Abstract
Background
In randomized, controlled trials of open-angle glaucoma (OAG) or ocular hypertension (OHT), bimatoprost 0.01 % improved tolerability while retaining the intraocular pressure (IOP)-lowering efficacy of bimatoprost 0.03 %. Given geographic/racial differences in glaucoma presentation, the APPEAL study assessed the occurrence and severity of hyperemia produced by bimatoprost 0.01 %, and its efficacy, in the Taiwanese clinical setting.
Methods
In this multicenter, open-label, observational study, treatment-naïve and previously treated patients with OHT or OAG received once-daily bimatoprost 0.01 % for 12 weeks. Hyperemia (primary endpoint) was graded at baseline, week 6, and week 12 using a photonumeric scale (0, +0.5, +1, +2, +3), grouped (≤ +1, none to mild; ≥ +2, moderate to severe), and reported as unchanged from baseline, improved, or worsened. IOP assessments followed the same schedule. Supplemental efficacy analyses were conducted based on previous therapies.
Results
The intent-to-treat population (N = 312) included treatment-naïve (13.5 %) and previously treated (86.5 %) patients; mean age was 53.3 years. At baseline, 46.3 % of previously treated patients were receiving prostaglandin analog (PGA) monotherapy. At week 12, 91.2 %, 5.9 %, and 2.9 % of treatment-naïve patients exhibited unchanged, worsened, and improved hyperemia from baseline, respectively; 77.9 %, 12.9 %, and 9.2 % of previously treated patients showed no change, worsening, and improvement, respectively. There were no statistically significant shifts in hyperemia severity in either group, or in subgroups based on previous use of any PGA, any non-PGA, latanoprost, or travoprost monotherapies. In treatment-naïve patients, mean IOP reduction from baseline (18.0 ± 3.8 mm Hg) was 3.6 mm Hg at week 12 (P < 0.0001); 83.3 % had baseline IOP ≤ 21 mm Hg. In previously treated patients, mean additional IOP reduction from baseline (17.8 ± 3.9 mm Hg) was 2.6 mm Hg (P < 0.0001); similar results were observed in patient subgroups based on previous therapies.
Conclusions
In the Taiwanese clinical setting, bimatoprost 0.01 % provided significant IOP lowering in treatment-naïve patients (regardless of baseline IOP) and previously treated patients (even those with relatively low IOP on other therapies), while causing no significant changes in hyperemia from baseline.
Trial registration
Clinicaltrials.gov NCT01814761. Registered 18 March 2013.
Similar content being viewed by others
Background
Glaucoma is second only to cataract as a leading cause of blindness worldwide [1, 2], and because the burden increases substantially with advanced disease [3–5], early detection and treatment is important. In addition, the characteristics and prevalence of glaucoma have been shown to vary with geography and race [6–11], emphasizing the need for clinical studies and management programs adapted to local populations [12]. For example, open-angle glaucoma (OAG) characterized by intraocular pressure (IOP) ≤ 21 mm Hg (i.e., normal-tension glaucoma [NTG]) is much more common in patients in Asian countries than Western ones [13]. A Korean study of adults over 50 years of age indeed showed that NTG accounted for 94.4 % of all OAG cases [14]. Similarly, another Korean study showed that 75.3 % of patients with OAG had baseline IOP ≤ 21 mm Hg [15]. However, whether OAG is associated with elevated or normal IOP, treatment options remain limited because the underlying mechanisms have yet to be elucidated. Current management of OAG relies on topical IOP-lowering agents [16, 17], and prostaglandin analogs (PGAs)/prostamides are often preferred as first-line therapy owing to their efficacy, safety, and convenience of use (once daily) [17–19]. In Taiwan, however, the National Health Insurance Administration reserves them for use as second-line therapy [20, 21].
The prostamide bimatoprost 0.03 % (Lumigan® 0.03 %; Allergan plc, Irvine, CA, USA) is an effective topical treatment with superior IOP-lowering effects (compared with other PGA monotherapies) and a favorable tolerability profile with long-term use [22–25]. Importantly, clinical studies have also demonstrated its efficacy and tolerability in patients with NTG [26–30]. Nonetheless, some patients experience adverse events (AEs) when treated with bimatoprost 0.03 %, the most common being conjunctival hyperemia. Considering that AEs experienced chronically can lead to nonadherence to therapy [31, 32] and impact disease progression [33, 34], bimatoprost 0.01 % (Lumigan® 0.01 %; Allergan plc, Irvine, CA, USA) [35] was developed and shown to improve tolerability while retaining the IOP-lowering efficacy of bimatoprost 0.03 % in clinical studies of patients with elevated IOP due to OAG or ocular hypertension (OHT) [36–39].
Given the regional differences in glaucoma presentation and treatment guidelines mentioned above [40], the Asia Pacific Patterns from Early Access of Lumigan 0.01 % study in Taiwan (APPEAL Taiwan) evaluated the occurrence and severity of hyperemia produced by bimatoprost 0.01 % monotherapy, as well as its IOP-lowering effects, in patients with OHT or OAG, including NTG, seen in the Taiwanese clinical practice setting.
Methods
Study design
This 12-week, open-label, noncomparative, observational study of bimatoprost 0.01 % in consecutive patients with OAG (including NTG) or OHT (ClinicalTrials.gov identifier: NCT01814761, registered on March 18, 2013) was conducted between May 2013 and August 2014, in accordance with the Guidelines for Good Clinical Practice and all applicable Taiwanese laws.
Study population
Eligible patients were at least 20 years of age, treatment-naïve or previously treated, and had been diagnosed before screening with OHT or OAG (including NTG), according to standard of care in the treating physician’s practice. OAG was defined as an eye with glaucomatous optic nerve head change and corresponding glaucomatous visual field defects, and the decision to prescribe topical bimatoprost 0.01 % was made by the treating physician prior to, and without consideration of study participation (per standard of care). Key exclusion criteria included a history of bimatoprost 0.01 % use; contraindications to bimatoprost 0.01 % use; hypersensitivity to any PGA or component of the study medication; concomitant use of topical, periorbital, intravitreal, or systemic steroid within 3 months of study initiation, or anticipated use during the study; and presence of any other abnormal ocular condition or symptom preventing study participation.
Study treatment
Bimatoprost 0.01 % was provided by Allergan Singapore Pte Ltd. At the baseline visit, patients were instructed to instill 1 drop of medication in the study eye each evening (at approximately 8 pm). If both eyes were eligible for inclusion, both were treated, but the eye with the higher baseline IOP was included in the analysis. If both eyes had identical baseline IOP, the right eye was designated as study eye. There was no washout of previous IOP-lowering medications prior to initiation of study treatment.
Outcomes and analyses
All outcomes were measured at approximately the same time of day at baseline, and weeks 6 and 12. The primary outcome variables were the occurrence and severity of ocular hyperemia at week 12, assessed and graded using a standard photonumeric bulbar conjunctival hyperemia grading scale: 0 (none; normal); +0.5 (trace; trace flush, reddish pink); +1 (mild; mild flush, reddish color); +2 (moderate; bright red color); and +3 (severe; deep, bright diffuse redness). Hyperemia grading was then collapsed into 2 categories: none to mild (i.e., 0 to +1) and moderate to severe (i.e., +2 and +3), as described by other groups [15, 38, 41, 42], and the shift in hyperemia severity/grading from baseline at weeks 6 and 12 was reported as improved, unchanged, or worsened [38].
The secondary outcome variables included the change in IOP from baseline (based on Goldmann applanation tonometry performed per standard of care in the treating physician’s practice) and response rates (i.e., percentage change in IOP from baseline) at 6 and 12 weeks in treatment-naïve patients and patients previously treated with monotherapy or combination therapy (i.e., switched). Safety assessments included biomicroscopy, visual acuity, ocular AEs, and the number of discontinuations due to AEs. For each discontinuation, every effort was made to contact the patient and document the outcomes.
All patients who received at least 1 dose of study medication were included in the intent-to-treat and safety populations. All individual hyperemia scores recorded for each grade (i.e., 0, +0.5, +1, +2, +3) were reported as frequency counts and percentages for all study visits, and the change from baseline was reported at weeks 6 and 12. The treatment effect was analyzed using a 2-sided McNemar test. Change and percentage change in IOP from baseline at weeks 6 and 12 were analyzed using the 2-sided Student paired t test. No statistical analyses were conducted comparing treatment-naïve and previously treated patients. Owing to the exploratory nature of the study, no sample size calculation was carried out.
Supplemental analyses of the occurrence and severity of hyperemia produced by bimatoprost 0.01 %, as well as its IOP-lowering effects, were conducted for subgroups of patients who at baseline were receiving PGA or non-PGA monotherapy, and latanoprost or travoprost monotherapy.
Results
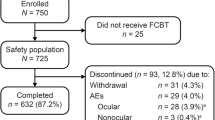
Eleven centers participated in the study. The intent-to-treat/safety population (N = 312) consisted of treatment-naïve patients and previously treated patients (Fig. 1). Among the latter subgroup, 91.1 % had been prescribed only 1 IOP-lowering therapy (monotherapy or fixed-combination therapy of any kind) at the time of enrollment. Most patients completed the study, and discontinuations were due to ocular AEs, lost to follow-up, or other non-AE-related reasons (Fig. 1).
Overall, 90.4 % of patients had a diagnosis of OAG. Baseline characteristics were similar between treatment-naïve and previously treated patients, except for the diagnosis and medical comorbidities (Table 1). Among previously treated patients, 83.0 % (n = 224) were receiving monotherapy at baseline (Table 1); 93.0 % were switched to bimatoprost 0.01 % monotherapy because of intolerance or insufficient IOP-lowering with previous therapy.
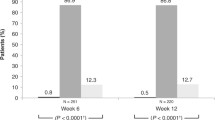
At week 12, 29 (85.3 %) treatment-naïve patients with available data had no hyperemia, compared with 36 (85.8 %) at baseline (Table 2). No severe cases were reported in this group of patients at any visit (Table 2). In the previously treated group, 170 (70.8 %) patients had no hyperemia at week 12, compared with 201 (74.4 %) at baseline (Table 2; Fig. 2a). There were no statistically significant shifts in hyperemia severity from baseline in either group (Fig. 2a) or in subgroups based on previous use of non-PGA versus PGA monotherapy (Fig. 2b), and latanoprost versus travoprost monotherapy (Fig. 2c). Similarly, there were no statistically significant shifts in hyperemia severity from baseline (P = 1.0000) in patients previously treated with > 1 therapy (not shown).
Shift in hyperemia severity grading from baseline at week 12 in (a) treatment-naïve and previously treated patients (P ≥ 0.2717 in both groups, compared with baseline), (b) patient subgroups previously treated with prostaglandin analog (PGA) or non-PGA monotherapy (P ≥ 0.2295 in both groups, compared with baseline), and (c) patient subgroups previously treated with latanoprost or travoprost monotherapy (P ≥ 0.1185 in both groups, compared with baseline)
In treatment-naïve patients, mean baseline IOP ± SD was 18.0 ± 3.8 mm Hg. A statistically significant reduction in mean IOP from baseline of approximately 20 % was observed at weeks 6 and 12 (P < 0.0001; Fig. 3). In the subgroup of patients who had a baseline IOP ≤ 21 mm Hg (mean ± SD, 16.6 ± 2.3 mm Hg; n = 35; 83.3 %), the mean IOP reduction from baseline was 2.8 mm Hg (P < 0.0001 at both timepoints) at both weeks 6 (16.2 %) and 12 (16.6 %). In the subgroup who had a baseline IOP > 21 mm Hg (mean ± SD, 24.9 ± 2.0 mm Hg; n = 7; 16.7 %), the mean IOP reduction from baseline reached 10.5 ± 4.2 mm Hg (P = 0.0154) at week 6 (41.0 %) and 10.0 ± 4.1 mm Hg (P = 0.0163) at week 12 (39.0 %).
In previously treated patients, mean IOP ± SD at baseline was 17.8 ± 3.9 mm Hg, and the mean additional IOP reduction from baseline (> 13 %) was also statistically significant at both post-baseline visits despite the high proportion of patients previously treated with PGA monotherapy (P < 0.0001; Fig. 3; Table 1). Similar results were observed in the patient subgroups previously treated with PGA versus non-PGA monotherapy (Fig. 4a), as well as those previously treated with latanoprost versus travoprost monotherapy (Fig. 4b). In patients who received > 1 therapy before switching to bimatoprost 0.01 %, mean IOP ± SD at baseline was 18.4 ± 3.8 mm Hg, and the mean additional IOP reduction from baseline was also statistically significant at weeks 6 (3.3 mm Hg; P = 0.0001) and 12 (2.4 mm Hg; P = 0.0093).
Mean intraocular pressure (IOP) reduction at week 12 in (a) patient subgroups previously treated with prostaglandin analog (PGA) or non-PGA monotherapy, and (b) patient subgroups previously treated with latanoprost or travoprost monotherapy. *P < 0.0001, compared with baseline; †P < 0.0002, compared with baseline
Overall, 26.2 % and 13.7 % of treatment-naïve and previously treated patients reported treatment-related AEs, respectively (Table 3); all were ocular in nature, none were serious, and conjunctival hyperemia was the most frequent in both groups (Table 3). Consistent with these results, more patients discontinued treatment owing to AEs in the treatment-naïve and previously treated groups (Table 3).
Discussion
The APPEAL Taiwan study was designed to assess the tolerability and efficacy of bimatoprost 0.01 % in patients with OHT or OAG (including NTG) evaluated in typical clinical practice settings. The results demonstrate that when administered once daily over 12 weeks, bimatoprost 0.01 % caused no significant shift in hyperemia severity from baseline in treatment-naïve and previously treated patients, but produced statistically significant IOP lowering from baseline in both groups.
At study end, hyperemia severity remained unchanged or improved in ≥ 87 % of patients in both groups. Moreover, mean IOP reduction from baseline reached 19 % in treatment-naïve patients, despite a low baseline mean IOP (i.e., 18.0 ± 3.8 mm Hg). Although the subgroup of patients with baseline IOP > 21 mm Hg and available data on hyperemia at week 12 was small (n = 4), mean IOP reduction from baseline reached 39 %, consistent with data from other studies of bimatoprost 0.01 % in treatment-naïve patients with baseline IOP > 21 mm Hg [15, 38, 39, 43]. Similarly, bimatoprost 0.01 % provided an additional 13 % reduction in mean IOP in previously treated patients, compared with baseline, despite the heterogeneity of this subgroup in terms of previous treatment.
In the supplemental analyses based on previous treatment, statistically significant reductions in mean IOP from baseline were observed in patient subgroups previously treated with non-PGA or PGA monotherapy, latanoprost or travoprost monotherapy, or > 1 therapy before switching to bimatoprost 0.01 %, but no significant shifts in hyperemia severity from baseline were observed in any of those subgroups.
Overall, our data indicate that treatment with bimatoprost 0.01 % can significantly lower IOP in treatment-naïve patients who have a low baseline IOP, as well as patients who have achieved some degree of IOP lowering with other therapies. These findings are consistent with those of the multicenter, open-label, observational, APPEAL Korea study of treatment-naïve [15] and previously treated [42] patients with OAG (including NTG) or OHT evaluated in the Korean clinical setting. The IOP-lowering efficacy and tolerability of bimatoprost 0.01 % observed herein also are consistent with the results of a 12-month, multicenter, randomized, double-masked, controlled clinical trial of bimatoprost 0.01 % in treatment-naïve and previously treated patients with elevated IOP due to glaucoma or OHT [37]. Similarly, our results are consistent with those of the 12-week, open-label, multicenter, observational, Canadian CLEAR study of treatment-naïve patients with OAG or OHT and elevated IOP who were monitored in the clinical setting [38].
The overall percentage of patients with ocular AEs leading to discontinuation (6.7 %) recorded herein is consistent with that reported in other studies (i.e., ≤ 5.4 %) [15, 37–39, 44].
As age is a primary risk factor for OAG and the population is aging across the globe, more patients are expected to require monitoring and therapy in the near future [1], making OAG an important clinical issue. In this regard, it is noteworthy that the study population evaluated herein had a relatively young mean age (53.3 years), compared with that in other studies of bimatoprost 0.01 % (mean range, 61.1–68.2 years) [37–39, 44]. In fact, mean age was even lower than the 58.0 and 59.5 years reported in the APPEAL Korea study of treatment-naïve [15] and previously treated patients [42], respectively, which included patients with NTG. Whether this difference is indicative of early onset or early detection is unknown at this time and requires further investigation.
Potential limitations of the study include the open-label design, lack of comparator, relatively short duration, and a possible Hawthorne effect in patients who switched to bimatoprost 0.01 % from a previous treatment [41], as well as the fact that inter-observer differences could have existed in the hyperemia grading despite use of a standard photonumeric bulbar conjunctival hyperemia grading scale. The absence of a washout period prior to initiation of study treatment should also be considered, although it was deliberately set to reflect typical clinical practice settings. It is also noteworthy that all outcome variables were assessed at 12 weeks, a timepoint at which no residual carry-over effects from previous treatments are expected.
Conclusions
This study of patients with OAG (including NTG) or OHT in the Taiwanese clinical setting showed that bimatoprost 0.01 % provides significant IOP lowering in treatment-naïve patients (regardless of baseline IOP), as well as previously treated patients (even those with relatively low IOP on other therapies), while causing no significant changes in hyperemia from baseline.
Abbreviations
- AEs:
-
Adverse events
- IOP:
-
Intraocular pressure
- NTG:
-
Normal-tension glaucoma
- OAG:
-
Open-angle glaucoma
- OHT:
-
Ocular hypertension
- PGA:
-
Prostaglandin analog
- SD:
-
Standard deviation
References
Quigley HA. New paradigms in the mechanisms and management of glaucoma. Eye. 2005;19:1241–8.
Glaucoma facts and stats. Glaucoma research foundation website. 2015. http://www.glaucoma.org/glaucoma/glaucoma-facts-and-stats.php. Accessed 18 Dec 2015.
Fiscella RG, Lee J, Davis EJ, Walt J. Cost of illness of glaucoma: a critical and systematic review. Pharmacoeconomics. 2009;27:189–98.
Lorenz K, Wolfram C, Breitscheidel L, Shlaen M, Verboven Y, Pfeiffer N. Direct cost and predictive factors for treatment in patients with ocular hypertension or early, moderate and advanced primary open-angle glaucoma: the CoGIS study in Germany. Graefes Arch Clin Exp Ophthalmol. 2013;251:2019–28.
Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152:515–22.
Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47:4254–61.
Kosoko-Lasaki O, Gong G, Haynatzki G, Wilson MR. Race, ethnicity and prevalence of primary open-angle glaucoma. J Natl Med Assoc. 2006;98:1626–9.
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Chua J, Tham YC, Liao J, Zheng Y, Aung T, Wong TY, Cheng CY. Ethnic differences of intraocular pressure and central corneal thickness: the Singapore epidemiology of eye diseases study. Ophthalmology. 2014;121:2013–22.
Cedrone C, Mancino R, Cerulli A, Cesareo M, Nucci C. Epidemiology of primary glaucoma: prevalence, incidence, and blinding effects. Prog Brain Res. 2008;173:3–14.
Chan EW, Li X, Tham YC, Liao J, Wong TY, Aung T, Cheng CY. Glaucoma in Asia: regional prevalence variations and future projections. Br J Ophthalmol. 2015;100:78-85.
Tsai CY, Woung LC, Chou P, Yang CS, Sheu MM, Wu JR, et al. The current status of visual disability in the elderly population of Taiwan. Jpn J Ophthalmol. 2005;49:166–72.
Cho HK, Kee C. Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol. 2014;59:434–47.
Kim JH, Kang SY, Kim NR, Lee ES, Hong S, Seong GJ, et al. Prevalence and characteristics of glaucoma among Korean adults. Korean J Ophthalmol. 2011;25:110–5.
Park KH, Simonyi S, Kim CY, Sohn YH, Kook MS. Bimatoprost 0.01 % in treatment-naïve patients with open-angle glaucoma or ocular hypertension: an observational study in the Korean clinical setting. BMC Ophthalmol. 2014;14:160.
American Academy of Ophthalmology. Primary open-angle glaucoma. www.aaojournal.org/article/S0161-6420(15)01276-2/pdf. Accessed 22 Dec 2015.
European Glaucoma Society. Terminology and guidelines for glaucoma. 2014. http://www.eugs.org/eng/EGS_guidelines4.asp. Accessed 22 Dec 2015.
LUMIGAN® (bimatoprost ophthalmic solution) 0.03 % for topical use [prescribing information]. 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021275s026lbl.pdf. Accessed 18 Dec 2015.
Lee AJ, McCluskey P. Clinical utility and differential effects of prostaglandin analogs in the management of raised intraocular pressure and ocular hypertension. Clin Ophthalmol. 2010;4:741–64.
Chiu SL, Chu CL, Muo CH, Chen CL, Lan SJ. Trends in glaucoma medication expenditures under universal health coverage: a national population-based longitudinal survey in Taiwan. J Ophthalmol. 2015;2015:243401.
Lin JC. The use of ocular hypotensive drugs for glaucoma treatment: changing trend in Taiwan from 1997 to 2007. J Glaucoma. 2015;24:364–71.
Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin analogs: a meta-analysis of randomized controlled clinical trials. J Glaucoma. 2008;17:667–73.
Cohen JS, Gross RL, Cheetham JK, VanDenburgh AM, Bernstein P, Whitcup SM. Two-year double-masked comparison of bimatoprost with timolol in patients with glaucoma or ocular hypertension. Surv Ophthalmol. 2004;49 Suppl 1:S45–52.
Simmons ST, Dirks MS, Noecker RJ. Bimatoprost versus latanoprost in lowering intraocular pressure in glaucoma and ocular hypertension: results from parallel-group comparison trials. Adv Ther. 2004;21:247–62.
Williams RD, Cohen JS, Gross RL, Liu CC, Safyan E, Batoosingh AL, For the Bimatoprost Study Group. Long-term efficacy and safety of bimatoprost for intraocular pressure lowering in glaucoma and ocular hypertension: year 4. Br J Ophthalmol. 2008;92:1387–92.
Inoue K, Shiokawa M, Fujimoto T, Tomita G. Effects of treatment with bimatoprost 0.03 % for 3 years in patients with normal-tension glaucoma. Clin Ophthalmol. 2014;8:1179–83.
Tsumura T, Yoshikawa K, Suzumura H, Kimura T, Sasaki S, Kimura I, Takeda R. Bimatoprost ophthalmic solution 0.03% lowered intraocular pressure of normal-tension glaucoma with minimal adverse events. Clin Ophthalmol. 2012;6:1547–52.
Sato S, Hirooka K, Baba T, Mizote M, Fujimura T, Tenkumo K, et al. Efficacy and safety of switching from topical latanoprost to bimatoprost in patients with normal-tension glaucoma. J Ocul Pharmacol Ther. 2011;27:499–502.
Chen MJ, Cheng CY, Chen YC, Chou CK, Hsu WM. Effects of bimatoprost 0.03 % on ocular hemodynamics in normal tension glaucoma. J Ocul Pharmacol Ther. 2006;22:188–93.
Dirks MS, Noecker RJ, Earl M, Roh S, Silverstein SM, Williams RD. A 3-month clinical trial comparing the IOP-lowering efficacy of bimatoprost and latanoprost in patients with normal-tension glaucoma. Adv Ther. 2006;23:385–94.
Kaštelan S, Tomić M, Metež Soldo K, Salopek-Rabatić J. How ocular surface disease impacts the glaucoma treatment outcome. Biomed Res Int. 2013;2013:696328.
Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28:267–82.
Cate H, Bhattacharya D, Clark A, Fordham R, Holland R, Broadway DC. Improving adherence to glaucoma medication: a randomised controlled trial of a patient-centred intervention (The Norwich Adherence Glaucoma Study). BMC Ophthalmol. 2014;14:32.
Hwang DK, Liu CJ, Pu CY, Chou YJ, Chou P. Persistence of topical glaucoma medication: a nationwide population-based cohort study in Taiwan. JAMA Ophthalmol. 2014;132:1446–52.
LUMIGAN® (bimatoprost ophthalmic solution) 0.01 % [prescribing information]. 2014. http://www.allergan.com/assets/pdf/lumigan_pi.pdf. Accessed 18 Dec 2015.
Figus M, Nardi M, Piaggi P, Sartini M, Guidi G, Martini L, Lazzeri S. Bimatoprost 0.01 % vs bimatoprost 0.03 %: a 12-month prospective trial of clinical and in vivo confocal microscopy in glaucoma patients. Eye. 2014;28:422–9.
Katz LJ, Cohen JS, Batoosingh AL, Felix C, Shu V, Schiffman RM. Twelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2010;149:661–71. e1.
Nixon DR, Simonyi S, Bhogal M, Sigouin CS, Crichton AC, Discepola M, et al. An observational study of bimatoprost 0.01 % in treatment-naïve patients with primary open angle glaucoma or ocular hypertension: the CLEAR trial. Clin Ophthalmol. 2012;6:2097–103.
Pfennigsdorf S, Ramez O, von Kistowski G, Mäder B, Eschstruth P, Froböse M, et al. Multicenter, prospective, open-label, observational study of bimatoprost 0.01 % in patients with primary open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:739–46.
Chung SD, Ho J, Lin HC, Kao LT, Tsai MC. Incremental healthcare service utilization for open-angle glaucoma: a population-based study. J Glaucoma. 2015;24:116–20.
Crichton AC, Nixon DR, Simonyi S, Bhogal M, Sigouin CS, Discepola MJ, et al. An observational study of bimatoprost 0.01 % in patients on prior intraocular pressure-lowering therapy: the Canadian Lumigan® RC Early Analysis Review (CLEAR) trial. Clin Ophthalmol. 2014;8:1031–8.
Kook MS, Simonyi S, Sohn YH, Kim CY, Park KH. Bimatoprost 0.01 % for previously treated patients with open-angle glaucoma or ocular hypertension in the Korean clinical setting. Jpn J Ophthalmol. 2015;59:325.
Tung JD, Tafreshi A, Weinreb RN, Slight JR, Medeiros FA, Liu JH. Twenty-four-hour effects of bimatoprost 0.01 % monotherapy on intraocular pressure and ocular perfusion pressure. BMJ Open. 2012;2; doi:10.1136/bmjopen-2012-001106.
Crichton AC, Vold S, Williams JM, Hollander DA. Ocular surface tolerability of prostaglandin analogs and prostamides in patients with glaucoma or ocular hypertension. Adv Ther. 2013;30:260–70.
Acknowledgements
Writing and editorial assistance was provided to the authors by Michele Jacob, PhD, of Evidence Scientific Solutions (Philadelphia, PA), and funded by Allergan plc (Dublin, Ireland). All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
We acknowledge the following institutions (researchers) for their participation in the study: Cathay General Hospital (Szu-Yuan Lin), China Medical University Hospital (Hsin-Yi Chen), Chung Shan Medical University Hospital (Mei-Ling Peng), Kaohsiung Medical University Hospital (Han-Yi Tseng), Macay Memorial Hospital (Yu-Wen Lan), Taichung Veterans General Hospital (Chun-Yuan Wang), and Taipei Veterans General Hospital (Yu-Chien Ko).
Funding
This study was sponsored by Allergan Singapore Pte Ltd (Singapore). The funding body was involved in the design, data analysis and interpretation, revision of the manuscript for intellectual content, and decision to submit the manuscript for publication.
Availability of data and materials
Data and associated protocols are available upon request to Allergan plc.
Author contributions
The authors met all the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). YYC, THW, CL, KYW, SLC, and DWL participated in the study design, data acquisition and interpretation, revised the manuscript critically for important intellectual content, and reviewed and approved the final version of the manuscript. SS participated in the study design and data interpretation, revised the manuscript critically for important intellectual content, and reviewed and approved the final version of the manuscript.
Competing interests
YYC, THW, CL, KYW, SLC, and DWL declare that they have no competing interests. SS is an employee of Allergan Singapore Pte Ltd (Singapore).
Consent to publish
Not applicable
Ethics approval and consent to participate
The study protocol was approved by the following Research Ethics Boards before study initiation: National Taiwan University Hospital Research Ethics Committee (National Taiwan University Hospital, Taipei City); Institutional Review Board of the Tri-Service General Hospital National Defense Medical Center (Tri-Service General Hospital, Taipei City); Changhua Christian Hospital Institutional Review Board (Changhua Christian Hospital, Changhua City); Kaohsiung Medical University Chung-Ho Memorial Hospital Institutional Review Board (Kaohsiung Medical University Hospital, Kaohsiung City); Kaohsiung V.G.H. Institutional Review Board (Kaohsiung Veterans General Hospital, Kaohsiung City); Institutional Review Board, Taipei Veterans General Hospital (Taipei Veterans General Hospital, Taipei City); Mackay Memorial Hospital Institutional Review Board (Mackay Memorial Hospital, Taipei City); The Institutional Review Board of Taichung Veterans General Hospital (Taichung Veterans General Hospital, Taichung City); Institutional Review Board Chung Shan Medical University Hospital (Chung Shan Medical University Hospital, Taichung City); Research Ethics Committee China Medical University & Hospital, (China Medical University Hospital, Taichung City); and Institutional Review Board of the Cathay General Hospital (Cathay General Hospital, Taipei City).
Study participants provided written informed consent before initiating or changing study treatment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, Y.Y., Wang, TH., Liu, C. et al. Tolerability and efficacy of bimatoprost 0.01 % in patients with open-angle glaucoma or ocular hypertension evaluated in the Taiwanese clinical setting: the Asia Pacific Patterns from Early Access of Lumigan 0.01 % (APPEAL Taiwan) study. BMC Ophthalmol 16, 162 (2016). https://doi.org/10.1186/s12886-016-0338-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-016-0338-6