Abstract
Purpose
To study the incidence and characteristics of bacillary layer detachment (BALAD) occurring with the two most common choroidal malignancies, choroidal metastasis and choroidal melanoma.
Methods
A retrospective multicentric record analysis. Eyes with a diagnosis of choroidal melanoma or choroidal metastasis that had good-quality fundus photography and spectral domain optical coherence tomography (OCT) scans of the macular and tumor regions allowing for delineation of the retinal layers were included for analysis. Qualitative image evaluation was done by two independent graders for the presence, location, and OCT features of BALAD, as well as any associated intraretinal or subretinal fluid. Demographic and clinical data were also retrieved.
Results
Of the 11 eyes with choroidal metastasis and 7 eyes with choroidal melanoma that were included in the final analysis, 6 (54.5%) and 1 (14.3%) had BALAD, respectively. The BALAD co-localized with the subretinal fluid in all cases and with the intraretinal fluid in 1/3 cases (33.3%), was foveal in location in 3 eyes (42.9%), was overlying the tumor in 6 eyes (85.7%), and varied in number and size. Reflectivity within the BALAD was consistently higher than the vitreous and adjacent subretinal fluid, and discernable suspended hyperreflective particles were noted in 5 eyes (71.4%).
Conclusion
BALAD is relatively common with choroidal metastasis. The OCT features described supplement our recognition of this new entity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Bacillary layer detachment (BALAD) is a recently recognized optical coherence tomography (OCT) finding that describes a split within the inner segments of the photoreceptors, just posterior to the external limiting membrane (ELM) [1]. While not pathognomonic for a specific disease, BALAD has been increasingly associated with inflammatory and infiltrative choroidal pathologies and has been suggested to occur due to an influx of fluid into the subretinal space that exceeds the pump capacity of the retinal pigment epithelium (RPE) with an intact ELM [1,2,3].
Special attention has recently been given to studying BALAD as a prognostic indicator in exudative macular pathologies, especially neovascular age-related macular degeneration [2,3,4,5], and in inflammatory choroidal conditions such as Vogt-Koyanagi-Harada disease [6]. This may have led to an overrepresentation of these cases within the literature [1]. Isolated case reports have described BALAD with other ocular pathologies, where the incidence and spectrum of the finding is less clear, including blunt eye trauma [7, 8], the pachychoroid spectrum of disorders [9, 10], choroidal tuberculous granuloma [11], and other causes of choroiditis [12, 13]. Two case reports [10, 14] described the occurrence of BALAD with choroidal metastasis, and another one [15] described it with the rare entity of unilateral diffuse uveal melanocytic proliferation. Herein, we study the incidence and characteristics of BALAD occurring with the two most common choroidal malignancies: choroidal metastasis and choroidal melanoma.
Methods
Study design and setting
This observational multicentric retrospective analysis involved two centers, the ophthalmology department at Ain Shams university hospitals and a private eye center (Al Mashreq Eye Center), both located in Cairo, Egypt. The study was approved by the research ethics committee at the Faculty of Medicine, Ain Shams University (FMASU MD120/2021). The extracted patient information was de-identified to ensure anonymity and the study adhered to the tenets of the Declaration of Helsinki.
Data extraction and inclusion criteria
Charts of patients with a confirmed diagnosis of choroidal melanoma or choroidal metastasis during the four-year interval of 2018 to 2022 were retrospectively reviewed for the availability of OCT imaging at the time of diagnosis. The diagnosis of choroidal melanoma was based on the modified criteria proposed by Shields et al. [16] in addition to documented growth on serial ultrasonography and/or fundus photography, while the diagnosis of choroidal metastasis was based on the clinical history, documentation of primary malignancy elsewhere, and multimodal imaging of the choroidal tumor. We only included eyes with adequate quality spectral-domain OCT B scans (signal strength ≥ 40) within the macula (centered around the fovea) and passing through the tumor region. The availability of fundus photography was another requirement. Eyes that had concurrent macular pathology that may result in intraretinal fluid (IRF) or subretinal fluid (SRF) accumulation (e.g., diabetic maculopathy, retinal vascular occlusive disease, and macular neovascularization) and those with ocular media opacity that prevented adequate identification of individual retinal layers on OCT were excluded from the analysis.
Extracted patient data included age, sex, laterality, choroidal melanoma tumor dimensions on presentation measured by B-scan ultrasonography, and primary tumor origin for choroidal metastases. Ocular clinical course, treatment modality, and follow up data were also retrieved when available. Best corrected visual acuity (BCVA) was recorded in Snellen format and later converted to the logarithm of the minimal angle of resolution (logMAR) with counting fingers, hand motion, light perception, and no light perception being designated as 2.10, 2.40, 2.70, and 3.00 logMAR, respectively. Intraocular pressure (IOP) measurements using an air puff tonometer on presentation were also recorded.
Image grading
The qualitative analysis of OCT scans was done by two independent retina specialists with open adjudication on any disagreements until full consensus was reached. Radial, raster or line scans passing through the fovea and through the tumor region were assessed for the presence of intraretinal fluid (IRF), subretinal fluid (SRF), and BALAD. The diagnosis of BALAD was based on the previously reported [1, 3] anatomical description of an abnormal dome-shaped elevation located within the outer retina, having an outer boundary corresponding to the ellipsoid zone (EZ) and an elevated inner boundary corresponding to the ELM. In cases with indistinct EZ along the suspected lesion, the margins (corners) of the elevation were evaluated and BALAD was diagnosed if a split between the EZ and the ELM could be detected. The reflectivity of the BALAD was compared to that of the vitreous cavity and adjacent SRF compartments. Subretinal hyperreflective material (SHRM) at the floor of the BALAD and discernable suspended hyperreflective particles within its lumen were documented. The number and size of the BALAD were also assessed. The size of the BALAD was considered small if its longest horizontal diameter was smaller than that of the optic disc, otherwise, it was considered of large size.
Statistical analysis
All data were anonymized and entries were made into an Excel (Microsoft 365) sheet. Data were analyzed using the Statistical Package for the Social Sciences version 25 (IBM, NY, USA). We presented numeric data as both mean ± standard deviation (SD) and median, and categorical variables as frequency (%). Where appropriate, the Student’s t-test was used to compare means between groups.
Results
Patient demographics
Our search initially identified 29 eyes, 17 with choroidal melanoma and 12 with choroidal metastasis. Of those, 10 eyes with choroidal melanoma and 1 eye with choroidal metastasis were excluded due to unavailability or poor-quality of OCT scans. A summary of the demographics and basic clinical information of the analyzed sample (17 patients, 18 eyes) are presented in Table 1. Two of the eyes with choroidal metastasis belonged to the same patient. The mean (± SD) age of the patients in the melanoma group was 57.1 (± 14) years, and in the metastasis group was 62.2 (± 10.4) years. Eyes with BALAD in both cohorts belonged to younger patients on average (melanoma: 38 years; metastasis: 58.3 ± 11 years) compared to eyes without BALAD (melanoma: 60.3 ± 12.2 years; metastasis: 66.8 ± 8.5 years, p = 0.193). For eyes with choroidal metastasis, BCVA was better on average in eyes with BALAD (1.4 ± 0.9 logMAR) compared to those without BALAD (2.0 ± 0.7 logMAR, p = 0.256). The most common primary sites of origin were the breast (7 eyes, 63.6%) and lung (2 eyes, 18.2%).
Choroidal melanoma with BALAD
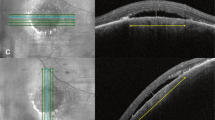
All 7 eyes with choroidal melanoma had SRF, and 2 eyes (28.6%) had IRF. We could detect BALAD in 1 eye (14.3%) that belonged to a 38-year-old male patient (Fig. 1). On examination, he had a BCVA of 20/20 in the right eye and 20/400 in the left eye. The fundus of the left eye had an amelanotic dome-shaped mass within the temporal macula (Fig. 1A), the height of the which was 4.9 mm, and the basal diameter was 11 mm on B-scan ultrasonography (Fig. 1B). Optical coherence tomography revealed localized SRF and cystoid IRF collections overlying the tumor, together with evidence of foveal BALAD adjacent to the tumor margin (Fig. 1C). Discernable suspended hyperreflective particles and SHRM were detected in the lumen and the floor of the BALAD, respectively. The patient elected to undergo plaque brachytherapy in his original care facility and no follow-up data was available.
Choroidal melanoma with BALAD. Montaged fundus photography of a left eye in panel (A) depicts a lower temporal macular amelanotic mass with overlying pigment deposition and cystic changes. On B-scan ultrasonography the tumor is dome shaped with low to moderate internal reflectivity (B). Optical coherence tomography line scan passing through the fovea and the nasal edge of the tumor (C) shows localized SRF (red asterisk), diffuse cystoid edema over sloping edge of the tumor (green asterisks), and BALAD (blue asterisks). The BALAD is noted in the foveal region, adjacent to the tumor margin, and co-localizing with other fluid compartments. Hyperreflective material can be seen at the floor of the BALAD obscuring part of the EZ and RPE underneath it
Choroidal metastasis with BALAD
All eyes with choroidal metastasis had SRF and 4/11 eyes (36.4%) had IRF overlying the tumor. Bacillary layer detachment could be detected in 6/11 eyes (54.5%). Eyes with BALAD had metastases that originated from primary breast malignancy in most cases (5/6, 83.3%), while 1 eye belonged to a patient that had a carcinoma of unknown primary. In all eyes with BALAD, the tumor involved the macular region (Table 1).
The BALAD was located underneath the fovea (2 eyes, 33.3%), in the perifoveal area (2 eyes), or in the peripapillary region (2 eyes). It co-localized with the SRF in all eyes and did not localize with the IRF in 2/2 eyes. The reflectivity within the BALAD was consistently higher than that of the vitreous and adjacent SRF compartments in all eyes, and discernable suspended hyperreflective particles could be detected within the BALAD in 4/6 eyes (66.6%). We could not detect SHRM at the floor of the BALAD in any of the cases. Regarding the morphology of BALAD, it was single and large in 4/6 eyes (66.6%), multiple and small in 1 eye (16.7%), and single and small in 1 eye. The inner boundary formed by the ELM and the outer boundary formed by the EZ were discernable along the course of BALAD in all eyes (Figs. 2 and 3). One eye (Fig. 3E, F) belonging to a 51-year-old female had multiple vertical hyperreflective lines traversing the thickness of the BALAD from the EZ to the ELM, dividing the BALAD into separate compartments.
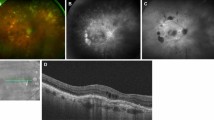
A case of choroidal metastasis with BALAD in a 40-year-old female patient with breast cancer. Fundus examination revealed multiple temporal choroidal masses with overlying SRF involving the foveal region (A). Radial scans centered over the fovea (B, C) and a raster scan over the lower macula (D) show SRF accumulation (red asterisks) involving the fovea and temporal macula, and BALAD (blue asterisks) localized to the lower parafoveal and lower temporal macular areas. White arrowheads point towards the site of splitting between the EZ and the ELM. Compared to SRF which appear as clear hyporeflective spaces, BALAD consistently demonstrates moderately hyperreflective material (turbidity) within the lumen. Yellow arrows point to the direction of the radial OCT B scan
Representative cases of OCT appearance of BALAD in eyes with choroidal metastasis. A 67-year-old female patient with multiple choroidal metastases in both eyes from breast cancer. The right eye scans (A,B) show SRF beneath the fovea and nasally (red asterisks), parafoveal IRF (green asterisk), and a single small BALAD in the nasal macula within the peripapillary region (blue asterisk). The left eye scans (C,D) show nasal SRF (red asterisk) and a single large BALAD in the upper peripapillary region (blue asterisk). A 51-year-old with choroidal metastasis in the left eye from breast cancer, with OCT scans (E,F) showing extensive SRF accumulation (red asterisk) and a single large BALAD in the upper perifoveal region (blue asterisk). Multiple vertical hyperreflective lines are seen traversing the thickness of the BALAD. Yellow arrows point to the direction of the OCT B scan
Two patients (3 eyes, 50%) were treated and followed up at the same study location, with a duration of follow up of 13 and 18 months. Treatment was by external beam radiotherapy in both patients. The three eyes showed improvement of BCVA from logMAR 2.4, 0.8 and 0.2 to logMAR 0.5, 0.5 and 0, respectively. Follow-up OCT scans were unavailable or inadequately tracked to original scans to allow comparison in two eyes; scans including the area with BALAD (foveal) were only available for 1 eye and showed complete resolution of the BALAD with partial resolution of the adjacent SRF 2 months after therapy.
Discussion
Our current understanding of BALAD is in its early stages, with multiple questions that remain unanswered regarding its etiology, pathology, incidence with different ocular diseases, and prognostic implications [1]. In this imaging analysis of 18 eyes with malignant choroidal tumors, we demonstrate that BALAD is not that uncommon in eyes with choroidal malignancy, occurring in over half of the studied eyes with choroidal metastasis and in 1 of 7 eyes with choroidal melanoma. We also analyzed the patient demographics, tumor characteristics and OCT features of BALAD among our studied cohort, the latter supplements our recognition of this new entity across different pathologies.
Studies on BALAD detection have majorly focused on its prognostic implications in macular neovascularization (reported incidence range, 4.5 − 7.4%) [2,3,4,5, 17] and inflammatory choroidal pathologies such as Vogt-Koyanagi-Harada disease (reported incidence, 94.9%) [6, 18]. Case reports or small case series have also described BALAD with other inflammatory ocular pathologies as acute posterior multifocal placoid pigment epitheliopathy [13, 19, 20] and serpiginous-like choroidopathy associated with tuberculosis [12, 21], infiltrative ones such as unilateral diffuse uveal melanocytic proliferation [15] and choroidal metastasis [14], degenerative lesions as macular telangiectasia that was associated with acute leakage and hemorrhage [22], and blunt eye trauma (reported incidence, 8% [7]). The incidence of BALAD with most of these pathologies is less recognized owing to the lack of structured analyses. Here, we provide preliminary incidence data on BALAD occurrence with the two most common types of choroidal malignancy and demonstrate an incidence of 54.5% and 14.3% with choroidal metastasis and melanoma, respectively. Recently, Guner et al. [23] reported on the SRF characteristics of a large sample of choroidal melanomas (236 patients). They found the incidence of BALAD to be 28.4%, and that it was more common in medium-sized tumors with larger SRF, which is consistent with our described case of medium-sized malignant melanoma and BALAD.
Agarwal [24] postulated that retinal acute fluid accumulation is responsible for the photoreceptor fracture seen with BALAD, and that the rapidity by which fluid collects in the outer retina is the most important determinant of BALAD occurrence. However, this does not explain the development of BALAD in eyes with gradual fluid accumulation, as would presumably occur with choroidal malignancy such as the cases described here. Ramtohul et al. [1] theorized that the pathophysiology of BALAD is related to breakdown of the RPE component of the outer blood-retinal barrier, which may be related to choroidal ischemia and compression of the choriocapillaris. Previous reports utilizing enhanced depth imaging OCT to study eyes with choroidal melanoma [25] and choroidal metastasis [26] have documented shared features between both tumor types, including choriocapillaris thinning/compression, RPE attenuation/atrophy, and structural loss of the photoreceptors’ interdigitation zone and EZ. Nevertheless, without sufficient histopathological evidence, the inciting feature for BALAD and the shared pathophysiology between both tumor types remains to be confirmed.
The appearance of BALAD in our cohort was consistent with the description found in previous reports [1, 6, 27]. The lesion could be described as a dome-shaped space that develops within the outer retina between two hyperreflective bands, the EZ externally and the ELM internally. Increased reflectivity within the BALAD compared to the vitreous and adjacent SRF was a consistent finding in all our cases. In the literature review by Ramtohul et al. [1], increased reflectivity within the BALAD was found in 90.8% of the reported cases. In the same review, suspended hyperreflective granules were noted in 77.1% of the cases; similarly, we could detect these particles in 5 of the 7 eyes with BALAD (71.4%). The BALAD co-localized with SRF in all 7 eyes and with IRF in 1/3 eyes (33.3%) across our sample. This is consistent with the findings by Ramtohul et al. [1] where BALAD was more commonly detected adjacent to SRF (77.5%) than IRF (5.6%). SHRM was only noted in our described case of BALAD with choroidal melanoma, which appeared to be continuous with hyperreflective material within the BALAD (Fig. 1C). In the recent report but Guner et al. [23] 58% of the eyes with BALAD were associated with heterogenous hyperreflective material. The size and number of BALAD varied among our cases, some were subtle and small (Figs. 1C and 2D), and others were very large (Fig. 3D, E), with the single large BALAD being the most detected morphology. Of note, one eye with choroidal metastasis and a single large overlying BALAD exhibited a distinct appearance of multiple vertical hyperreflective lines or septa, dividing the BALAD into separate compartments (Fig. 3E, F). These septa were long recognized in the OCT scans of eyes with Vogt-Koyanagi-Harada disease [28], and were recently described as part of the BALAD presentation. Hypotheses regarding their composition include fibrin-like material or migrating mitochondria [1, 13].
Our study provides preliminary information on the incidence and imaging features of BALAD with two different ocular malignancies. The study of BALAD in malignant choroidal tumors provides a unique opportunity for its longitudinal analysis with the slowly evolving fluid accumulation and gradual resolution after therapy. Furthermore, enucleated eyes for large choroidal tumors that had BALAD could serve in the histopathological analysis of these lesions. There are, however, limitations to our study. This was a retrospective study, which is a common theme to all currently available studies on BALAD owing to its recent description. Considering the dynamic evolution of BALAD, the retrospective review of records would be limited in picking up all cases and determining true incidence, as well as in following up the lesions and determining their prognostic implications. Further, the small sample size limits the accurate derivation of incidence rates and underpowers the analysis in detecting small differences between eyes that developed BALAD and those that did not in order to suggest any prognostic implications of BALAD. Future prospective studies or post-hoc analyses of existing datasets with larger samples are warranted to address these issues.
In conclusion, we demonstrate the occurrence of BALAD with the two most common types of intraocular malignancy. We report an incidence rate of 54.5% in eyes with choroidal metastasis and 14.3% in eyes with choroidal melanoma. The reported OCT features of BALAD in the setting of choroidal malignancy supports our recognition of this new feature across different ocular pathology.
Data Availability
Derived data supporting the findings of this study are available from the corresponding author on request.
Abbreviations
- BALAD:
-
bacillary layer detachment
- BCVA:
-
best corrected visual acuity
- ELM:
-
external limiting membrane
- EZ:
-
ellipsoid zone
- IOP:
-
intraocular pressure
- IRF:
-
intraretinal fluid
- LogMAR:
-
logarithm of the minimal angle of resolution
- mm:
-
millimeters
- OCT:
-
optical coherence tomography
- RPE:
-
retinal pigment epithelium
- SD:
-
standard deviation
- SRF:
-
subretinal fluid
References
Ramtohul P, Engelbert M, Malclès A, Gigon E, Miserocchi E, Modorati G, et al. Bacillary Layer Detachment: Multimodal Imaging and Histologic Evidence of a Novel Optical Coherence Tomography Terminology: literature review and proposed theory. Retina. 2021;41:2193–207.
Yordi S, Sarici K, Cetin H, Lunasco LM, Le TK, Sevgi DD et al. Bacillary Detachment in Neovascular Age-Related Macular Degeneration: Incidence, Clinical Features, and Response to Anti-VEGF Therapy. Ophthalmol Retin. 2022.
Jung JJ, Soh YQ, Yu DJG, Rofagha S, Lee SS, Freund KB et al. Bacillary Layer Detachment Because of Macular Neovascularization. Retina. 2021;41:2106–14.
Ramtohul P, Malclès A, Gigon E, Freund KB, Introini U, Bandello F, et al. Long-term outcomes of Bacillary Layer detachment in Neovascular Age-Related Macular Degeneration. Ophthalmol Retin. 2022;6:185–95.
Kim JH, Kim JW, Kim CG, Bacillary Layer Detachment in. A Korean Cohort with Neovascular Age-Related Macular Degeneration. Retina. 2022;42:1028–37.
Agarwal A, Freund KB, Kumar A, Aggarwal K, Sharma D, Katoch D, et al. Bacillary Layer Detachment in Acute Vogt-Koyanagi-Harada Disease: a Novel swept-source Optical Coherence Tomography Analysis. Retina. 2021;41:774–83.
Venkatesh R, Agrawal S, Reddy NG, Pereira A, Yadav NK, Chhablani J. Bacillary layer detachment in acute nonpenetrating ocular trauma. Can J Ophthalmol. 2021.
Tekin K, Teke MY. Bacillary layer detachment: a novel optical coherence tomography finding as part of blunt eye trauma. Clin Exp Optom. 2019;102:343–4.
Fuganti RM, Casella AM, Roisman L, Zett C, Maia M, Farah ME, et al. Case series bacillary layer detachment associated with acute central serous chorioretinopathy in patients with COVID-19. Am J Ophthalmol Case Reports. 2022;28:101690. https://doi.org/10.1016/j.ajoc.2022.101690.
Cicinelli MV, Giuffré C, Marchese A, Jampol LM, Introini U, Miserocchi E, et al. The Bacillary detachment in posterior segment ocular Diseases. Ophthalmol Retin. 2020;4:454–6. https://doi.org/10.1016/j.oret.2019.12.003.
Markan A, Aggarwal K, Gupta V, Agarwal A. Bacillary layer detachment in tubercular choroidal granuloma: a new optical coherence tomography finding. Indian J Ophthalmol. 2020;68:1944–6.
Socci da Costa D, Gomes E, Silva A, Melichar A, Neves DB, Correa PA, Moraes RT. Bacillary layer detachment in serpiginous-like choroiditis of presumed intraocular tuberculosis: report of two cases. Am J Ophthalmol case Rep. 2022;27:101653.
Kohli GM, Bhatia P, Shenoy P, Sen A, Gupta A. Bacillary Layer Detachment in Hyper-acute stage of acute posterior multifocal Placoid Pigment Epitheliopathy: a Case Series. Ocul Immunol Inflamm. 2022;30:703–6.
Gaillard M-C, Vaclavik V, DeGottrau P, Odermatt R, Schiappacasse L, Schalenbourg A. Bacillary Layer detachment (BALAD) in Macular Choroidal Metastasis of a low-grade rectal adenocarcinoma. Klin Monbl Augenheilkd. 2022;239:582–5.
Tekchandani U, Singh R, Dogra M. Bacillary Layer detachment in unilateral diffuse Uveal Melanocytic Proliferation. Ophthalmol Retin. 2021;5:760.
Shields CL, Lim L-AS, Dalvin LA, Shields JA. Small choroidal melanoma: detection with multimodal imaging and management with plaque radiotherapy or AU-011 nanoparticle therapy. Curr Opin Ophthalmol. 2019;30:206–14.
Venkatesh R, Reddy NG, Agrawal S, Pereira A, Yadav NK, Chhablani J. Bacillary layer detachment on optical coherence tomography in exudative age-related macular degeneration. Eur J Ophthalmol. 2021:11206721211064016.
Atas F, Kaya M, Saatci AO. The effect of pulse steroid treatment of ten days’ long on the improvement of bacillary layer detachment in a patient with Vogt-Koyanagi Harada disease. Romanian J Ophthalmol. 2021;65:183–6.
Ramtohul P, Denis D, Gascon P. Bacillary Layer detachment in Acute posterior multifocal Placoid Pigment Epitheliopathy: a Multimodal Imaging Analysis. Retina. 2021;41:e12–4.
Kuroiwa DAK, Ribeiro LZ, Regatieri CVS. Acute posterior multifocal placoid pigment epitheliopathy with bacillary layer detachment. Am J Ophthalmol. 2022;235:e345–6.
Rodríguez-Vidal C, Galletero Pandelo L, Artaraz J, Fonollosa A. Bacillary layer detachment in a patient with serpiginoid choroiditis. Indian J Ophthalmol. 2022;70:2687–9.
Ramtohul P, Comet A, Denis D, Gascon P. Hemorrhagic Bacillary Layer detachment in Macular Telangiectasia Type 2. Retina. 2021;41:e42–3.
Guner MK, Ferenchak K, Olsen TW, Dalvin LA. Optical Coherence Tomography Findings in Choroidal Melanoma-Associated Subretinal Fluid. Retina. 2022;42:2159–68.
Agarwal A, Commentary. Bacillary layer detachment in retinochoroidal pathologies: the concept of retinal acute fluid accumulation. Indian J Ophthalmol - Case Reports. 2021;1:665–6.
Shields CL, Kaliki S, Rojanaporn D, Ferenczy SR, Shields JA. Enhanced depth imaging Optical Coherence Tomography of Small Choroidal Melanoma. Arch Ophthalmol. 2012;130:850.
Al-Dahmash SA, Shields CL, Kaliki S, Johnson T, Shields JA. Enhanced Depth Imaging Optical Coherence Tomography of Choroidal Metastasis in 14 Eyes. Retina. 2014;34:1588–93.
Mehta N, Chong J, Tsui E, Duncan JL, Curcio CA, Freund KB, Presumed Foveal Bacillary Layer Detachment in a Patient with Toxoplasmosis Chorioretinitis and Pachychoroid Disease, et al. Retinal cases & brief reports. 2021;15:391–8.
Lin D, Chen W, Zhang G, Huang H, Zhou Z, Cen L, et al. Comparison of the optical coherence tomographic characters between acute vogt-koyanagi-harada disease and acute central serous chorioretinopathy. BMC Ophthalmol. 2014;14:87.
Acknowledgements
None.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
YAF conceived the idea and design. YAF & NA collected the data and graded the images. MN adjudicated conflict in image analysis. All authors contributed to the data interpretation. YAF drafted the manuscript with contributions from DMA and WME. AGS supervised the work and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the research ethics committee at the Faculty of Medicine, Ain Shams University (FMASU MD120/2021). The extracted patient information were de-identified to ensure anonymity, and, thus, informed consent was waived by the research ethics committee at the Faculty of Medicine, Ain Shams University (FMASU MD120/2021). All methods were carried out in accordance with the Declaration of Helsinki. The study is retrospective and did not include any experimental protocols.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fouad, Y.A., Salman, A.G., Ashour, D.M. et al. Bacillary layer detachment with malignant choroidal tumors: a case series. BMC Ophthalmol 23, 142 (2023). https://doi.org/10.1186/s12886-023-02892-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-023-02892-7