Abstract
Background
To develop a dynamic prediction model for diabetic retinopathy (DR) using systemic risk factors.
Methods
This retrospective study included type 2 diabetes mellitus (T2DM) patients discharged from the Second Affiliated Hospital of Kunming Medical University between May 2020 and February 2022. The early patients (80%) were used for the training set and the late ones (20%) for the validation set.
Results
Finally, 1257 patients (1049 [80%] in the training set and 208 [20%] in the validation set) were included; 360 (28.6%) of them had DR. The areas under the curves (AUCs) for the multivariate regression (MR), least absolute shrinkage and selection operator regression (LASSO), and backward elimination stepwise regression (BESR) models were 0.719, 0.727, and 0.728, respectively. The Delong test showed that the BESR model had a better predictive value than the MR (p = 0.04899) and LASSO (P = 0.04999) models. The DR nomogram risk model was established according to the BESR model, and it included disease duration, age at onset, treatment method, total cholesterol, urinary albumin to creatinine ratio (UACR), and urine sugar. The AUC, kappa coefficient, sensitivity, specificity, and compliance of the nomogram risk model in the validation set were 0.79, 0.48, 71.2%, 78.9%, and 76.4%, respectively.
Conclusions
A relatively reliable DR nomogram risk model was established based on the BESR model.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Diabetes mellitus (DM) is a chronic metabolic disease caused by multiple factors, where patients with type 2 DM (T2DM) account for the greatest number of those with DM [1]. Systemic complications of T2DM can greatly shorten life expectancy, leading to disability [2], and even endanger patients’ lives [3]. Diabetic retinopathy (DR) is one of the most common and serious complications of diabetic microangiopathy in T2DM. A previous study showed that the prevalence of DR varies across regions, reaching approximately 18.7% in southwest China [4].
Currently, the diagnosis of DR relies on a combination of the slit lamp, color fundus photography, and fundus fluorescein angiography (FFA) by physicians experienced in fundus disease or skilled technicians, which might reduce the accuracy of DR diagnosis in primary community hospitals [5]. In addition, the likelihood of a clinical cure for DR is low, and early detection and intervention are of essential importance for reducing DR-induced visual loss [6]. Therefore, developing a simple and feasible DR prediction tool is urgent.
It is well known that some factors are associated with DR, such as hyperglycemia (a 1% reduction in HbA1C level was reported to be associated with a 35% reduced risk of DR, 15-25% reduced risk of DR progression, 25% reduced risk of vision-threatening DR, and 1% reduced risk of blindness), diabetes duration (duration ≥ 20 years was reported to be associated with DR in 50-90% of patients), hypertension (a 10 mm Hg reduction in systolic blood pressure was reported to be associated with a 40-50% reduced risk in DR progression), cataract surgery, nephropathy, and pregnancy [4, 7,8,9]. A recent study described a machine learning model based on 17 variables; however,such models are complicated to use in the everyday clinical setting [10].
Therefore, this study aimed to develop a convenient and dynamic prediction model for DR using RGMS-II parameters in combination with conventional systemic risk factors.
Methods
Study design and populations
This retrospective study included T2DM patients from the electronic medical record system of the Second Affiliated Hospital of Kunming Medical University between May 2020 and February 2022. The inclusion criteria were: 1) > 18 years of age; 2) diagnosis of T2DM; 3) complete medical information. Patients with a history of vitreoretinal diseases such as vitreoretinal surgery, retinal laser photocoagulation, glaucoma, or poor-quality fundus photography images that affected DR assessment were excluded.
This study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University. Due to the retrospective nature of the study, the ethics committee waived the requirement for informed consent.
80% of the patients included in the early phase of this study were selected as the training set, while the other 20% of patients included in the later phase were selected as the validation set.
Data collection
Detailed data were collected for medical records, including demographic characteristics (e.g., sex and age), laboratory indications [e.g., urine albumin-creatinine ratio (UACR), serum insulin, lipid accumulation product (LAP), and high-density lipoprotein cholesterol (HDL-C)], ambulatory glucose data, color fundus photography images, and medical history, based on previous literature and expert recommendations (Supplementary Table 1). LAP was calculated according to the formulas described by Kahn [11]: LAP male = [waist circumference (cm) − 65] × TG (mmol/L), LAP female = [waist circumference (cm) − 58] × TG (mmol/L). For relatively thin patients (waist circumference < 65 and 58 cm for men and women, respectively), waist circumference was manually modified to 66 cm and 59 cm, respectively, to avoid negative LAP values. The duration of DM was determined according to clinical experience. The age of onset of T2DM (AOO) was classified as described by Yeung et al. [12], and AOO ≤ 40 years was classified as early-onset DM. The diastolic blood pressure (DBP) and systolic blood pressure (SBP) were calculated as the mean value of the first 3 days of hospitalization and were classified as normal blood pressure, high normal blood pressure, and hypertension according to the 2018 Chinese Guidelines for the Management of Hypertension [13]. Urinary albumin abnormality was classified according to the American Diabetes Association (ADA) criteria [14].
DR was diagnosed according to the 2002 International Clinical Diabetic Retinopathy Disease Severity Scale [15]. Nonproliferative abnormalities included microaneurysms, intraretinal hemorrhage, venous beading, and intraretinal microvascular abnormalities. Proliferative abnormalities included neovascularization, vitreous hemorrhage, and anterior retinal hemorrhage (Supplementary Fig. 2). This study used a 45˚ 6.3-megapixel digital wheal-free camera (Canon, Japan) to acquire images of each eye, separately for the macula and the center of the optic disc. In order to ensure the accuracy of the input data, all data and fundus photography images were independently collected by two clinicians and checked and summarized by a third clinician.
Statistical analysis
SPSS 23.0 (IBM, Armonk, NY, USA) and R 4.1.1 software (The R Project for Statistical Computing, www.r-project.org) were used for statistical analysis. Continuous data that conformed to normal distribution were described as means ± SD, and compared with the independent t-test. Continuous data with skewed distribution were described as median [interquartile range (IQR)] and compared using the Mann-Whitney U-test. Categorical data were described as frequencies (percentages) and analyzed using the chi-square test. Pearson and Spearman correlation analyses were used to explore the correlations between independent variables. Univariable logistic regression analyses were used to assess the significance of independent variables in the training set. The variables with statistical significance (P < 0.05) were analyzed by multivariable logistic regression to establish the multivariate regression (MR) model. The “glmnet” package was used for least absolute shrinkage and selection operator (LASSO) variable selection. The “glm” function and the “MASS” package were used in the backward mode to establish the backward elimination stepwise regression (BESR) model. The receiver operating characteristics (ROC) of the DR prediction model were compared using Delong’s test. The “rms” package was used to plot the DR nomogram risk model according to the DR prediction model with the highest prediction value. All statistical analyses were two-sided, and P < 0.05 was considered statistically significant.
Results
Characteristics of the patients
A total of 1456 T2DM patients were screened, of which 199 (13.7%) were excluded due to incomplete medical records, and 1257 (86.3%) patients were finally included: 468 (37.2%) women and 789 (62.8%) men (p = 0.393). DR was found in 297 (28.0%) patients in the training set (n = 1049) and 66 (31.7%) in the validation set (n = 208) (Table 1; Fig. 1).
Establishment of DR prediction model
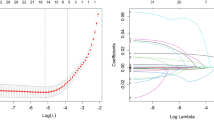
Figure 2 and Supplementary Table 2 show the correlations between variables. Univariable regression analysis was performed first (Supplementary Table 3), and the variables associated with DR were analyzed by MR (Table 2). The results showed that longer duration of disease (10–19 years vs. <10 years: OR = 1.64 [1.14–2.34]; 20–29 years vs. <10 years, OR = 3.64 [2.13–6.26]), treatment (insulin vs. no treatment: OR = 2.48 [1.49–4.19]; oral antidiabetic drugs vs. no treatment: OR = 2.01 [1.28–3.22]; combination therapy vs. no treatment: OR = 2.38 [1.43–4.01]), UACR (UACR 30–300 vs. <30: OR = 1.82 [1.20–2.73]; UACR > 300 vs. <30: OR = 2.91 [1.07–8.14]), and urine sugar positivity compared to negative (OR = 1.65 [1.20–2.28]) were potential risk factors for the development of DR, while AOO > 40 years (OR = 0.65 [0.46–0.92]) was a potential protective factor for concomitant DR. The independent variables screened by the LASSO model were disease duration (OR = 1.99 [1.61–2.46]), treatments (OR = 1.23 [1.06–1.42]), TC (OR = 1.18 [1.06–1.32]), UACR (OR = 2.04 [1.61–2.57]), and urine sugar positive (OR = 1.65 [1.20–2.28]) (Table 2 and Supplementary Fig. 1).
The BESR was used to select the best model according to the minimal Akaike information criterion (AIC, 1111.39), which included 11 variables. After excluding non-significant independent variables, the remaining six variables independently associated with DR and included in the final model (Table 2) were longer disease duration (10–19 years vs. <10 years: OR = 1.83 [1.28–2.61]; and 20–29 years vs. <10 years: OR = 4.11 [2.44-7.00]), AOO > 40 years (OR = 0.68 [0.48–0.97]), treatment method (insulin vs. no treatment: OR = 2.52 [1.52–4.25]; oral antidiabetic drugs vs. no treatment: OR = 1.84 [1.17–2.94]; and combination therapy vs. no treatment: OR = 2.27 [1.37–3.79]), TC (OR = 1.18 [1.06–1.32]), UACR (UACR 30–300 vs. <30: OR = 1.87 [1.28–2.73]; and UACR > 300 vs. <30: OR = 3.49 [2.00-6.15]), and urine sugar positive (OR = 1.54 [1.10–2.16]).
The ROC curves for the three regression models were plotted (Fig. 3). The AUCs for MR, LASSOR, and BESR were 0.719 (0.684, 0.755), 0.727 (0.693, 0.761), and 0.728 (0.694, 0.762), respectively. The Delong test showed that the BESR model had a better predictive value than the MR (P = 0.04899) and LASSO (P = 0.04999) models, indicating that the BESR model had the highest ability to predict DR.
The comparison regarding area under receiver operating curves of the three regression clinical models in the training set. Fit1: Multivariable Regression Analysis; fit2: Least Absolute Shrinkage and Selection Operator Regression Analysis; fit3: Backward Elimination Stepwise Regression Analysis; ROC: receiver operator characteristic; AUC: area under the ROC curve
Construction and verification of the DR nomogram risk model
The nomogram risk model was established according to the BESR model (Fig. 4). In the validation set, the nomogram showed high predictive performance (Table 3). Specifically, the AUC, kappa coefficient, optimal cutoff, sensitivity, specificity, and compliance were 0.79, 0.48, 0.35, 71.2%, 78.9%, and 76.4%, respectively. The McNemar test showed no significant difference between the positivity rate reported by the prediction model and the actual positivity rate derived from the diagnostic criteria (P = 0.152).
(a) Nomogram Prediction Model for DR in the training test. (b) Dynamic Nomogram Prediction Model for DR in the training set. https://yixueyanjiu.shinyapps.io/DynNomapp/
Discussion
Over recent years, there has been an increasing number of studies on clinical prediction models for DR [10, 17,18,19]. However, the complexity of the pathogenesis of DR and the heterogeneity of the population limit the clinical dissemination of relevant research results. In this study, the BESR-based model showed high predictive performance in an independent validation set. The model indicated that younger AOO, long disease duration, history of insulin or oral antidiabetic drug therapy, elevated TC, elevated UACR, and positive urine sugar were independent risk factors for developing DR in patients with T2DM. This study might help improve the efficiency of DR screening and benefit more patients with T2DM. In the past, it was the view was believed that T2DM is a metabolic disease mainly associated with aging [20]; however, due to increasing social pressures and lifestyle changes, T2DM has begun to occur at an ever younger age, which has become a global trend, especially in the Chinese population with the highest disease burden of T2DM [21]. Most studies have shown that a younger age of T2DM onset is associated with an increased risk of DR [22, 23]. The results of the present study showed that late-onset DM (i.e., AOO > 40 years) [24] was a protective factor for DR. In their study, Huang et al. [23] confirmed that a younger AOO of T2DM increases the risk of DR and that such patients might also have metabolic disorders of glucose, lipids, and amino acids due to long-term hyperglycemia. The “high glucose metabolic memory” after returning blood glucose to normal levels can induce and exacerbate the inflammatory response through the histone deacetylase sirtuin-1 (SIRT1)-mediated LKB1/AMPK/ROS signaling pathway and promote apoptosis of retinal vascular endothelial cells [25]. The duration of the disease is a non-modifiable risk factor. This study showed a 1.83-fold increased risk of DR in T2DM patients with a disease duration > 10 years and a further increased risk of DR in patients with a disease duration > 20 years, thus confirming the key role of T2DM disease duration in DR progression. Bek [22] retrospectively investigated the relationship between the course of T2DM and the onset and progression of DR in 17,461 patients and showed that the risk of DR was very low in the first 10 years after onset; however, in patients with a disease course > 10 years, the risk of DR increased with time after the onset of T2DM. In order to reduce the occurrence of blinding DR, age should be moderately limited at the screening to help identify the presence of retinal microvascular damage early so that patients can be provided with appropriate intervention or guidance early in the course of DR.
As a chronic metabolic disease, conventional treatment strategies for T2DM include oral antidiabetic drugs, insulin injections, and lifestyle interventions. Still, it remains unclear whether the various treatments promote microvascular complications in patients with T2DM. Metformin is the first-line drug for treating T2DM [26], inhibiting retinal neovascularization and inflammation [27]. This study showed an increased risk of DR development in patients treated with oral antidiabetic drugs but also in patients treated with insulin. These results suggest that the treatment of T2DM is also a risk factor for developing DR. In agreement with our findings, Alemu et al. [28] showed that patients treated with insulin only had a higher risk of DR than T2DM patients treated with glycemic combination therapy. Bain et al. [29] suggested that the occurrence of DR is related to the “permeability theory”. When poor glycemic control rapidly changes to tight control, with the rapid decrease of HBA1c, intravascular osmotic pressure decreases sharply and the gradient of intra- and extracellular osmotic pressure increases, leading to changes in the retinal microvasculature, which is sensitive to pressure changes and tends to result in retinal edema. However, some scholars believe that this theory is not a good explanation for the proliferative changes in DR patients and have proposed the “synergistic hypothesis” [30]. This theory suggests that exogenous insulin acts synergistically with VEGF to cause proliferative changes in retinal microvasculature. In vitro studies also seem to support the hypothesis that Nox4-derived ROS regulate insulin-induced hypoxia-inducible factor-1α (HIF-1a) and VEGF expression through PI3K/AKT and ERK1/2 pathways; however, increased VEGF levels are involved in neovascularization and blood-retinal barrier disruption, leading to microangioma formation and vascular leakage [31]. Since all subjects in this study were hospitalized, most patients had suboptimal glycemic control. Therefore, we hypothesized that insulin therapy is associated with a high risk of developing DR and may be associated with a rapid reduction in blood glucose. Paradoxically, the early Diabetes Control and Complications Trial (DCCT) [32] looked at 726 patients with T2DM without DR and 715 patients with T2DM and mild-to-moderate nonproliferative diabetic retinopathy (NPDR) and showed a relatively higher risk of DR development and progression in the intensive treatment group; however, at subsequent longer follow-up, the risk of DR progression was lower in the intensive treatment group than in the conventional treatment. In addition, once DR progression occurred, visual improvement was more pronounced in the patients in the intensive treatment group. Therefore, it could be hypothesized that insulin treatment is associated with a high risk of DR development and possibly related to the rapid reduction of blood glucose [33]. Future studies should examine these hypotheses in order to further confirm the relationship between diabetes treatment and DR risk. DM and diabetic nephropathy (DN) are the two most common microvascular complications in patients with T2DM, and there are many similarities in the pathogenesis and progression between DN and DR [34]. The present study showed high UACR and urine sugar positivity were risk factors for developing DR in patients with T2DM. Romero et al. [35] conducted a 10-year prospective study showing that both eGFR and UACR were important risk factors for DR, with UACR having a more prominent effect on DR than eGFR. In addition, the levels of other renal function-related indicators, such as urea, creatinine, uric acid, and urine metal analysis, were higher in the DR group than in the NDR group in the univariable analyses, which could also indicate the possibility of renal injury. Therefore, a regular review should be performed for the early detection of diabetic microvascular complications.
Furthermore,our results showed that the BESR model had a significantly higher predictive value than the MR and LASSO models and that the BESR model had the highest ability to predict DR. The nomogram showed high predictive performance of DR, which is consistent with previous studies [17, 18]. The nomogram by Li et al. [17] included diabetic peripheral neuropathy (DPN), age, neutrophilic granulocyte (NE), HDL-C, hemoglobin A1c (HbA1C), duration of T2DM, and glycosylated serum protein (GSP). Still, GSP is not routinely measured in many hospitals, limiting the applicability of the nomogram. Chen et al. [18] developed a nomogram for DR based on age, diabetes duration, HbA1c, albuminuria, and triglycerides, all of which are routine variables;however, their AUC was lower than ours. Although the predictive performance of the model developed in this study was not particularly excellent (Table 4), an online DR prediction tool based on several easily accessible clinical indicators may help to improve the efficiency of DR screening and benefit more patients with T2DM (https://yixueyanjiu.shinyapps.io/DynNomapp/).
There are some limitations in this study. Although the data used to validate the prediction model in this study were independent of the modeling data, patient selection bias might exist since the data were from a single medical center. In future studies, we plan to collaborate with other centers and use their clinical data to conduct a more extensive and in-depth external validation of the prediction model.Moreover, although the GV parameters were calculated and analyzed, they were not included in the final nomogram risk model. The association between GV and DR needs to be further explored.
Conclusions
A relatively reliable DR nomogram risk model was established according to the BESR model, including disease duration, age at onset, treatment method, total cholesterol, UACR, and urine sugar.
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- DM:
-
Diabetes mellitus
- T2DM:
-
Type 2 DM
- DR:
-
Diabetic retinopathy
- MR:
-
Multivariate Regression
- BESR:
-
Backward elimination stepwise regression
- FFA:
-
Fundus fluorescein angiography
- UACR:
-
Urine albumin-creatinine ratio
- LAP:
-
Lipid accumulation product
- HDL-C:
-
High-density lipoprotein cholesterol
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
- ADA:
-
American Diabetes Association
- LASSO:
-
Least absolute shrinkage and selection operator
- ROC:
-
Receiver operating characteristics
- NPDR:
-
Nonproliferative diabetic retinopathy
- DN:
-
Diabetic nephropathy
- NE:
-
Neutrophilic granulocyte
- GSP:
-
Glycosylated serum protein
References
Allende-Vigo MZ. Diabetes mellitus prevention. Am J Ther. 2015;22(1):68–72.
Rodrigues GR, Rao MV, Fernandes M, Mendonca T. Diabetes and vision loss. Diabet medicine: J Br Diabet Association. 2021;38(1):e14402.
Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2020;162:108086.
Song P, Yu J, Chan KY, Theodoratou E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010803.
Wong TY, Sabanayagam C. Strategies to Tackle the Global Burden of Diabetic Retinopathy: from epidemiology to Artificial Intelligence. Ophthalmologica. 2020;243(1):9–20.
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on Diabetic Eye Care: the International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608–22.
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012.
Ng SM, Ayoola OO, McGuigan MP, Chandrasekaran S. A multicentre study evaluating the risk and prevalence of diabetic retinopathy in children and young people with type 1 diabetes mellitus. Diabetes Metab Syndr. 2019;13(1):744–6.
Schreur V, van Asten F, Ng H, Weeda J, Groenewoud JMM, Tack CJ, et al. Risk factors for development and progression of diabetic retinopathy in dutch patients with type 1 diabetes mellitus. Acta Ophthalmol. 2018;96(5):459–64.
Li W, Song Y, Chen K, Ying J, Zheng Z, Qiao S, et al. Predictive model and risk analysis for diabetic retinopathy using machine learning: a retrospective cohort study in China. BMJ Open. 2021;11(11):e050989.
Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26.
Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2(12):935–43.
Liu J. Highlights of the 2018 chinese hypertension guidelines. Clin Hypertens. 2020;26:8.
American Diabetes Association Professional Practice C. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):17–S38.
Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–82.
Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446–55.
Li Y, Li C, Zhao S, Yin Y, Zhang X, Wang K. Nomogram for Prediction of Diabetic Retinopathy among Type 2 Diabetes Population in Xinjiang, China. Diabetes Metab Syndr Obes. 2022;15:1077–89.
Chen X, Xie Q, Zhang X, Lv Q, Liu X, Rao H. Nomogram Prediction Model for Diabetic Retinopathy Development in Type 2 Diabetes Mellitus Patients: A Retrospective Cohort Study. J Diabetes Res. 2021;3825155.
Cichosz SL, Johansen MD, Knudsen ST, Hansen TK, Hejlesen O. A classification model for predicting eye disease in newly diagnosed people with type 2 diabetes. Diabetes Res Clin Pract. 2015;108(2):210–5.
Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019;13(2):1165–72.
Pan J, Jia W. Early-onset diabetes: an epidemic in China. Front Med. 2018;12(6):624–33.
Bek T. Systemic risk factors contribute differently to the development of proliferative diabetic retinopathy and clinically significant macular oedema. Diabetologia. 2020;63(11):2462–70.
Huang JX, Liao YF, Li YM. Clinical features and microvascular complications risk factors of early-onset type 2 diabetes Mellitus. Curr Med Sci. 2019;39(5):754–8.
Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–82.
Zheng Z, Chen H, Li J, Li T, Zheng B, Zheng Y, et al. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61(1):217–28.
Hsu SK, Cheng KC, Mgbeahuruike MO, Lin YH, Wu CY, Wang HD et al. New Insight into the Effects of Metformin on Diabetic Retinopathy, Aging and Cancer: Nonapoptotic Cell Death, Immunosuppression, and Effects beyond the AMPK Pathway. Int J Mol Sci. 2021;22(17).
Han J, Li Y, Liu X, Zhou T, Sun H, Edwards P, et al. Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS ONE. 2018;13(3):e0193031.
Alemu Mersha G, Alimaw YA, Woredekal AT. Prevalence of diabetic retinopathy among diabetic patients in Northwest Ethiopia-A cross sectional hospital based study. PLoS ONE. 2022;17(1):e0262664.
Bain SC, Klufas MA, Ho A, Matthews DR. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: a review. Diabetes Obes Metab. 2019;21(3):454–66.
Jingi AM, Tankeu AT, Ateba NA, Noubiap JJ. Mechanism of worsening diabetic retinopathy with rapid lowering of blood glucose: the synergistic hypothesis. BMC Endocr Disord. 2017;17(1):63.
Meng D, Mei A, Liu J, Kang X, Shi X, Qian R, et al. NADPH oxidase 4 mediates insulin-stimulated HIF-1alpha and VEGF expression, and angiogenesis in vitro. PLoS ONE. 2012;7(10):e48393.
Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16.
Ricard N, Bailly S, Guignabert C, Simons M. The quiescent endothelium: signalling pathways regulating organ-specific endothelial normalcy. Nat Reviews Cardiol. 2021;18(8):565–80.
Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus - A comprehensive review. J Diabetes Complications. 2020;34(8):107613.
Romero-Aroca P, Baget-Bernaldiz M, Navarro-Gil R, Moreno-Ribas A, Valls-Mateu A, Sagarra-Alamo R et al. Glomerular Filtration Rate and/or Ratio of Urine Albumin to Creatinine as Markers for Diabetic Retinopathy: A Ten-Year Follow-Up Study. J Diabetes Res. 2018;5637130.
Funding
This work is supported by the National Natural Science Foundation of China (Grant No. 81860173).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: CH Z, X Z, YP Z. Analyzed the data: CH Z, X Z. Contributed materials/analysis tools: CH Z, LQ Z, MJ M, YN Y. Wrote the paper: CH Z, YP Z, X Z. We thank the Natural Science Foundation of China (Approval No. 81860173) for the financial support.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University (Review - PJ − 2020 − 132). As a retrospective study, the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University waived the requirement for individual informed consent. The study was carried out in accordance with the applicable guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., Zhou, L., Ma, M. et al. Dynamic nomogram prediction model for diabetic retinopathy in patients with type 2 diabetes mellitus. BMC Ophthalmol 23, 186 (2023). https://doi.org/10.1186/s12886-023-02925-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-023-02925-1