Abstract
Background
Several techniques for fixation of the posterior chamber intraocular lens (IOL) have been developed. We evaluate long-term functional outcomes and safety of posterior chamber IOL implantation using Hoffman scleral haptic fixation and sutureless Sharioth technique in patients with posttraumatic and postoperative aphakia.
Methods
This retrospective case-series included 42 eyes operated by one surgeon. The data including demographic data, ocular history, preoperative, early postoperative and final best corrected visual acuity (BCVA), rate of complications as well as postoperative IOL position were collected. The mean follow-up was 14.5 months. Hoffman haptic scleral fixation was performed in 31 eyes, Sharioth technique—in 11 eyes. Aphakia was due to eye trauma (19) or complicated cataract surgery (23).
Results
Overall, the final BCVA improved in 26 eyes, did not change in 5 eyes, and worsened in 11 eyes. No significant differences in BCVA were found between groups operated with Hoffman scleral fixation and Sharioth technique. Postoperatively, we noticed two dislocations of IOL fixated using Sharioth technique and none after Hoffman technique. No severe complications were observed.
Conclusion
Both transscleral fixation techniques are feasible methods of secondary IOL implantation in posttraumatic and postoperative aphakia. with low incidence of complications, however visual outcomes are diverse.
Similar content being viewed by others
Background
Surgical secondary artificial intraocular lens (IOL) implantation is a standard procedure both in posttraumatic and postoperative aphakia. The status of the posterior capsule may vary from intact to partially deficient or totally absent. Thus, the technique of implantation of IOL may vary from putting the lens into the bag [1] to suturing of IOL to iris or implantation to the anterior or posterior chamber [2–7]. If the anterior capsule is not damaged, the lens may be implanted to the sulcus [1]. Anterior chamber IOL carry high risk of postoperative complications as corneal endothelial damage, uveitis, glaucoma, hyphema (UGH) and cystoid macular edema [8, 9]. Suturing the IOL to the iris may result in iris chafing, uveitis, and pupillary constriction [10]. Furthermore, iris fixation is impossible in cases of significant iris trauma. Currently, if the posterior capsule is not present and if there is lack of iris tissue, most of IOLs are placed into posterior chamber and sutured to the sclera through the ciliary sulcus or pars plana.
The aim of this study was to estimate the visual outcomes and safety of two methods of secondary posterior chamber IOL implantation-a transscleral IOL haptic fixation using Hoffman technique or Sharioth scleral suturing technique-in patients with deficient posterior capsule support due to trauma or complicated cataract surgery.
Methods
This retrospective study included patients, who had secondary IOL implantation surgery performed between March 2011 and December 2014 in the Department of General Ophthalmology in Lublin, Poland. The study was approved by the independent Ethics Committee at the Medical University in Lublin, Poland and performed in accordance with the Declaration of Helsinki.
This study included 42 eyes of 42 patients (15 women, 27 males). The mean age was 53.5 years ± 21.5 (SD) (range 13–85 years). The mean follow-up was 14.5 months ± 2.2 (SD) (range 12–16 months). Inclusion criteria were as follows: (1) total absence of capsular bag, (2) history of eye trauma or complicated cataract surgery causing aphakia, (3) regular 1 year follow-up. The preoperative diagnosis was as follows: post pars plana vitrectomy (PPV) due to intraocular foreign body (IOFB)-2 eyes, post PPV due to endophthalmitis-5 eyes, post blunt eye trauma-12 eyes and after complicated cataract surgery-23 eyes. Data collected included demographic data, ocular history, indication for surgery, preoperative and postoperative best corrected visual acuity (BCVA), intraocular pressure, detailed anterior and posterior segment evaluation using stereoscopic slit lamp biomicroscopy and indirect ophthalmoscopy. Patients were evaluated on the day 1, day 3, day 14, 3 months, and 12 months postoperatively. Intraocular lens position was assessed by a slit lamp examination with a dilated pupil, nonvisibility of the optic edge in a mid-dilated pupil of 4 mm was considered as a good centration. Final BCVA was the principal visual outcome indicator (expressed in Snellen decimal letters). It was reported as the percentage of eyes achieving BCVA of 0.5 or better, BCVA of 0.2–0.4, and BCVA of 0.1 or worse. Data were analyzed using t-test.
Surgical techniques
All eyes were operated in local anesthesia (peribulbar injection of mixture of lignocaine, bupivacain and hylasis). Postoperative medication included topical drops (combined antibiotic and steroid) given 5 times daily and taped slowly for 4 weeks. Three kinds of IOL were implanted: Alcon MA60AC and Alcon MA60BM 3-piece, acrylic foldable IOL (Alcon International, United States) as well as Rayner 570C acrylic injectable, hydrophilic IOL (Rayner Intraocular Lenses, Ltd., Hove, East Sussex, United Kingdom).
Each procedure was performed by one surgeon (DH)- 31 patients underwent Hoffman technique and 11—Sharioth technique. All eyes underwent anterior vitrectomy during the primary surgery as a routine accompaniment. Additionally, a constant infusion was needed.
-
1.
Hoffman technique (Fig. 1)
Fig. 1 Hoffman technique-1) hollowing the tunnel in the sclera 2) a straight needle attached to a 10–0 polypropylene suture is passed through the conjuctiva and sclera in the pocket, posterior to the iris to the pupil, out through the opposite paracentes 3) the needle is passed back using hollow 26-G needle 4) after tying the haptic, the suture is tied into pocket
-
First, a 3–4 mm wide corneal incision is made, 0.5 mm anterior to the limbus, at a depth of 0.3 mm. Using a crescent knife the tunnel is made 2–3 mm posterior to the limbus, creating the reverse sclera pocket. A straight needle attached to a 10–0 polypropylene suture is passed through the roof of the scleral pocket, posterior to the iris and to the pupil area, out through the opposite paracentesis. The needle is passed back in the barrel of a hollow 26-gauge needle. The same procedure is made on the opposite side. A corneal incision of 2.6 mm is performed for lens implantation. The loops of 10–0 polypropylene sutures are externalized through corneal incision and sutured to the haptics. Then the sutures outgoing through the sclera, are drawn out through the incisions (in scleral pockets) and tied. The knots are buried in sclera pockets and no conjunctiva suturing is needed [11].
-
-
2.
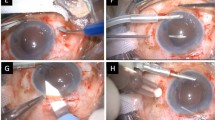
Sharioth technique (Fig. 2)
Fig. 2 Scharioth technique-1) hollowing the intrascleral tunnel 2) inserting a 25-G needle at the end of the opposite intrascleral tunnel, insert the haptic of the PCIOL (posterior chamber intraocular lens) into the hollow needle (this maneuver can be performed using micropincet 25-G) 3) removal of the haptic out by withdrawing the needle, the other haptic is retrieved using tweezers 4) inserting the second haptic into opposite intrascleral tunnel via the barrel of the hollow needle
-
After peritomies are done, sclerectomy is made using a 25-gauge needle. Two 2.00–3.00 mm scleral tunnels are created parallel to the limbus 180° from each other. After the PCIOL (posterior chamber intraoocluar lens) is inserted, a 25-gauge needle is inserted at the end of one intrascleral tunnel, the haptic of the PCIOL is inserted into the hollow needle (this maneuver can be performed using micropincet 25-gauge).
-
The haptic is removed out by withdrawing the needle and inserted into the scleral tunnel. The other haptic is retrieved using tweezers. This haptic is inserted into the opposite intrascleral tunnel. Finally, the haptics are suspended through the sclerectomies into these scleral tunnels and the peritomies are closed [12].
Results
Functional results
Overall, mean preoperative BCVA was 0.279 (range 0.025–1.0) whereas early postoperative BCVA was 0.249 (range 0.025–0.6) and late postoperative 0.354 (range 0.010–1.0) (p = 0.176) (Table 1). Mean preoperative BCVA was 0.291 (range 0.025–1.0) in the group operated with Hoffman technique and 0.245 (range 0.1–0.6) in the group managed with Sharioth technique. Mean early postoperative BCVA was 0.239 (range 0.025–0.600) and 0.279 (range 0.025–0.600), respectively (p = 0.557). Mean late postoperative BCVA was 0.297 (range 0.010–1.000) and 0.454 (range 0.010–0.900), respectively (p = 0.161).
Overall, eighteen eyes (43 %) had a final BCVA of 0.5 or better, eighteen eyes (43 %) of 0.2 to 0.4, and six eyes (14 %) of worse than 0.1. Overall, the final BCVA improved in 26 eyes (62 %), did not change in 5 eyes (12 %), and worsened in 11 eyes (26 %). In the group of eyes with worsening of the visual acuity most of them (8 eyes) were posttraumatic.
Complications
In the present study we noticed two dislocations of PCIOL fixated using Sharioth technique. First patient suffered from severe ocular trauma. After 2 months, dislocation of PCIOL was found. The Siepser knot was tied to the haptic and it was suspended through the scleral flap. Second patient had an ocular history of TPPV surgery after endophthalmitis. Originally, implant was fixated using Scharioth technique. After 1 month the PCIOL became luxated. Reoperation was performed using Siepser knot to tie the haptic to the scleral bed.
We have not observed any dislocation after Hoffman technique. Severe complications (eg. expulsive hemorrhage, retinal or choroidal detachment, prolonged inflammation, or secondary glaucoma) were not observed in our group of patients. No evidence of suture erosion was found.
Discussion
In the present study we have shown the functional results and complication rate after two secondary PCIOL implantation methods in patients with posttraumatic and postoperative aphakia after 1-year follow-up. Many authors highlight the fact that transscleral fixation provides the most physiological placement of IOL in cases of absence of the lens capsule [5, 11, 12]. Sulcus placement without fixation to the sclera ensures early satisfactory outcomes, but significant complications (eg, erosion of the iris, UGH syndrome, iris pigment epithelial defects, decentration) may occur later [13, 14].
Angle-supported anterior chamber IOL have been rarely used due to numerous complications (endothelial cell loss, corneal decompensation, UGH). However, many of the problems were associated with the older closed loop anterior chamber IOLs and are not common in the newer open-loop single-piece anterior chamber IOLs [15].
Iris-claw IOLs may be a good alternative, however higher costs limit their extensive usage [16].
A large prospective study (176 patients) comparing different secondary IOL implantation techniques combined with penetrating keratoplasty showed that iris-suture fixation provided less complications (cystid macular oedema, glaucoma escalation, IOL dislocation, graft failure) than transscleral fixation [17].
Relatively new technique is fibrin-glue assisted sutureless fixation described by Argawal [18]. In this technique scleral flaps are made horizontally at 3 and 9 o’ clock (as also described for standard trans-scleral suture fixation procedures). One-year results showed good outcomes [19].
Several techniques for fixation of the posterior chamber IOL to the ciliary sulcus have been developed [2–5, 20]. Malbran and co-authors were the first to report transscleral sulcus fixation with sutures of posterior chamber lenses in aphakic patients who had had previous intracapsular cataract extraction [8]. Sharioth technique [12], performed in some of our patients, does not require any suturing. Two scleral tunnels created for suspension of the haptics of the PCIOL seem to be effective for transscleral fixation. This method avoids intraocular knots with free suture ends and minimises potential risk of iris chafing. Sharioth technique is technically more difficult than Hoffman technique, but allows to minimise intraocular manipulations. However, we noticed two cases of decentration of the PCIOL after 1 and 2 months (5 %) in eyes operated with Sharioth technique (one patient after severe ocular trauma and one after complicated retinal detachment surgery). Re-interventions were needed in both cases.
In our study most cases the PCIOL were stable without tilt. It is known that erosion of suture knots through the conjunctiva creates a communication between the extra- and intraocular environments, increasing the risk of contamination [2]. When knots are tied under the conjunctiva alone, the risk is up to 24 %, even scleral flaps are associated with the risk of 15 % [21].
Nottage and colleagues in a study of 69 patients after transscleral fixation observed glaucoma (5.8 %), cystic macular oedema (5.8 %), bullous keratopathy (4.3 %), retinal detachment (1.4 %), uveitis (1.4 %), keratitis (1.4 %) and choroidal haemorrhage (1.4 %) after 14 months of the follow-up [22]. They observed 1 suture erosion 2 years after surgery.
In the present series of 42 patients we observed deterioration of the visual acuity in one-fourth of cases. It is quite high percentage, although most of these cases were posttraumatic. However, 43 % of our patients had a final BCVA of 0.5 or better . Other studies describe better functional results. For example Kjeka and colleagues [23] report the mean preoperative BCVA 0.37, which improved to 0.5 postoperatively. At the end of follow-up, BCVA was unchanged or improved in 81 eyes (89.0 %), reduced by 2 Snellen lines in four eyes (4.4 %), and between finger counting and light perception in four eyes (4.4 %). The most serious complication was suprachoroidal haemorrhage, which occurred in two eyes, retinal detachment occurred in three eyes. In a study of Lanzetta [24] mean visual acuity was 0.29 preoperatively and 0.71 postoperatively after a mean follow-up of 14.2 months. A best corrected visual acuity of 0.5 or better was obtained in 12 eyes. In the recent study by Agrawal [25] the percentage of eyes with vision worsening after scleral fixation was 6.9 %. In our study we did not observe any severe complications such as corneal decompensation, cystoid macular edema, vitreous hemorrhage, retinal detachment, suprachoroidal hemorrhage, endophthalmitis, or glaucoma escalation.
Conclusion
Both Hoffman and Sharioth techniques of posterior chamber IOL implantation are feasible methods of managing posttraumatic and postoperative aphakia. However, functional outcomes are diverse, especially in posttraumatic cases. Longer follow-up on a large population is required. Careful selection of patients and surgical method should be made before operation.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Medical University in Lublin, Poland. A written informed consent was obtained from all the participants after the study protocol had been explained.
Consent to publish
A written informed consent to publish person’s data was sought along with the consent for participation into the study.
Availability of data and materials
Data can be shared upon request.
Abbreviations
- BCVA:
-
best corrected visual acuity
- IOFB:
-
intraocular foreign body
- IOL:
-
intraocular lens
- PCIOL:
-
posterior chamber intraocular lens
- PPV:
-
pars plana vitrectomy
References
Gimbel HV, Sun R, Ferensowicz M, Anderson Penno E, Kamal A. Intraoperative management of posterior capsule tears in phacoemulsification and intraocular lens implantation. Ophthalmology. 2001;108:2186–9.
Por YM, Lavin MJ. Techiques of intraocular lens suspension in the absence of capsular/ zonular support: diagnostic and surgical techniques: Marco zarbin and Chu David. Surv Ophthalmol. 2005;50(5):429–61.
Chang DF. Siepser slipknot for McCannel iris-suture fixation of subluxated intraocular lenses. J Cataract Refract Surg. 2004;30:1170–6.
Teichmann KD. Pars Plana fixation of posterior chamber intraocular lenses. Ophthalmic Surg. 1994;25:549–53.
Smiddy WE, Sawusch MR, O'Brien TP, Scott DR, Huang SS. Implantation of scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 1990;16:691–6.
Apple DJ, Price FW, Gwin T, Imkamp E, Daun M, Casanova T, et al. Sutured retropupillary posterior chamber intraocular lenses for exchange or secondary implantation; the 12th annual binkhorst lecture. Ophthalmology. 1989;96:1241–7.
Wagoner MD, Cox TA, Ariyasu RG, Jacobs DS, Karp CL. Intraocular lens implantation in the absence of capsular support; a report by the American academy of ophthalmology. Ophthalmic technology assessment. Ophthalmol. 2003;110:840–59.
McCluskey P, Harrisberg B. Long-term results using scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 1994;20:34–9.
Shapiro A, Leen MM. External transscleral posterior chamber lens fixation. Arch Ophthalmol. 1991;109:1759–60.
Menezo JL, Martinez MC, Cisneros AL. Iris-fixated worst claw versus sulcus-fixated posterior chamber lenses in the absence of capsular support. J Cataract Refract Surg. 1996;22:1476.
Hoffman RS, Fine H, Packer M, Rozenberg I. Scleral fixation using suture retrieval through a scleral tunnel. J Cataract Refract Surg. 2006;32:1259–63.
Scharioth GB, Prasad S, Georgalas I, Tataru C, Pavlidis M. Intermediate results of sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2010;36(2):254–9.
Amino K, Yamakawa R. Long-term results of out-of-the-bag intraocular lens implantation. J Cataract Refract Surg. 2000;26:266–70.
Piette S, Canlas OAQ, Tran HV, Ishikawa H, Liebmann JM, Ritch R. Ultrasound biomicroscopy in uveitis-glaucoma-hyphema syndrome. Am J Ophthalmol. 2002;133:839–41.
Hassan TS, Soong HK, Sugar A, Meyer RF. Implantation of kelman-style, open-loop anterior chamber lenses during keratoplasty for aphakic and pseudophakic bullous keratopathy. A comparison with iris-sutured posterior chamber lenses. Ophthalmology. 1991;98(6):875–80.
De Silva SR, Arun K, Anandan M, Glover N, Patel CK, Rosen P. Iris-claw intraocular lenses to correct aphakia in the absence of capsule support. J Cataract Refract Surg. 2011;37(9):1667–72.
Schein OD, Kenyon KR, Steinert RF, Verdier DD, Waring 3rd GO, Stamler JF, Seabrook S, Vitale S. A randomized trial of intraocular lens fixation techniques with penetrating keratoplasty. Ophthalmology. 1993;100(10):1437–43.
Agarwal A, Kumar DA, Jacob S, Baid C, Agarwal A, Srinivasan S. Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J Cataract Refract Surg. 2008;34:1433–8.
Kumar DA, Agarwal A, Prakash G, Jacob S, Sarvanan Y, Agarwal A. Glued posterior chamber IOL in eyes with deficient capsular support: a retrospective analysis of 1-year post-operative outcomes. Eye. 2010;24:1143–8.
Osher RH, Cionni RJ. The torn posterior capsule: its intraoperative behavior, surgical management, and long-term consequences. J Cataract Refract Surg. 1990;16:490–4.
Stark WJ, Gottsch JD, Goodman DF, Goodman GL, Pratzer K. Posterior chamber intraocular lens implantation in the absence of capsular support. Arch Ophthalmol. 1989;107:1078–83.
Nottage JM, Bhasin V, Nirankari VS. Long-term safety and visual outcomes of transscleral sutured posterior chamber IOLs and penetrating keratoplasty combined with transscleral sutured posterior chamber IOLs. Trans Am Ophthalmol Soc. 2009;107:242–50.
Kjeka O, Bohnstedt J, Meberg K, Seland JH. Implantation of scleral-fixated posterior chamber intraocular lenses in adults. Acta Ophthalmol. 2008;86(5):537–42.
Lanzetta P, Bandello FM, Virgili G, Crovato S, Menchini U. Is scleral fixation a safe procedure for intraocular lens implantation? Doc Ophthalmol. 1999;97(3–4):317–24.
Agrawal S, Singh V, Gupta SK, Misra N, Srivastava RM. Transscleral fixation of closed loop haptic acrylic posterior chamber intraocular lens in aphakic nonvitrectomized eyes. Indian J Ophthalmol. 2015;63(8):649–53.
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DH-performing surgery; KN-analysis and interpretation of the data, writing the manuscript, AO, JM-W, MF-acquisition of the data, writing the manuscript, AGJ, CF and RR-analysis of the data and revision of the manuscript for important intellectual content, general supervision. RM, KM-M-critical analysis of the results, revision of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Haszcz, D., Nowomiejska, K., Oleszczuk, A. et al. Visual outcomes of posterior chamber intraocular lens intrascleral fixation in the setting of postoperative and posttraumatic aphakia. BMC Ophthalmol 16, 50 (2016). https://doi.org/10.1186/s12886-016-0228-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-016-0228-y