Abstract
Background
To identify the cut-off values for the number of metastatic lymph nodes (nMLN) and lymph node ratio (LNR) that can predict outcomes in patients with FIGO 2018 IIICp cervical cancer (CC).
Methods
Patients with CC who underwent radical hysterectomy with pelvic lymphadenectomy were identified for a propensity score-matched (PSM) cohort study. A receiver operating characteristic (ROC) curve analysis was performed to determine the critical nMLN and LNR values. Five-year overall survival (OS) and disease-free survival (DFS) rates were compared using Kaplan–Meier and Cox proportional hazard regression analyses.
Results
This study included 3,135 CC patients with stage FIGO 2018 IIICp from 47 Chinese hospitals between 2004 and 2018. Based on ROC curve analysis, the cut-off values for nMLN and LNR were 3.5 and 0.11, respectively. The final cohort consisted of nMLN ≤ 3 (n = 2,378) and nMLN > 3 (n = 757) groups and LNR ≤ 0.11 (n = 1,748) and LNR > 0.11 (n = 1,387) groups. Significant differences were found in survival between the nMLN ≤ 3 vs the nMLN > 3 (post-PSM, OS: 76.8% vs 67.9%, P = 0.003; hazard ratio [HR]: 1.411, 95% confidence interval [CI]: 1.108–1.798, P = 0.005; DFS: 65.5% vs 55.3%, P < 0.001; HR: 1.428, 95% CI: 1.175–1.735, P < 0.001), and the LNR ≤ 0.11 and LNR > 0.11 (post-PSM, OS: 82.5% vs 76.9%, P = 0.010; HR: 1.407, 95% CI: 1.103–1.794, P = 0.006; DFS: 72.8% vs 65.1%, P = 0.002; HR: 1.347, 95% CI: 1.110–1.633, P = 0.002) groups.
Conclusions
This study found that nMLN > 3 and LNR > 0.11 were associated with poor prognosis in CC patients.
Similar content being viewed by others
Background
Cervical cancer (CC) is a significant global health concern. In 2020, there were 604,000 new patients with CC globally, and 342,000 patients died from CC. CC has become the most lethal cancer in 36 countries [1]. Tumor staging indicates the extent of disease, guiding treatment options, as well as in prognostication. Several studies have demonstrated lymph node metastasis to be a poor prognostic factor [2,3,4,5]; therefore, FIGO 2018 staging includes metastasis to pelvic lymph nodes as IIIC1 and to para-aortic lymph nodes as IIIC2, whereby, the annotation "r" or "p" indicates radiological or pathological involvement [6].
Metastatic lymph node is closely related to the prognosis of stage IIIC, and researchers have attempted to use various parameters related to lymph node (LN), including the number of metastatic lymph nodes (nMLN), number of examined lymph nodes, size of metastatic lymph node, lymph node ratio (LNR), and log odds of metastatic lymph nodes, as prognostic factors in CC. Log odds of metastatic lymph nodes is defined as logarithm of the probability of being a metastatic lymph node versus the probability of being a negative LN (the log of [metastatic lymph node ± 0.5/the number of removed LNs − metastatic lymph node + 0.5]). While nMLN is one of the most commonly used features to predict prognosis [7,8,9,10,11,12,13,14,15]; recently, the LNR which is defined as the number of metastatic lymph nodes divided by the total number of removed lymph nodes (nMLN/the number of removed LNs) has also gained attention as a prognostic factor [11, 15,16,17,18,19,20,21,22,23,24,25,26]. Nevertheless, the specific values of nMLN and LNR are still being explored for stage IIIC CC, with cut-off values of nMLN ranging from ≥ 2 to > 5 [10, 12,13,14,15] and those of LNR ranging from 0.05 to 0.2 [11, 15, 18,19,20, 23,24,25,26]. However, the predictive outcomes based on nMLN and LNR in patients with stage IIIC CC remain controversial and warrant further exploration, especially regarding cut-off values [7]. Therefore, this study aims to explore the specific cut-off values of nMLN and LNR that can accurately predict survival outcomes in patients with FIGO 2018 stage IIICp CC. The findings will contribute to survival risk stratification and the development of individualized treatment strategies for these patients, providing real-world evidence for clinical decision-making.
Methods
Study design
This retrospective study aimed to explore the specific cut-off values of nMLN and LNR that can accurately predict survival outcomes in patients with FIGO 2018 stage IIICp CC. We used data obtained from the Chinese Cervical Cancer Clinical Research Database developed through a clinical trial (Project 1538; Ethics Clearance NFEC-2017–135; Clinical Trial Registration Number: CHiCTR1800017778, http://apps.who.int/trialsearch/). The database includes 63,926 cases of CC from 47 hospitals in China and contains patients’ clinical information, pretreatment biopsy findings, laboratory and imaging information, treatment-related information, treatment complications, and postoperative pathology. Data were collected by two gynecologists who received specific training for the clinical trial using EpiData 3.1 (EpiData Association, Odense, Denmark) for dual data entry and standard interviews for follow-up data by telephone or outpatient visits. Details of the data collection and follow-up methods have been previously described [26,27,28].
Inclusion and exclusion criteria
The inclusion criteria for eligible cases were: age ≥ 18 years; CC detected through biopsy of the uterine cervix; histological confirmation; adenocarcinoma, adenosquamous cell carcinoma, or squamous cell carcinoma type CC; FIGO 2018 stage IIICp; underwent Q-M type-B or type-C radical hysterectomy with pelvic ± para-aortic lymphadenectomy; had complete information on LN dissection and postoperative pathology; underwent standardized adjuvant therapy; and had complete follow-up history.
The exclusion criteria include violation of selection criteria, missed visits, cancer of the uterine cervix stump, and CC combined with other malignancies or pregnancy.
Diagnostic criteria for metastatic lymph nodes
The diagnosis of metastatic LNs was determined by two pathologists with 15 years of practice. The pathologist tests all surgically resected LNs and diagnoses metastatic LNs when a tumor lesion is found.
Outcomes
The outcomes for this study were 5-year disease-free survival (DFS) and overall survival (OS) after treatment. DFS is the terminal time point from diagnosis to follow-up, relapse, or death. OS was defined as the terminal time point from diagnosis to a valid follow-up or death for any reason.
Statistical analysis
LNR (%) is the ratio of the nMLN to the total number of removed LNs (values are totals for both left and right sides). Receiver operating characteristic (ROC) curve analysis was used to determine the optimal nMLN and LNR values. If in normal distribution, aequential variables were characterized by mean ± standard deviation, and an independent sample t-test was employed for comparison between groups. If in non-normal distribution, the continuous measurement data were expressed as median and interquartile range and tested with the Mann–Whitney U test. Categorical data was presented as frequency and percentage values, and comparison was done using Chi square or Fischer's exact test as appropriate. Survival analysis was performed using the Kaplan–Meier method. Independent risk factors were identified through Cox proportional hazards models, and hazard ratios (HRs) and 95% confidence intervals (95% CI) were calculated. Propensity score matching (PSM) was applied to decrease the effect of baseline discrepancies within groups. The analyses were all conducted using SPSS (version 29; IBM Corp., Armonk, N.Y., USA), with significance defined at a P-value < 0.05.
Results
Case screening results and cut-off value determination
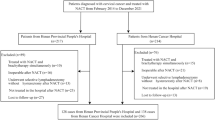
From the database, 3,135 CC cases with FIGO 2018 stage IIICp (FIGO 2009 stages I-IIB with metastatic lymph node) met our inclusion and exclusion criteria, with no missing values. The selection process for these cases is illustrated in Fig. 1. The sequential variables were in normal distribution. The median number of removed LNs was 20 (range, 7–116). The ROC curve analysis showed that the optimal cut-off values of nMLN and LNR to predict 5-year OS and DFS were 3.5 and 0.11, respectively. Therefore, patients were divided into nMLN ≤ 3 (n = 2,378) and nMLN > 3 (n = 757) groups, and LNR ≤ 0.11 (n = 1,748) and LNR > 0.11 (n = 1,387) groups. Baseline comparisons of patients in the nMLN ≤ 3 vs nMLN > 3 groups and LNR ≤ 0.11 vs LNR > 0.11 groups (pre-PSM and post-PSM) are shown in Tables 1 and 2.
Survival analysis of all patients (pre-PSM)
Before PSM, the HRs for lower survival in the nMLN > 3 groups were as follows: OS: 1.437, 95% CI: 1.155–1.789, P = 0.001, and DFS: 1.468, 95% CI: 1.228–1.754, P < 0.001, while those in the LNR > 0.11 group were as follows: OS: 1.454, 95% CI: 1.181–1.790, P < 0.001; DFS: 1.392, 95% CI: 1.177–1.646, P < 0.001. Adenocarcinoma correlated with worse 5-year OS and DFS than squamous cell carcinoma (P < 0.001), while adenosquamous cell carcinoma correlated with worse 5-year OS (P = 0.006) but not with DFS (P = 0.069). Tumor diameter > 4 cm and unknown tumor diameter correlated with worse 5-year OS and DFS (P < 0.05) compared to tumor diameter ≤ 4 cm. Compared to negative lymphovascular space invasion, positive lymphovascular space invasion correlated with a worse 5-year OS (P = 0.005) but not with DFS (P = 0.471). Compared to cervical stromal invasion ≤ 1/2, cervical stromal invasion > 1/2 correlated with a worse 5-year OS and DFS (P < 0.001), while unknown cervical stromal invasion correlated with a worse 5-year OS (P = 0.041) but not with DFS (P = 0.065). Compared to negative parametrial involvement, positive parametrial involvement correlated with worse 5-year DFS (P < 0.001) but not with OS (P = 0.149). Additionally, there were no effects of age, hysterectomy type, vaginal margin, or para-aortic lymph node metastasis on either 5-year OS or DFS (P > 0.05) (Table 3).
Comparison of oncological outcomes in nMLN ≤ 3 and nMLN > 3 groups
The Kaplan–Meier analysis revealed significant differences in survival between the nMLN ≤ 3 and nMLN > 3 groups with OS: 81.0% vs 66.0%, P < 0.001, and DFS: 70.3% vs 51.5%, P < 0.001 (pre-PSM); OS: 76.8% vs 67.9%, P = 0.003 and DFS: 65.5% vs 55.3%, P < 0.001 (post-PSM) (Fig. 2).
After PSM, the HRs for lower survival for the nMLN > 3 group were as follows, OS: 1.411, 95% CI: 1.108–1.798, P = 0.005, and DFS: 1.428, 95% CI: 1.175–1.735, P < 0.001). LNR > 0.11 was associated with a worse 5-year OS (P = 0.026) but not DFS (P = 0.114), compared to LNR ≤ 0.11. While adenocarcinoma correlated with worse 5-year DFS than squamous cell carcinoma (P = 0.047) but not with OS (P = 0.129), adenosquamous cell carcinoma did not affect either 5-year OS or DFS (P > 0.05). Compared to Type QM-B, Type QM-C2 was associated with poorer 5-year OS and DFS (P < 0.05). However, Type QM-C1 and unknown hysterectomy types were not associated with worse 5-year OS or DFS (P > 0.05). Compared to tumor diameter ≤ 4 cm, tumor diameter > 4 cm correlated with a worse 5-year OS (P = 0.035) but not with DFS (P = 0.050); unknown tumor diameter were not associated with worse 5-year OS or DFS (P > 0.05). Cervical stromal invasion > 1/2 correlated with a worse 5-year DFS (P = 0.035) but not OS (P = 0.174) compared to cervical stromal invasion ≤ 1/2, while unknown cervical stromal invasion did not affect either 5-year OS or DFS (P > 0.05). Positive parametrial involvement correlated with worse 5-year OS and DFS (P < 0.05) when compared to negative parametrial involvement. Moreover, before and after PSM there were no effects of age, lymphovascular space invasion, vaginal margin, or para-aortic lymph node metastasis on either 5-year OS or DFS (P > 0.05) (Table 4).
Comparison of oncological outcomes in LNR ≤ 0.11 and LNR > 0.11 groups
The Kaplan–Meier analysis revealed significant differences in survival between the LNR ≤ 0.11 and LNR > 0.11 groups as follows: OS: 81.9% vs 70.6%, P < 0.001, and DFS: 72.3% vs 57.9%, P < 0.001 (pre-PSM); OS: 82.5% vs 76.9%, P = 0.010; and DFS: 72.8% vs 65.1%, P = 0.002 (post-PSM) (Fig. 3).
After PSM, the HRs for lower survival for the LNR > 0.11 group were as follows, OS: 1.407, 95% CI: 1.103–1.794, P = 0.006, and DFS: 1.347, 95% CI: 1.110–1.633, P = 0.002. While adenocarcinoma correlated with a worse 5-year OS and DFS compared to squamous cell carcinoma (P = 0.001 and 0.110, respectively), adenosquamous cell carcinoma did not affect either the 5-year OS or DFS (P > 0.05). Tumor diameter > 4 cm correlated with a worse 5-year OS and DFS (P = 0.013 and 0.012, respectively) compared to tumor diameter ≤ 4 cm, while unknown tumor diameter did not affect either the 5-year OS or DFS (P > 0.05). Compared to cervical stromal invasion ≤ 1/2, cervical stromal invasion > 1/2 correlated with a worse 5-year OS and DFS (P = 0.008 and 0.001, respectively), while unknown cervical stromal invasion did not affect either 5-year OS or DFS (P > 0.05). Positive parametrial involvement correlated with a worse 5-year OS and DFS (P = 0.025 and 0.001, respectively) compared to negative parametrial involvement. Compared to negative vaginal margins, positive vaginal margins correlated with a worse 5-year DFS (P = 0.012) but not with OS (P = 0.889). Additionally, before and after PSM there were no effects of age, nMLN, hysterectomy type, lymphovascular space invasion, or para-aortic lymph node metastasis on either 5-year OS or DFS (P > 0.05) (Table 5).
Discussion
Summary of main results
Metastatic lymph node is a poor prognostic indicator for CC [2,3,4,5]. The current study determined the impact of nMLN and LNR on survival outcomes in patients with FIGO 2018 stage IIICp CC. We demonstrated that nMLN > 3 indicated a worse prognosis than nMLN ≤ 3, while LNR > 0.11 was a poor prognostic indicator compared to LNR ≤ 0.11.
Predictive value of the nMLN and LNR
The LN staging system based on nMLN has been extensively applied to breast, gastric, and rectal carcinomas [21]. In CC, high nMLN is negatively correlated with prognosis [7,8,9,10,11,12,13,14,15]. While cut-off values for nMLN ranging from ≥ 2 to > 5 have been reported [10, 12,13,14,15], the optimal cut-off value is still being explored. Several studies have demonstrated that the prognosis of patients with only one metastatic lymph node was comparable to that of those without metastatic lymph nodes [12,13,14]. However, patients with ≥ 2 metastatic lymph nodes had poorer survival than those without an metastatic lymph node (87% vs 61%, P < 0.001) [14] and a worse 5-year DFS than those with 1–2 metastatic lymph nodes (54.7% vs 78.1%, P = 0.006) [10]. A 10-year multi-center study by Kwon et al. in Korea included 249 patients with FIGO 2009 stages IB-IIA CC, all of whom underwent radical hysterectomy with pelvic ± para-aortic lymphadenectomy [7]. All patients had pathologically confirmed metastatic lymph node and underwent postoperative standardized adjuvant therapy. The mean number of removed LNs was 26 (range, 4–85), and the Evaluate Cut Points application was used to determine the cut-off values. Kwon et al. found that nMLN > 3, lymphovascular space invasion, and non-squamous cell carcinoma were risk factors for poor distant metastasis-free survival and DFS in early-stage CC, while there was no significant survival difference between patients without an metastatic lymph node and those with 1–3 metastatic lymph nodes [7]. Olthof et al. studied CC patients (FIGO 2009 stages IA2-IIA1) with metastatic lymph nodes diagnosed after radical hysterectomy and pelvic lymphadenectomy and demonstrated that nMLN ≥ 4 was independently associated with poor survival [15]. Another study by Inoue and Morita included CC patients (FIGO 2009 stages IB-IIB) with pathologically confirmed metastatic lymph node after radical hysterectomy and bilateral pelvic lymphadenectomy [12]. They concluded that the nMLN was a better prognostic factor than the presence of metastatic lymph node, with 5-year OS of 81%, 41%, and 23% in patients with 1, 2–3, and ≥ 4 metastatic lymph nodes, respectively (P < 0.001). Patients with ≥ 4 metastatic lymph nodes had markedly lower survival rates than those without any metastatic lymph node. Consistent with the studies by Kwon et al., Olthof et al., and Inoue and Morita, we found that the nMLN > 3 group had a poorer prognosis than the nMLN ≤ 3 group [7, 12, 15]. All these studies included patients with similar stages of CC. While our study included patients with FIGO 2009 stages IA-IIB CC, Kwon et al., Inoue et al., and Olthof et al. studied those with FIGO 2009 stages IB-IIA, FIGO 2009 stages IB-IIB, and FIGO 2009 stages IA2-IIA1 CC [7, 12, 15]. Additionally, patients in all the studies underwent radical hysterectomy with pelvic ± para-aortic lymphadenectomy. Finally, the metastatic lymph nodes were diagnosed through postoperative pathological confirmation in all these studies. These factors also account for the inconsistent results from other studies.
The LNR is the ratio of the nMLN to the total number of removed LNs, which avoids the effects of confounding factors [29]. LNR is a newly validated predictor of CC, however, the exact cut-off value for LNR remains unclear, with the values reported in the literature ranging from 0.05 to 0.2 [11, 15, 18,19,20, 23,24,25,26]. In a study by Aslan et al. [19] that included 138 patients with stage IIIC1 and 47 with stage IIIC2 CC, patients with LNR < 0.05 had better 5-year OS and DFS than those with LNR ≥ 0.05 (OS: 80.6% vs 61.2%, P = 0.007; DFS: 78.2% vs 48.4%, P < 0.001). However, 23.2% of the patients underwent para-aortic lymphadenectomy, which limited the number of removed LNs (pelvic LNs > 10 and para-aortic LNs > 5). In another study on FIGO 2009 stage I or II CC, Fleming et al. [20] demonstrated that LNR > 0.066 was associated with poorer progression-free survival, whereas LNR > 0.076 was associated with poorer OS. However, only 95 patients were included in the study. Similar results were reported by Li et al. LNR ≥ 0.08 was an independent prognostic factor for OS (P = 0.001) [24]. Their study included 273 patients with FIGO 2018 stage IIICp CC; only a few received postoperative adjuvant therapy. Another study reported that CC patients with LNR > 0.2 had a 2.56-fold higher risk of poor OS and a 2.404-fold higher risk of poor DFS than those with LNR < 0.2 [18]. Their study, which included 198 squamous cell carcinoma patients (42.4% of whom without postoperative adjuvant therapy), showed that the LNR had a higher predictive value than the cervical stromal invasion depth [18]. Several studies have reported cut-off values of LNR comparable to the value (0.11) in the current study [11, 15, 23, 25]. In a study including 80 patients with CC and metastatic lymph nodes (FIGO 2009 stages IIB to IVA) who underwent concurrent platinum-based radiotherapy after surgical staging, Polterauer et al. [23] concluded that LNR > 0.1 was an independent factor associated with poor DFS and OS (P = 0.01). Chen et al. [25] studied 120 CC patients with metastatic lymph nodes (FIGO 2009 stages IA2 to IIB) and found that patients with LNR > 0.1 had markedly poorer 5-year OS than those with LNR ≤ 0.1 (42.9% and 11.8%, respectively). In addition, as a prognostic evaluator, LNR was verified to be superior to nMLN. In Chen’s study, 75% of the patients received neoadjuvant chemotherapy before surgery. Guo et al. [11] showed that for squamous cell carcinoma patients with FIGO 2018 stage IIIC CC (928IIIC1/97IIIC2), the LNR > 0.16 was an independent risk factor. The stage was confirmed by radical abdominal hysterectomy, pelvic lymphadenectomy, bilateral salpingo-oophorectomy, and para-aortic lymphadenectomy. Additionally, Olthof et al. [15] found that LNR ≥ 0.177 was associated with a poorer 5-year OS compared with LNR < 0.177.
The accuracy of imaging such as computed tomography, magnetic resonance imaging, and positron emission tomography for the diagnosis of metastatic lymph node requires further improvement [30]. The sensitivity and specificity of computed tomography were 57% and 91%, magnetic resonance imaging were 52% and 94%, and positron emission tomography were 66% and 97%, respectively [31]. Therefore, pathological confirmation appears to be the most accurate criterion for assessing LN status [32]. Meanwhile, laparoscopic treatment of CC has shown that minimally invasive radical hysterectomy has a worse prognosis than open surgery [33]. Therefore, only patients who underwent open surgery and had pathologically confirmed metastatic lymph node were included in the current study. Currently, there is no definitive opinion on the minimum number of LNs that should be removed during lymphadenectomy. In 2009, the European Organization for Research and Treatment of Cancer proposed a standard of 11 LNs to be removed during surgery for CC [34]. However, according to the European Society of Gynecologic Oncology Quality Indicators for the Management of Cervical Cancer Surgery, there is no specific recommendation regarding the number of LNs to be removed during lymphadenectomy [35]. Therefore, the number of LNs removed was not limited in this study. Theoretically, the higher the number of examined LNs, the higher the value of the assessment of LN status [36]. The number of examined metastatic lymph nodes positively correlates with the number of removed LNs [7, 37]. Therefore, inconsistencies in the cut-off values of nMLN and LNR may be due to the diagnosis of metastatic lymph node and number of removed LNs.
Predictive value of other prognostic predictors
The prognostic risk factors for CC include both high-risk and intermediate-risk factors. Pelvic LN metastasis, parametrial involvement, and positive vaginal margin represent the main high-risk factors, while the intermediate-risk ones include lymphovascular space invasion, deep cervical stromal invasion, and tumor diameter > 4 cm [38, 39]. A meta-analysis by Huang et al. [39] demonstrated a significant association between deep cervical stromal invasion, parametrial involvement, lymphovascular space invasion, tumor diameter, age, and pelvic LN metastasis. A report by Salvo et al. [40] disputed this, claiming that only the effect of metastatic LN was considered in cases of stage IIIC diseases, without any consideration given to factors such as tumor diameter or parametrial, vaginal, and ovarian invasion. The present study revealed that, in addition to nMLN and LNR, adenocarcinoma, adenosquamous cell carcinoma, lymphovascular space invasion, tumor diameter > 4 cm, cervical stromal invasion > 1/2, positive vaginal margin, and positive parametrial involvement were significant prognostic predictors—which is in agreement with the results of the above two studies.
The most common CC tissue type is squamous carcinoma (75%), followed by adenocarcinoma (10–25%), with other types (including adenosquamous carcinoma) being far less frequent (< 5%) [41]. Hence, we were only able to analyze small number of adenosquamous carcinoma cases in this study. Some studies have suggested that tissue type correlates with prognosis [42,43,44,45]. Nakanishi et al. and Kodama et al. [44, 45] both demonstrated that LN metastasis can influence outcomes, being associated with a significantly worse prognosis for adenocarcinoma than for squamous cell carcinoma. A study on stage IIIC disease also showed that the prognoses of adenocarcinoma and adenosquamous cell carcinoma were worse than that of squamous carcinoma [43]. In this study, both adenocarcinoma (OS HR: 1.590, 95% CI: 1.226–2.063, P < 0.001; DFS HR: 1.498, 95% CI. 1.217–1.845, P < 0.001) and adenosquamous cell carcinoma (OS HR: 1.779, 95% CI: 1.177–2.690, P = 0.006) had worse prognoses than squamous cell carcinoma before PSM. After PSM only adenocarcinoma had a worse prognosis than squamous carcinoma (DFS HR: 1.385, 95% CI: 1.004–1.910, P = 0.047 in the nMLN ≤ 3 and nMLN > 3 groups; OS HR: 1.860, 95% CI: 1.289–2.683, P = 0.001; DFS HR: 1.500, 95% CI: 1.104–2.039, P = 0.010 in the LNR ≤ 0.11 and LNR > 0.11 groups), which may be related to the lower number of adenosquamous cell carcinoma cases in these groups after PSM.
Lymphovascular space invasion manifests as one or more clusters of tumor cells in the interstitial space (ie., the lymphatic or vascular interstitial space) surrounded by endothelial cells, suggesting invasion of tumor cells into blood and lymphatic vessels. These tumor cells may spread and implant at other sites throughout surrounding blood or lymphatic vessels, leading to tumor metastasis. However, current studies on the relationship between lymphovascular space invasion and prognosis are conflicting. Kwon et al. and Widschwendter et al. both showed that lymphovascular space invasion strongly correlated with LN metastasis and resulted in a poor prognosis in cases of CC [7, 22]. However, a study by Wang et al. [46] reported that this phenomenon was an independent predictor of pelvic LN metastasis, but that there is currently no evidence to suggest that it can guide early-stage CC prognosis. However, that study also included cases without metastatic LN. In this study, lymphovascular space invasion correlated with lower OS (OS HR: 1.283, 95% CI: 1.080–1.524, P = 0.005) before PSM, but not afterward. Therefore, the relationship between lymphovascular space invasion and prognosis in stage IIIC merits further investigation through prospective studies.
Tumor size was found to be positively associated with pelvic LN metastasis, as a tumor diameter of > 4 cm increased the rate of pelvic LN metastasis by as much as as 43.5%. Tumor diameter was also found to correlate significantly with prognosis [47]. In their study, Park et al. confirmed that a tumor diameter of > 4 cm had a significant impact on patient prognoses [48]. In this study, a tumor diameter of >CI: 1.1111–2.825 4 cm was associated with poorer prognosis (OS HR: 1.408, 95% CI: 1.183–1.675, P < 0.001; DFS HR: 1.365, 95% CI: 1.187- 1.569, P < 0.001 pre-PSM; post-PSM, in the nMLN ≤ 3 and nMLN > 3 groups, OS HR: 1.320, 95% CI: 1.020–1.708, P = 0.035; in the LNR ≤ 0.11 and LNR > 0.11 groups, OS HR: 1.383, 95% CI: 1.072–1.785, P = 0.013; DFS HR: 1.297, 95% CI: 1.060–1.587, P = 0.012). It is therefore possible that increased tumor size correlates with increased risk of parametrial involvement and LN metastasis, as well as decreased survival [49].
Paracervical lymphoid tissue is abundant. The paracervix represents the first site of extracervical dissemination, and parametrial involvement tends to lead to LN metastasis. Nanthamongkolkul et al. suggested that deep parametrial involvement and deep cervical stromal invasion correlated with LN metastasis, resulting in a decreased survival rate [50, 51]. This study also found that positive parametrial involvement correlated with poorer prognoses ( DFS HR: 1.562, 95% CI: 1.223–1.995, P < 0.001 pre-PSM; post-PSM, in the nMLN ≤ 3 and nMLN > 3 groups, OS HR: 1.772, 95% CI: 1.1111–2.825, P = 0.016; DFS HR: 1.805, 95% CI: 1.245–2.618, P = 0.002; in the LNR ≤ 0.11 and LNR > 0.11 groups, OS HR: 1.720, 95% CI: 1.071–2.760, P = 0.025; DFS HR: 1.859, 95% CI: 1.291–2.678, P = 0.001). A cervical stromal invasion of > 1/2 also correlated with poorer prognosis compared to ≤ 1/2 (OS HR: 1.738, 95% CI: 1.313–2.301, P < 0.001; DFS HR: 1.581, 95% CI: 1.278–1.957, P < 0.001 pre-PSM; post-PSM, in the nMLN ≤ 3 and nMLN > 3 groups, DFS HR: 1.367, 95% CI: 1.023–1.828, P = 0.035; in the LNR ≤ 0.11 and LNR > 0.11 groups, OS HR: 1.729, 95% CI: 1.151–2.598, P = 0.008; DFS HR: 1.639, 95% CI: 1.208–2.223, P = 0.001).
Surgical staging of CC is currently based on Querleu-Morrow (QM) staging, which is divided into four major categories: A, B (B1, B2), C (C1, C2), and D (D1, D2). The standard surgical procedure for CC is radical hysterectomy accompanied by possible pelvic and para-aortic lymphadenectomy. Radical hysterectomies are staged according to QM as type QM-C2. Type QM-C2 hysterectomies involve the removal of the entire parametrium and pelvic autonomic nerves, which can lead to significant complications [52]. In contrast, type QM-B hysterectomies involve relatively narrow resections that may reduce complications, potentially with similar therapeutic outcomes to those of type QM-C [53]. A study by Chen et al. [54], which analyzed data from 9135 CC cases, showed that, before PSM, patients who underwent type QM-C2 hysterectomies had lower 5-year OS (89.5 vs 92.0%, HR: 1.393) and DFS (84.3 vs 87.4%, HR: 1.342) rates compared to those who underwent type QM-B ones. After PSM, there was no difference in OS between the two groups, but the 5-year DFS was lower in the patients who underwent type QM-C2 procedures (82.1 vs 84.8%, HR: 1.144). Therefore, type Q-MB hysterectomies may be useful for the treatment of stage IA1 (lymphovascular space invasion)-IIA2 CCs. In this study, only patients in the nMLN ≤ 3 and nMLN > 3 groups who underwent type QM-C2 hysterectomies had poorer prognoses than those who had type QM-B procedures, after PSM (OS HR: 1.329, 95% CI: 1.021–1.729, P = 0.034; DFS HR: 1.293, 95% CI: 1.044–1.601, P = 0.019). That study by Chen et al. included patients without LN metastasis, whereas all patients in the present study had LN metastasis, which may explain the differences in the results obtained between the two studies. Whether type QM-B hysterectomy procedures can be effectively used to treat stage IIIC malignancies merits further exploration.
Strengths and weaknesses
The strength of the current study is that it includes real-world data using a large sample size from a developing country with a high prevalence of CC. In addition, we accurately calculated the cut-off values using ROC curves. The effect of intra-group baseline differences was reduced using the statistically accepted PSM. However, due to the retrospective nature of the analysis, the current study has certain limitations. First, retrospective studies do not allow for rigorous screening of study subjects, resulting in significant population heterogeneity in the study cohort. Second, all patients in this study received standard postoperative adjuvant therapy; however, detailed adjuvant regimens, drug doses, and radiation doses were not differentiated. All of these factors may have had an impact on survival rates. Third, inter-institutional heterogeneity might exist, especially in surgical techniques, despite the two institutions sharing training programs for gynecologic oncologists. Finally, the high number of cases without para-aortic lymphadenectomy and the lack of metastasis-related information such as metastatic lesions, para-aortic or pelvic topography, type of LN metastasis (size, micrometastasis) etc., as well as the lack of detailed protocols for the evaluation of LN by the pathologists and information on the status of HPV infection may have led to some bias in our results.
Implications for practice and future research
In conclusion, for patients with FIGO 2018 stage IIICp CC, nMLN > 3 and LNR > 0.11 appeared to be associated with inferior oncological outcomes. In clinical practice, patients with FIGO 2018 stage IIICp CC need to be staged and appropriately treated to stratify the survival risk according to the impact of the nMLNs and LNR. A multi-center, large-sample prospective study is needed to further confirm the results of the current study.
Conclusions
Patients with nMLN > 3 have a worse prognosis than those with nMLN ≤ 3, while patients with LNR > 0.11 have a poorer prognosis than those with LNR ≤ 0.11. Treatment and management of patients with FIGO 2018 stage IIICp CC need to be differentiated by nMLN and LNR.
Availability of data and materials
Data supporting the findings of this study are available upon request from the corresponding authors. The data are not publicly available because of privacy and ethical restrictions. These data are also related to unpublished studies. However, these data involve privacy issues for some patients and are not publicly shared.
Abbreviations
- ARH:
-
Abdominal radical hysterectomy
- CC:
-
Cervical cancer
- CI:
-
Confidence intervals
- DFS:
-
Disease-free survival
- FIGO:
-
International Federation of Gynecology and Obstetrics
- HR:
-
Hazard ratio
- LN:
-
Lymph node
- LNR:
-
Lymph node ratio
- nMLN:
-
Number of metastatic lymph nodes
- OS:
-
Overall survival
- PSM:
-
Propensity score matching
- QM:
-
Querleu-Morrow
- ROC:
-
Receiver operating characteristic
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Miyahara S, Tsuji K, Shimada M, Shibuya Y, Shigeta S, Nagai T, et al. The impact of histological subtype on survival outcome of patients with stage IIB-IVA cervical cancer who received definitive radiotherapy. Tohoku J Exp Med. 2021;55(4):303–13. https://doi.org/10.1620/tjem.255.303.
Chen W, Xiu S, Xie X, Guo H, Xu Y, Bai P, et al. Prognostic value of tumor measurement parameters and SCC-Ag changes in patients with locally-advanced cervical cancer. Radiat Oncol. 2022;17(1):6. https://doi.org/10.1186/s13014-021-01978-0. Published 2022 Jan 10.
Qin F, Pang H, Yu T, Luo Y, Dong Y. Treatment strategies and prognostic factors of 2018 FIGO stage IIIC cervical cancer: a review. Technol Cancer Res Treat. 2022;21: 15330338221086403. https://doi.org/10.1177/15330338221086403.
Brodeur MN, Dejean R, Beauchemin MC, Samouëlian V, Cormier B, Bacha OM, et al. Oncologic outcomes in the era of modern radiation therapy using FIGO 2018 staging system for cervical cancer. Gynecol Oncol. 2021;162(2):277–83. https://doi.org/10.1016/j.ygyno.2021.05.023.
Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl 1(Suppl 1):28–44. https://doi.org/10.1002/ijgo.13865.
Kwon J, Eom KY, Kim YS, Park W, Chun M, Lee J, et al. The prognostic impact of the number of metastatic lymph nodes and a new prognostic scoring system for recurrence in early-stage cervical cancer with high risk factors: a multicenter cohort study (KROG 15–04). Cancer Res Treat. 2018;50(3):964–74. https://doi.org/10.4143/crt.2017.346.
Lee YJ, Kim DY, Lee SW, Park JY, Suh DS, Kim JH, et al. A postoperative scoring system for distant recurrence in node-positive cervical cancer patients after radical hysterectomy and pelvic lymph node dissection with para-aortic lymph node sampling or dissection. Gynecol Oncol. 2017;144(3):536–40. https://doi.org/10.1016/j.ygyno.2017.01.001.
Yan DD, Tang Q, Tu YQ, Chen JH, Lv XJ. A comprehensive analysis of the factors of positive pelvic lymph nodes on survival of cervical cancer patients with 2018 FIGO stage IIIC1p. Cancer Mana Res. 2019;11:4223–30. https://doi.org/10.2147/CMAR.S204154.
Pedone AL, Carbone V, Gallotta V, Fanfani F, Cosentino F, Turco LC, et al. Should the number of metastatic pelvic lymph nodes be integrated into the 2018 figo staging classification of early stage cervical cancer? Cancers. 2020;12(6): 1552. https://doi.org/10.3390/cancers12061552.
Guo Q, Zhu J, Wu Y, Wen H, Xia L, Ju X, et al. Validation of the prognostic value of various lymph node staging systems for cervical squamous cell carcinoma following radical surgery: a single-center analysis of 3,732 patients. Ann Transl Med. 2020;8(7):485. https://doi.org/10.21037/atm.2020.03.27.
Inoue T, Morita K. The prognostic significance of number of positive nodes in cervical carcinoma stages IB, IIA, and IIB. Cancer. 1990;65(9):1923–7. https://doi.org/10.1002/1097-0142(19900501)65:9%3c1923::aid-cncr2820650909%3e3.0.co;2-m.
Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85(7):1547–54. https://doi.org/10.1002/(sici)1097-0142(19990401)85:7%3c1547::aid-cncr16%3e3.0.co;2-2.
Tsai CS, Lai CH, Wang CC, Chang JT, Chang TC, Tseng CJ, et al. The prognostic factors for patients with early cervical cancer treated by radical hysterectomy and postoperative radiotherapy. Gynecol Oncol. 1999;75(3):328–33. https://doi.org/10.1006/gyno.1999.5527.
Olthof EP, Mom CH, Snijders MLH, Wenzel HHB, van der Velden J, van der Aa MA. The prognostic value of the number of positive lymph nodes and the lymph node ratio in early-stage cervical cancer. Acta Obstet Gynecol Scand. 2022;101(5):550–7. https://doi.org/10.1111/aogs.14316.
Voordeckers M, Vinh-Hung V, Van de Steene J, Lamote J, Storme G. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol. 2004;70(3):225–30. https://doi.org/10.1016/j.radonc.2003.10.015.
Kim YS, Kim JH, Yoon SM, Choi EK, Ahn SD, Lee SW, et al. Lymph node ratio as a prognostic factor in patients with stage III rectal cancer treated with total mesorectal excision followed by chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;74(3):796–802. https://doi.org/10.1016/j.ijrobp.2008.08.065.
Li C, Liu W, Cheng Y. Prognostic significance of metastatic lymph node ratio in squamous cell carcinoma of the cervix. Onco Targets Ther. 2016;9:3791–7. https://doi.org/10.2147/OTT.S97702.
Aslan K, Meydanli MM, Oz M, Tohma YA, Haberal A, Ayhan A. The prognostic value of lymph node ratio in stage IIIC cervical cancer patients triaged to primary treatment by radical hysterectomy with systematic pelvic and para-aortic lymphadenectomy. J Gynecol Oncol. 2020;31(1): e1. https://doi.org/10.3802/jgo.2020.31.e1.
Fleming ND, Frumovitz M, Schmeler KM, dos Reis R, Munsell MF, Eifel PJ, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol. 2015;136(1):48–53. https://doi.org/10.1016/j.ygyno.2014.11.010.
Joo JH, Kim YS, Nam JH. Prognostic significance of lymph node ratio in node-positive cervical cancer patients. Medicine. 2018;97(30):e11711. https://doi.org/10.1097/MD.000000000001171.
Widschwendter P, Polasik A, Janni W, de Gregorio A, Friedl TWP, de Gregorio N. Lymph node ratio can better predict prognosis than absolute number of positive lymph nodes in operable cervical carcinoma. Oncol Res Treat. 2020;43(3):87–95. https://doi.org/10.1159/000505032.
Polterauer S, Hefler L, Seebacher V, Rahhal J, Tempfer C, Horvat R, et al. The impact of lymph node density on survival of cervical cancer patients. Br J Cancer. 2010;103(5):613–6. https://doi.org/10.1038/sj.bjc.6605801.
Li A, Wang L, Jiang Q, Wu W, Huang B, Zhu H. Risk stratification based on metastatic pelvic lymph node status in stage IIIC1p cervical cancer. Cancer Manag Res. 2020;12:6431–9. https://doi.org/10.2147/CMAR.S253522.
Chen Y, Zhang L, Tian J, Fu X, Ren X, Hao Q. Significance of the absolute number and ratio of metastatic lymph nodes in predicting postoperative survival for the International Federation of Gynecology and Obstetrics stage IA2 to IIA cervical cancer. Int J Gynecol Cancer. 2013;23(1):157–63. https://doi.org/10.1097/IGC.0b013e318277.
Li Z, Duan H, Guo J, Yang Y, Wang W, Hao M, et al. Discussion on the rationality of FIGO 2018 stage IIIC for cervical cancer with oncological outcomes: a cohort study. Ann Transl Med. 2022;10(2):122. https://doi.org/10.21037/atm-21-6374.
Ye Y, Li Z, Kang S, Zhan X, Zhang Y, Xu Y, et al. Impact of different postoperative adjuvant therapies on the survival of early-stage cervical cancer patients with one intermediate-risk factor: a multicenter study of 14 years. J Obstet Gynaecol Res. 2023;49(6):1579–91. https://doi.org/10.1111/jog.15632.
Ye Y, Li Z, Kang S, Yang Y, Ling B, Wang L, et al. Treatment of FIGO 2018 stage IIIC cervical cancer with different local tumor factors. BMC cancer. 2023;23(1):421. https://doi.org/10.1186/s12885-023-10801-w.
Zhou J, Wu SG, Sun JY, Liao XL, Li FY, Lin HX, et al. Incorporation of the number of positive lymph nodes leads to better prognostic discrimination of node-positive early stage cervical cancer. Oncotarget. 2017;8(16):26057–65. https://doi.org/10.18632/oncotarget.15220.
Atri M, Zhang Z, Dehdashti F, Lee SI, Ali S, Marques H, et al. Utility of PET-CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: Results of ACRIN6671/GOG0233 trial. Gynecol Oncol. 2016;142(3):413–9. https://doi.org/10.1016/j.ygyno.2016.05.002.
Liu B, Gao S, Li S. A Comprehensive comparison of CT, MRI, positron emission tomography or positron emission tomography/ct, and diffusion weighted imaging-MRI for detecting the lymph nodes metastases in patients with cervical cancer: a meta-analysis based on 67 studies. Gynecol Obstet Invest. 2017;82(3):209–22. https://doi.org/10.1159/000456006.
Zigras T, Lennox G, Willows K, Covens A. Early cervical cancer: current dilemmas of staging and surgery. Curr Oncol Rep. 2017;19(8):51. https://doi.org/10.1007/s11912-017-0614-5.
Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895–904. https://doi.org/10.1056/NEJMoa1806395.
Verleye L, Vergote I, Reed N, Ottevanger PB. Quality assurance for radical hysterectomy for cervical cancer: the view of the European Organization for Research and Treatment of Cancer-Gynecological Cancer Group (EORTC-GCG). Annals of oncology : official journal of the European Society for Medical Oncology. 2009;20(10):1631–8. https://doi.org/10.1093/annonc/mdp196.
Cibula D, Planchamp F, Fischerova D, Fotopoulou C, Kohler C, Landoni F, et al. European society of gynaecological oncology quality indicators for surgical treatment of cervical cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2020;30(1):3–14. https://doi.org/10.1136/ijgc-2019-000878.
Zhou J, Zhang WW, Wu SG, He ZY, Sun JY, Wang Y, et al. The impact of examined lymph node count on survival in squamous cell carcinoma and adenocarcinoma of the uterine cervix. Cancer management and research. 2017;9:315–22. https://doi.org/10.2147/CMAR.S141335.
Kesic V. Management of cervical cancer. Eur J Surg Oncol. 2006;32(8):832–7. https://doi.org/10.1016/j.ejso.2006.03.037.
Ramireza PT, Frumovitza M, Parejab R, Lopezc A, Vieirad MA, Ribeiroe RA. Phase III randomized trial of laparoscopic or robotic versus abdominal radical hysterectomy in patients with early stage cervical cancer: LACC trial. Gynecol Oncol. 2018;149(1):245. https://doi.org/10.1016/j.ygyno.2018.04.552.
Bedford S. Cervical cancer: physiology, risk factors, vaccination and treatment. Br J Nurs. 2009;18(2):80–4. https://doi.org/10.12968/bjon.2009.18.2.37874.
Huang BX, Fang F. Progress in the study of lymph node metastasis in early-stage cervical cancer. Current medical science. 2018;38(4):567–74. https://doi.org/10.1007/s11596-018-1915-0.
Salvo G, Odetto D, Pareja R, Frumovitz M, Ramirez PT. Revised 2018 International Federation of Gynecology and Obstetrics (FIGO) cervical cancer staging: a review of gaps and questions that remain. Int J Gynecol Cancer. 2020;30(6):873–8. https://doi.org/10.1136/ijgc-2020-001257.
Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. https://doi.org/10.1002/ijgo.12611.
Pan X, Yang W, Wen Z, Li F, Tong L, Tang W. Does adenocarcinoma have a worse prognosis than squamous cell carcinoma in patients with cervical cancer? A real-world study with a propensity score matching analysis. J Gynecol Oncol. 2020;31(6): e80. https://doi.org/10.3802/jgo.2020.31.e80.
Ye Y, Zhang G, Li Z, et al. Initial treatment for FIGO 2018 stage IIIC cervical cancer based on histological type: A 14-year multicenter study. Cancer Med. 2023;12(19):19617–32. https://doi.org/10.1002/cam4.6586.
Nakanishi T, Ishikawa H, Suzuki Y, Inoue T, Nakamura S, Kuzuya K. A comparison of prognoses of pathologic stage Ib adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2000;79(2):289–93. https://doi.org/10.1006/gyno.2000.5935.
Kodama J, Seki N, Masahiro S, Kusumoto T, Nakamura K, Hongo A, et al. Prognostic factors in stage IB-IIB cervical adenocarcinoma patients treated with radical hysterectomy and pelvic lymphadenectomy. J Surg Oncol. 2010;101(5):413–7. https://doi.org/10.1002/jso.21499.
Wang W, Jia HL, Huang JM, et al. Identification of biomarkers for lymph node metastasis in early-stage cervical cancer by tissue-based proteomics. Br J Cancer. 2014;110(7):1748–58. https://doi.org/10.1038/bjc.2014.92.
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379(20):1905–14. https://doi.org/10.1056/NEJMoa1804923.
Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Further stratification of risk groups in patients with lymph node metastasis after radical hysterectomy for early-stage cervical cancer. Gynecol Oncol. 2010;117(1):53–8. https://doi.org/10.1016/j.ygyno.2009.12.006.
Kubota S, Kobayashi E, Kakuda M, et al. Retrospective analysis for predictors of parametrial involvement in IB cervical cancer. J Obstet Gynaecol Res. 2019;45(3):679–85. https://doi.org/10.1111/jog.13855.
Nanthamongkolkul K, Hanprasertpong J. Predictive factors of pelvic lymph node metastasis in early-stage cervical cancer. Oncol Res Treat. 2018;41(4):194–8. https://doi.org/10.1159/000485840.
Turan T, Kimyon Comert G, Boyraz G, et al. What is the impact of corpus uterine invasion on oncologic outcomes in surgically treated cervical cancer? J Obstet Gynaecol Res. 2021;47(10):3634–43. https://doi.org/10.1111/jog.14953.
Wang X, Chen C, Liu P, Li W, Wang L, Liu Y. The morbidity of sexual dysfunction of 125 Chinese women following different types of radical hysterectomy for gynaecological malignancies. Arch Gynecol Obstet. 2018;297(2):459–66. https://doi.org/10.1007/s00404-017-4625-0.
Chen C, Wang W, Liu P, et al. Survival after abdominal Q-M type B versus C2 radical hysterectomy for early-stage cervical Cancer. Cancer Manag Res. 2019;11:10909–19. https://doi.org/10.2147/CMAR.S220212. Published 2019 Dec 31.
Acknowledgements
We thank Min Hao (The Second Hospital of Shanxi Medical University), Bin Ling (China-Japan Friendship Hospital), Lixin Sun and Hongwei Zhao (Shanxi Cancer Hospital), Jihong Liu and Lizhi Liang (Sun Yat-sen University Cancer Center), Lihong Lin and Yu Guo (Anyang Tumor Hospital), Li Wang (The Affiliated Tumor Hospital of Zhengzhou University), Weidong Zhao (Anhui Provincial Cancer Hospital), Yan Ni (The Yuncheng Central Hospital of Shanxi Province), Wentong Liang and Donglin Li (Guizhou Provincial People’s Hospital), Xuemei Zhan and Mingwei Li (Jiangmen Central Hospital), Weifeng Zhang (Ningbo Women & Children’s Hospital), Peiyan Du (The Affiliated Cancer Hospital and Institute of Guangzhou Medical University), Ziyu Fang (Liuzhou Workers’ Hospital), Rui Yang (Shenzhen Hospital of Peking University), Long Chen (Qingdao Municipal Hospital), Encheng Dai and Ruilei Liu (Linyi People’s Hospital), Yuanli He and Mubiao Liu (Zhujiang Hospital, Southern Medical University), Jilong Yao and Zhihua Liu (Shenzhen Maternity & Child Health Hospital), Xueqin Wang (The Fifth Affiliated Hospital of Southern Medical University), Anwei Lu (Maternal and Child Health Hospital of Guiyang Province), Shuangling Jin (Peace Hospital affiliated to Changzhi Medical College), Ben Ma (Guangzhou First People’s Hospital), Zhonghai Wang (Shenzhen Nanshan People’s Hospital), Lin Zhu (The Second Hospital of Shandong University), Hongxin Pan (The Third Affiliated Hospital of Shenzhen University), Qianyong Zhu (No. 153. Center Hospital of the Liberation Army/Hospital No. 988 of the Chinese People’s Liberation Army Joint Support Force), Dingyuan Zeng and Zhong Lin (Maternal and Child Health Care Hospital of Liuzhou), Xiaohong Wang (Laiwu People’s Hospital/Jinan City People’s Hospital), and Bin Zhu (The Affiliated Yiwu Women and Children Hospital of Hangzhou Medical College) for their contributions to data collection.
Registry and the registration No. of the Study/Trial
Clinical trial number CHiCTR1800017778; International Clinical Trials Registry Platform Search Port, https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1800017778, registered at 14/08/2018.
Funding
This study was funded by the National Science and Technology Support Program of China (Grant No. 2014BAI05B03), National Natural Science Fund of Guangdong (Grant No. 2015A030311024), Dongguan Sci-tech Commissioner Program (Grant No. 20221800500562), Guangdong Higher Vocational Education Teaching Reform Research and Practice Project (Grant No. GDJG 2021008), Teaching and Learning Reform Programs Established by the Cantonese Continuing Education and Vocational Training Council (Grant No.2022YJZW005), Education and Teaching Reform Subjects for 2022 of Guangdong Higher Vocational Colleges and Universities Teaching Steering Committee for Medicine and Health Professions(Grant No.2022LX069), and Science and Technology Plan of Guangzhou (Grant No. 158100075).
Author information
Authors and Affiliations
Contributions
All authors approved the final version of the study. Yanna Ye and Rui Lian contributed equally to the work. Yanna Ye conceptualized, engineered, and monitored the study, construed data, and formulated and amended the manuscript. Rui Lian conceived and implemented the literature search, performed the data analysis and interpretation, and developed and refined the manuscript. Zhiqiang Li conceptualized and engineered the study, and gathered and explained the data. Xiaolin Chen explained the data and developed and refined the manuscript. Yahong Huang conducted the documentation search, analyzed and interpreted data, and drew graphs and figures. Jilong Yao and Anwei Lu provided resources, performed the data analysis and interpretation, and validation and participated in writing the original draft of the manuscript. Jinghe Lang orchestrated this study. Ping Liu and Chunlin Chen conceived of, designed, and supervised the study. All authors contributed to editorial changes in the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki of 1964. And it was approved by the Ethics Committee of the Nanfang Hospital of Southern Medical University (approval number NFEC-2017–135), who deemed that written informed consent was not necessary owing to the retrospective nature of the research and the concealment of patient information.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, Y., Lian, R., Li, Z. et al. Predictive value of number of metastatic lymph nodes and lymph node ratio for prognosis of patients with FIGO 2018 stage IIICp cervical cancer: a multi-center retrospective study. BMC Cancer 24, 1005 (2024). https://doi.org/10.1186/s12885-024-12784-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12784-8