STTA treatment.
To administer IVTA, the patient was placed in a supine position, and topical 0.5% proparacaine (Alcaine, Alcon, Geneve, Switzerland) anesthetic eye drops were applied. We mixed 1 mL of normal saline with preservative-free TA (Maqaid, Wakamoto Pharmaceutical Co., Ltd., Tokyo, Japan) (40 mg/1 bottle), extracted a volume of 0.1 ml (4 mg/0.1 mL) using a 30-gauge needle on a 1-mL syringe, and injected it through the superotemporal pars plana (3.0-mm posterior to the limbus) area. After injection, the patients were prescribed topical 0.5% moxifloxacin eye drops (Vigamox, Novartis, Basel, Switzerland) for 5 days.
To administer STTA, the patient was placed in the supine position, and topical 0.5% proparacaine anesthesia eye drops were applied. A volume of 1.0 mL (40 mg/mL) of TA (DongKwang, Seoul, Korea) was injected into the inferotemporal sub-Tenon’s capsule area using a 30-gauge needle on a 1-mL syringe. After injection, the patients were prescribed topical 0.5% moxifloxacin eye drops for 5 days.
To assess the efficacy of IVTA and STTA, all patients underwent ophthalmological examination, including slit-lamp evaluation, best-corrected visual acuity (BCVA), IOP, and swept-source OCT (Topcon DRI OCT, Topcon, Tokyo, Japan) at baseline and at 1, 2, and 3 months after treatment. BCVA was measured using a standard Snellen chart and was converted to the logarithm of the minimum angle of resolution (logMAR). OCT was performed after pupil dilatation. OCT was used to detect CMT using a macular cube scan protocol (central 6 × 6 mm2 area).
Statistical analysis
Changes in BCVA, CMT, and IOP from baseline to the 1-, 2- and 3-month visits were analyzed using Wilcoxon’s signed-rank test. Changes in CMT and BCVA between the IVTA and STTA groups were assessed using the Mann–Whitney U test. Statistical analyses were conducted using SPSS (version 24.0; SPSS Inc., Chicago, IL, USA).
Results
Of the 108 participants, 68 were excluded owing to the following reasons: an HbA1c level of > 8% (58 participants), a follow-up period of < 3 months (5 participants), and the presence of an accompanying retinal vein occlusion (5 participants). Finally, 40 individuals were included in this study. Forty eyes of 40 patients who received IVTA (20 eyes of 20 patients, 9 males and 11 females) or STTA (20 eyes of 20 patients, 8 males and 12 females) for the treatment of bevacizumab-resistant DME. The inclusion and exclusion criteria were predefined for participant selection. Patient demographic and disease characteristics are presented in Table 1. No significant differences in demographic and disease characteristics were observed between the IVTA and STTA groups (Table 1). There was no significant difference in the mean CMT at baseline between the STTA and IVTA groups.
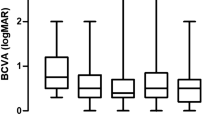
The mean CMT of both the IVTA group and the STTA group improved significantly over the course of 3 months (Table 2). The mean BCVA of the IVTA group improved from 0.75 ± 0.55 logMAR at baseline to 0.6 ± 0.45 logMAR at 1 month after treatment (p = 0.036), while the mean BCVA of the STTA group improved from 0.6 ± 0.36 logMAR at baseline to 0.53 ± 0.35 logMAR at 1 month after treatment (p = 0.016) (Fig. 1). Even though seven of the 40 eyes required anti-glaucoma drugs, no significant changes in the mean IOP of both groups were noted at 1, 2, and 3 months after treatment as compared to baseline (Table 2). SD-OCT images of representative patients are presented in Fig. 2.
Representative images comparing results of IVTA and posterior STTA injections in patients with bevacizumab-resistant DME. (A) Initial presentation of the left eye of a 59-year-old male patient who showed no response to IVB injections. SD-OCT reveals diffuse retinal thickening and serous retinal detachment associated with diabetic macular edema. (B) Resolution of macular edema and serous retinal detachment at 1 month after IVTA injection. (C) Complete resolution of serous retinal detachment and cystic change at 2 months after treatment. (D) Slight aggravation of serous retinal detachment and macular edema at 3 months after treatment. (E) Initial presentation of the left eye of a 57-year-old male patient who showed no response to IVB injections. SD-OCT shows diffuse retinal thickening and serous retinal detachment associated with diabetic macular edema. (F) Complete resolution of serous retinal detachment and macular edema at 1 month after posterior STTA injection. (G) Sustained macular edema at 2 months after treatment. (H) Slight aggravation of macular edema at 3 months after treatment. DME, diabetic macular edema; IVB, intravitreal bevacizumab; IVTA, intravitreal triamcinolone acetonide; SD-OCT, Spectral-domain optical coherence tomography; STTA, sub-Tenon’s capsule triamcinolone acetonide
Comparing the changes in CMT and BCVA between the IVTA and STTA groups at baseline and at 1, 2, and 3 months of treatment, no statistically significant difference was observed (Table 2).
Additionally, 1 month after injection, four patients in the IVTA group and three patients in the STTA group experienced a minor elevation in IOP, ranging from 22 to 25 mmHg. Nevertheless, all patients showed a return to normal IOP after the administration of a combination of topical carbonic anhydrase inhibitors and beta-blocker eye drops. No injection-related adverse events, such as secondary glaucoma, sterile or infectious endophthalmitis, and retinal detachment, were observed during the 3-month follow-up period.
Discussion
In this study, we demonstrated that preservative-free IVTA and STTA exhibited equivalent anatomical and functional effects in bevacizumab-resistant DME. Although no statistically significant differences were observed between the two groups, when observed over the course of 3 months after a single injection, IVTA exhibited approximately a 60-µm greater reduction in CMT and showed an improvement in BCVA of approximately 0.06 logMAR, equivalent to around three additional ETDRS letters. Increased IOP was noted in four cases in the IVTA group and in three cases in the STTA group. Therefore, the proportions of patients using anti-glaucoma medication were similar between the two groups. While the mean IOP in the IVTA group decreased slightly, the mean IOP increased in the STTA group. In the IVTA group, the mean IOP decreased by 0.25 mmHg at 3 months compared to baseline, which may have been due to the use of anti-glaucoma medication. In the STTA group, on the other hand, the mean IOP increased by 0.1 mmHg at 3 months compared to baseline. Although the change in mean IOP was not statistically significant (p = 0.342), the increase in IOP was numerically greater in the STTA group than in the IVTA group. Therefore, in patients with bevacizumab-resistant DME, both IVTA and STTA were effective. Although differences were not statistically significant, IVTA showed better reduction in CMT, better BCVA improvement, and a decrease in IOP compared to STTA.
Steroids have been used to treat DME owing to their anti-inflammatory mechanisms, inhibition of VEGF synthesis, and stabilization of vascular hyper-permeability [22,23,24,25]. Among the various steroids, TA is widely used for DME treatment because of its excellent anti-angiogenic and anti-inflammatory properties and its ability to stabilize blood–retinal barrier effects [26]. In real-world settings of DME treatment, switching to steroids for cases resistant to anti-VEGF therapy is a commonly used and effective strategy, with favorable results [27, 28]. In addition, STTA has also been reported to be effective in treating other diseases causing cystoid macular oedema, such as Irvine-Gass syndrome [29].
Both IVTA and STTA commonly use high local steroid concentrations, minimizing the risk of systemic side effects. Various studies have compared the efficacy of IVTA and STTA in patients with DME. Previous systematic reviews have indicated that, in a short-term follow-up period of 3 months, IVTA generally outperformed STTA in terms of CMT and BCVA [26]. However, no previous study has compared bevacizumab-resistant DME focusing on a limited number of patients with long-term unresponsiveness to anti-VEGF therapy. Interestingly, our findings revealed no significant differences in the effects of IVTA and STTA. This may be attributable to the underlying pathogenesis of DME. The lack of response to anti-VEGF therapy suggested the presence of low VEGF levels.
Similar to previous studies, our study demonstrated that both IVTA and STTA were most effective in terms of CMT reduction during the first month after injection, with effectiveness gradually diminishing thereafter [20]. However, in terms of BCVA, a beneficial effect was observed only in the first month after treatment with no subsequent improvement, indicating that the functional effect was not as pronounced as the anatomical effect.
Inoue et al. recently reported that IVTA injection results in significantly higher vitreous concentrations of the steroid (1.22 ± 0.24 µg/ml) compared to STTA injection (< 0.001 µg/ml) [30]. IVTA directly delivers the drug, whereas STTA passes through the choroid and sclera differently. This difference in the administration routes can lead to inadequate drug delivery. Owing to the higher concentration of IVTA, the incidence of side effects such as intraocular pressure elevation, glaucoma, and cataracts has been found to be higher with IVTA than with STTA [31, 32]. However, in this study, STTA showed a similar effect to IVTA, despite its lower concentration, which suggests that the integrity of the blood–retinal barrier may have been compromised, leading to a reduced therapeutic effect.
Pharmacologic studies have demonstrated that after IVTA injection, the drug concentration is high at 1 month, resulting in effective outcomes, and that this effect is maintained for up to 3 months [33]. Similarly, animal model experiments have demonstrated that the effect of STTA was maintained pharmacokinetically for months [34]. Therefore, side effects, such as elevated IOP, can be attributed most to the first month. In our study, 7 of 40 patients (17.5%) showed an increased IOP in the first month after injection. This is similar to the previously reported occurrence rate of elevated IOP after steroid injection, which ranged from 10 to 30% [35,36,37].
There are several limitations in our study. First, we employed a retrospective, nonrandomized study design. Second, the inclusion of only a small number of cases may have affected the generalizability of our findings. Third, although all patients received at least three consecutive bevacizumab injections before steroid injection, other anti-VEGF agents may have been considered for the treatment of bevacizumab-resistant DME. Lastly, due to the short observation period, we were unable to ascertain long-term complications, such as the potential occurrence of severely increased IOP > 25 mmHg, secondary glaucoma, or cataract, which are common side effects of steroid injections.
Conclusions
In this study, we demonstrated that IVTA and STTA exhibited statistically equivalent anatomical and functional effects in patients with bevacizumab-resistant DME. Given that STTA is less invasive compared with IVTA, STTA may be considered a preferred treatment approach for the management of DME. However, conducting a future study with a larger sample size and long-term follow-up would yield valuable insights into this topic.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- BCVA:
-
best-corrected visual acuity
- CMT:
-
central macula thickness
- DM:
-
diabetes mellitus
- DME:
-
diabetic macular edema
- ETDRS:
-
Early Treatment Diabetic Retinopathy Study
- HbA1c:
-
hemoglobin A1c
- IOP:
-
intraocular pressure
- IVB:
-
intravitreal bevacizumab
- IVTA:
-
preservative-free intravitreal TA
- logMAR:
-
logarithm of the minimum angle of resolution
- OCT:
-
optical coherence tomography
- STTA:
-
posterior sub-Tenon capsule TA
- TA:
-
triamcinolone acetonide
- VEGF:
-
vascular endothelial growth factor
References
Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91:1464–74.
Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806.
Scholl S, Augustin A, Loewenstein A, Rizzo S, Kupperman B. General pathophysiology of macular edema. Eur J Ophthalmol. 2011;21(Suppl 6):10–9.
Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77e35.
Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123:2376–85.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25.
Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, et al. The REVEAL study: Ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology. 2015;122:1402–15.
Prünte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, Studnička J, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100:787–95.
Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–9.
Patelli F, Fasolino G, Radice P, Russo S, Zumbo G, FM DIT, et al. Time course of changes in retinal thickness and visual acuity after intravitreal triamcinolone acetonide for diffuse diabetic macular edema with and without previous macular laser treatment. Retina. 2005;25:840–5.
Jonas JB, Kreissig I, Degenring R. Secondary chronic open-angle glaucoma after intravitreal triamcinolone acetonide. Arch Ophthalmol. 2003;121:729–30.
Gillies MC, Islam FM, Larsson J, Pasadhika S, Gaston C, Zhu M, et al. Triamcinolone-induced cataract in eyes with diabetic macular oedema: 3-year prospective data from a randomized clinical trial. Clin Exp Ophthalmol. 2010;38:605–12.
Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121:2473–81.
Jonas JB, Kreissig I, Söfker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121:57–61.
Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–24. discussion 24– 5.
Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol. 1995;120:55–64.
Bakri SJ, Kaiser PK. Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am J Ophthalmol. 2005;139:290–4.
Ozdek S, Bahçeci UA, Gürelik G, Hasanreisoğlu B. Posterior subtenon and intravitreal triamcinolone acetonide for diabetic macular edema. J Diabetes Complications. 2006;20:246–51.
Craig DB, Habib GG. Flaccid paraparesis following obstetrical epidural anesthesia: possible role of benzyl alcohol. Anesth Analg. 1977;56:219–21.
Bonini-Filho MA, Jorge R, Barbosa JC, Calucci D, Cardillo JA, Costa RA. Intravitreal injection versus Sub-tenon’s infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Invest Ophthalmol Vis Sci. 2005;46:3845–9.
Cardillo JA, Melo LA Jr., Costa RA, Skaf M, Belfort R Jr., Souza-Filho AA, et al. Comparison of intravitreal versus posterior Sub-tenon’s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2005;112:1557–63.
Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–9.
Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006;27:413–26.
Andrés-Blasco I, Gallego-Martínez A, Machado X, Cruz-Espinosa J, Di Lauro S, Casaroli-Marano R et al. Oxidative stress, inflammatory, angiogenic, and apoptotic molecules in proliferative diabetic retinopathy and diabetic macular edema patients. Int J Mol Sci. 2023;24.
Saklatvala J. Glucocorticoids: do we know how they work? Arthritis Res. 2002;4:146–50.
Qi HP, Bi S, Wei SQ, Cui H, Zhao JB. Intravitreal versus subtenon triamcinolone acetonide injection for diabetic macular edema: a systematic review and meta-analysis. Curr Eye Res. 2012;37:1136–47.
Busch C, Zur D, Fraser-Bell S, Laíns I, Santos AR, Lupidi M, et al. Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol. 2018;55:789–96.
Hong IH, Choi W, Han JR. The effects of intravitreal triamcinolone acetonide in diabetic macular edema refractory to anti-VEGF treatment. Jpn J Ophthalmol. 2020;64:196–202.
Karasu B, Kesim E, Kaskal M, Celebi ARC. Efficacy of topical dexamethasone eye drops in preventing ocular inflammation and cystoid macular edema following uncomplicated cataract surgery with or without injection of a single dose perioperative subtenon triamcinolone acetonide. Cutan Ocul Toxicol. 2022;41:310–7.
Inoue M, Takeda K, Morita K, Yamada M, Tanigawara Y, Oguchi Y. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol. 2004;138:1046–8.
Choi YJ, Oh IK, Oh JR, Huh K. Intravitreal versus posterior subtenon injection of triamcinolone acetonide for diabetic macular edema. Korean J Ophthalmol. 2006;20:205–9.
Cellini M, Pazzaglia A, Zamparini E, Leonetti P, Campos EC. Intravitreal vs. subtenon triamcinolone acetonide for the treatment of diabetic cystoid macular edema. BMC Ophthalmol. 2008;8:5.
Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–6.
Park HJ, Lee JE, Kim SI, Pak KY, Oum BS, Lee JS, et al. Intravitreal pharmacokinetics after posterior subtenon triamcinolone acetonide injection in vitrectomized rabbit eyes. Retina. 2014;34:801–6.
Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–7.
Wingate RJ, Beaumont PE. Intravitreal triamcinolone and elevated intraocular pressure. Aust N Z J Ophthalmol. 1999;27:431–2.
Maeda Y, Ishikawa H, Nishikawa H, Shimizu M, Kinoshita T, Ogihara R, et al. Intraocular pressure elevation after subtenon triamcinolone acetonide injection; Multicentre retrospective cohort study in Japan. PLoS ONE. 2019;14:e0226118.
Funding
No funding or grant support.
Author information
Authors and Affiliations
Contributions
All authors attest that they meet the current ICMJE criteria for Authorship. Collect and analyzed data: SHJ, MK, YJR; Wrote the paper: SHJ, MK, YJR; Approved final version of the manuscript: SHJ, YJR.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study adhered to the tenets of the Declaration of Helsinki, and data collection was conducted in compliance with and approved by the Institutional Review Board of Incheon St. Mary’s Hospital, Korea. All patients provided written informed consent after receiving an explanation of the potential risks associated with IVTA or STTA.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jeon, S.H., Kim, M. & Roh, YJ. Comparison of intravitreal preservative-free triamcinolone versus posterior sub-tenon triamcinolone acetonide injection for bevacizumab-resistant diabetic macular edema. BMC Ophthalmol 24, 25 (2024). https://doi.org/10.1186/s12886-024-03291-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03291-2