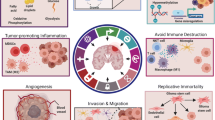
Abstract
The intricate interplay between cancer cells and their surrounding microenvironment has emerged as a critical factor driving the aggressive progression of various malignancies, including gliomas. Among the various components of this dynamic microenvironment, the extracellular matrix (ECM) holds particular significance. Gliomas, intrinsic brain tumors that originate from neuroglial progenitor cells, have the remarkable ability to actively reform the ECM, reshaping the structural and biochemical landscape to their advantage. This phenomenon underscores the adaptability and aggressiveness of gliomas, and highlights the intricate crosstalk between tumor cells and their surrounding matrix.
In this review, we delve into how glioma actively regulates glioma ECM to organize a favorable microenvironment for its survival, invasion, progression and therapy resistance. By unraveling the intricacies of glioma-induced ECM remodeling, we gain valuable insights into potential therapeutic strategies aimed at disrupting this symbiotic relationship and curbing the relentless advance of gliomas within the brain.
Graphical Abstract

Similar content being viewed by others
Introduction
The extracellular matrix (ECM) is an intricate three-dimensional non-cell network of proteoglycans (PGs) and fibrous proteins that surrounds and supports cells within tissues [1]. This complex scaffolding not only provides structural integrity but also plays a pivotal role in regulating various cellular processes, including proliferation, migration, differentiation, and apoptosis. Its composition and organization are dynamically modulated in response to developmental cues, physiological changes, and pathological conditions. In the context of cancer, such as gliomas, the ECM takes center stage as a crucial microenvironmental component that profoundly influences disease progression, metastasis, and therapeutic responses.
Gliomas, intrinsic brain tumors that originate from neuroglial progenitor cells, are characterized by their aggressive infiltrative behavior and propensity for recurrence [2]. Glioma cells exploit the ECM’s dynamic properties to their advantage. Through an array of molecular mechanisms, gliomas actively remodel the ECM, transforming it into a permissive environment that facilitates tumor growth, invasion, and evasion of therapeutic interventions. This remodeling involves the synthesis, deposition, degradation, and modification of ECM components, all of which collectively contribute to the establishment of a niche that supports glioma recurrence and progression.
Despite recent advances in surgery combined with radiotherapy and chemotherapy, patient survival rates have increased only slightly [3, 4]. One of the primary reasons for this poor prognosis is the diffuse infiltration of small clusters of tumor cells into the brain tissue, which prevents complete surgical tumor resection [5]. There is usually no clear boundary between the tumor and the surrounding brain parenchyma, which complicates complete surgical resection. Consequently, within months after surgery, recurrent neoplasms are triggered in the proximity of the resection zone, mostly within 2 cm of the original region [6, 7]. Individual or small clusters of tumor cells can migrate centimeters from the gross tumor into the surrounding healthy brain tissue [8].These roaming glioma cells actively remodel the ECM around them by degrading current ECM and replacing with Glioma-Modified ECM (GM-ECM). A total RNA-seq analysis showed an enhancement of cell-ECM interaction gene expression in recurrent versus parental cell populations [9]. The GM-ECM helps roaming gliomas survive adjuvant treatment after surgery and furtherly promotes residual roaming glioma to migrate, recurrent and progress.
Residual roaming glioma cells create and remodel ECM to be more abundant, denser and stiffer
The GM-ECM in gliomas significantly differs in composition and architecture from that in normal tissue. Considering its physical properties, the GM-ECM is more abundant, denser, and stiffer. Glioma cells have the ability to extensively remodel their microenvironment both through deposition of new matrix components and degradation of existing ones [10]. One key class of proteins responsible for regulating the turnover of collagens are the matrix metalloproteinases (MMPs). MMPs possess a broad range of overlapping substrate specificities and family members are able to degrade diverse substrates including collagens, fibronectin, laminin, proteoglycans, cell surface proteins, and pro-forms of growth factors such as TGF-β [11]. In the context of collagen turnover, 14 MMPs have been reported to degrade various collagens [12] (Fig. 1)(Table 1).
GM-ECM volume in the brain increases from 20 to 48% in high-grade gliomas, mainly due to Hyaluronan (HA). HA is a non-sulfated glycosaminoglycan present in the extracellular matrix, composed of N-acetylglucosamine/glucuronic acid disaccharide repeats of variable length. Hyaluronan is one of the most highly anionic substances in the body, attracting Na+ and other cations which bring with them an osmotic influx of water. Thus, the extracellular space in the brain expands to a volume approximately 10 times greater than that of the matrix substance itself, ensuring a water-rich environment.
Hyaluronan is increased approximately four-fold in primary brain tumors, reaching levels comparable to those present during the central nervous system (CNS) development [13]. The encoded gene for HA synthase (HAS), especially HAS2, was aberrantly up-regulated in tumor, relative to HAS genes in normal tissue, consistent with the finding that HAS overexpression results in increased tumorigenic features [14].
ECM glycoproteins such as fibronectin, tenascin, laminin, vitronectin and collagen of different types have been shown to be produced by glioma cells [13, 29, 30]. These overproduced glycoproteins increase GM-ECM density. Tenascins are a family with 4 known members in vertebrates: tenascin-W, tenascin-C (TNC), tenascin-X and tenascin-R (TNR). TNR is produced by normal healthy neurons and released into the ECM [31]. It is expressed in the ECM of non-tumoral brain parenchyma but exhibits a loss of expression in glioma, which may reflect deregulation of the normal ECM [15]. TNC is a highly expressed tenascin during normal fetal development and usually undetectable in adult brains. However, persistent levels of TNC have been characterized in adult neural stem cell niches such as the radial glia and astrocyte stem cell compartment of the subventricular zone, or associated with chronic neuropathological conditions, like glioma. Glioma cells are the main source of TNC production, and overexpression of TNC involves Notch pathway, which is a common upregulated pathway in glioma [16, 17]. TNCs are very large multimeric glycoproteins that consist of six polypeptide monomers and are combined into the hexamer at their N-termini. Nearly all kinds of solid tumors express high levels of TNC, but the highest concentrations have been found in gliomas. In fact, TNC was originally discovered as a major glioma cell secretion product. Its enrichment in human glioma ECM is correlated with malignancy.
Normal mature tissue cells rarely express TNC but in gliomas, TNC is expressed by malignant tumor cells [21]. TNC acts by binding to chondroitin sulfate proteoglycans(CSPGs) such as brevican or neurocan, which are also special in brain development [22]. By knocking down TNC in primary GBM cells and injecting into nude mice then, the patient derived model (PDX) with glioma shows not only increased survival, but also softer tumor area and lower mechanosignaling, which demonstrates that TNC acts as a driver molecular in increasing ECM stiffness.
Immunohistochemistry in patient glioma samples revealed that the elevated ECM stiffness in the patients with poorer prognosis was accompanied by a substantial increase in HA expression as well as increased levels of TNC. Additionally, ECM stiffness did not correlate with levels or distribution of type I collagen, vasculature or cellularity [18]. GBM that had TNC immunopositivity survived for a significantly shorter period than those in which TNC expression was absent, TNC in gliomas can be identified as a predictor of poor prognosis and disease progression [19, 20].
There is no consensus of fibronectin’s (FN) function in glioma, but its presence is well recognized in glioma tumors and their surrounding GM-ECM. A clear positive IHC staining of FN is found in all malignant gliomas, a much lower intensity was observed in glioma with lower-grade malignancy, and no staining was observed in healthy brain tissues [23].Glioma cells can promote fibronectin fibrillogenesis through CD93, a transmembrane receptor which is often overexpressed in tumor vessels in many cancers including glioma [24].
The GM-ECM is filled with overproduced glycoproteins and other components that drive the stiffening phenomena through various physical and chemical approaches. Normal brain is a flexible, soft organ with ECM stiffness of 0.2 to 1.2 kPa, which increases up to 45 kPa around the GM-ECM [32].Another clinical study, using Intraoperative Shear Wave Elastography for in vivo measurement of brain tumor stiffness shows that normal brain tissue has been characterized by a reproducible mean stiffness of 7.3 ± 2.1 kPa, that lower-grade glioma stiffness is different from high-grade glioma stiffness (p = 0.01) and that normal brain stiffness is very different from low-grade gliomas stiffness (p < 0.01) [33]. The increase of stiffness in glioma is also supported by the atomic force microscopy (AFM), which measured the stiffness of freshly removed human brain tumor tissue and indicated a significant stiffness difference (p ≤ 0.001) between glioblastoma and healthy tissue distributions, with stiffness levels of glioblastoma tissue almost threefold higher [34].
It’s difficult to obtain fresh human brain tissue samples for stiffness measurements due to logistical and regulatory challenges. However, we can still study the stiffness around glioma in several ways. There are some up/down-regulated pathways or gene alterations from glioma that influence the ECM to make it stiffer, one of which is PIEZO1. PIEZO1 is overexpressed in aggressive human gliomas and its expression inversely correlates with patient survival. PIEZO1 localizes at focal adhesions to activate integrin-FAK signaling, regulate the ECM and reinforce tissue stiffening. In turn, a stiffer mechanical microenvironment elevates PIEZO1 expression to promote glioma aggression [35]. The PIEZO1 promoter is generally hyper-methylated in the IDH mutant gliomas, correlating with decreased PIEZO1 mRNA expression in these tumors. This finding suggests that generally more aggressive IDH wild-type gliomas are epigenetically more poised to overexpress PIEZO1.
Another influencing pathway is Cancer-Associated Fibroblasts (CAFs) and its downstream. CAFs promote ECM stiffness in response to signals from yes-associated protein 1 (YAP1), which is one of the key effectors in the Hippo pathway acting in cooperation with other oncogenic factors like COX-2 for promotion of immunosuppression and drug resistance in cancer cells [36].
There is s potential feedback mechanism in gliomas when regulating the stiffness in the GM-ECM and then sensing it, possibly through the connection between TNC expression and NOTCH pathway. TNC can bind to integrin α2β1 on the glioma cell and upregulates JAG1 expression which interacts with its receptor NOTCH [22]. Glioma cells can sense the stiffness of the ECM through TNC by working as a ligand for integrins α2/7/8/9β1 and αvβ1/3/6 [37].
GM-ECM protects residual glioma cells from adjuvant therapy by building a safe harbor
Alterations that increase ECM abundance, density, and stiffness can negatively affect response to therapy in multiple ways. One of the most obvious effect is that an excessive accumulation of dense and rigid ECM, which histologically often encapsulates clusters of tumor cells, can act as a barrier, shielding the cells from therapeutic agents [38, 39] (Fig. 2).
When culturing GBM cells in a 3D hydrogel system with a gradient of stiffness, the cells in stiffer regions of the gradient hydrogel show less proliferation and spreading. These stiffer regions lead to the formation of more densely packed cellular spheroids. Because these spheroids have a compact structure, they can act as a barrier to drug diffusion. This physical barrier may prevent chemotherapeutic agents, such as Temozolomide (TMZ), from effectively penetrating the spheroid and killing the cancer cells inside, leading to the drug resistance [40].
High concentration of microproteins in the ECM such as chondroitin sulfate proteoglycans (CSPGs) can cause accumulating interstitial pressure and severe tortuosity in the extracellular space within the tumor, further limiting the penetration and distribution of therapeutic agents, especially those with larger molecular sizes [41].
Hyaluronan (HA), upregulated and enriched in the GM-ECM, can bind large amounts of water in the ECM which leads to an increase in interstitial fluid pressure (PIF). Some studies indicate that transcapillary transport and diffusion within the tumor might be hindered by high PIF resulting from high HA contend and/or vessel leakage. In two of these studies [42, 43], improved vascular perfusion and reduced vessel collapse were observed after hyaluronidase treatment. This might indicate that the high PIF in hyaluronan-rich tumors restricts drug transport mainly by compressing the supplying vessels and rather than interfering with interstitial drug diffusion. By depleting HA, tumor harbors increased macromolecular permeability by a trigger of fenestrations and interendothelial junctional gaps in tumor endothelia.
The GM-ECM not only acts as a physical barrier obstructing various pharmaceutical agents to protect residual glioma cells from destruction, but it also acts as an active biocomponent to cloak these cells, shielding them from immune cell detection and assault. Neither T cells nor dendritic cells are able to penetrate dense fibrils in GM-ECM. T-lymphocyte phenotype is dependent upon collagen fiber density: loose matrices support cytotoxic T-lymphocytes and dense matrices or those incorporating specific glycoproteins leads to immune-inhibitory phenotypes. T-lymphocyte movement is driven by chemokine gradients in loose ECM, but in dense ECMs, T-lymphocytes doesn’t demonstrate chemokine-directed movement. The clinical relevance of this shielding function of the stromal ECM that keeps immune cells at distance from the tumor cells was most strikingly demonstrated by previous research using a urothelial cancer patient cohort that shows non-response to PD-L1 checkpoint inhibition correlated with CTL’s low activity in the stromal ECM [44].
Beside the physical shielding, changes and regulatory mechanisms at the molecular and pathway levels are also involved in the protection of residual glioma cells. High ECM density causes tumor hypoxia, potentially increasing collagen deposition in the ECM, leading to higher levels of ECM density and stiffness [45]. An excessive accumulation of dense and stiff ECM, which histologically often encapsulates clusters of tumor cells, can act as a barrier. This effect is directly linked to a reduced overall supply, as this barrier also impairs diffusion of oxygen, nutrients, and metabolites. Increased hypoxia and metabolic stress lead to activation of antiapoptotic and drug resistance pathways. Presence of hypoxia reprograms tumor cells through multiple proteins, such as hypoxia-inducible factor (HIF)-1α, which can protect glioma cells in multiple ways [46,47,48].
In addition to passive defense, the GM-ECM can also take the initiative to resist recognition and attack by immune cells. TNC secreted by tumor cells in GM-ECM does not only promoting stiffness, but also protects glioma cells from T cell attack. By deposited in the vicinity of T cells in the GM-ECM and interacting with α5β1 and αvβ6 integrins on T lymphocytes associated with reduced mTOR signaling, TNC inhibits T cell proliferation and protects the glioma cell from the immune cell [49]. TNC can also provide drug resistance, experiment shows TNC-knockdown GBM neurospheres found to be more sensitive to temozolomide (TMZ) treatment [50].
Integrins belong to a large family of heterodimeric transmembrane adhesion receptors, named after their roles in “integrating” cell function with the surrounding stroma [51]. The expression of integrins is upregulated in various cancers, including GBM [52]. Cells can sense tissue stiffness through signals from FAK that again cooperates with integrins. FAK signals increase pro-survival pathways like AKT and MAPK. This has been shown to confer resistance to glioma treatments such as, rapamycin, an mTOR inhibitor [53].
FN is a high-molecular-weight glycoprotein in the ECM that is known for being over-expressed in several cancers [54]. Research looking into FN and the glioma stem-like cells (GSCs) indicates that FN can suppressed p53-mediated apoptosis and upregulated P-glycoprotein expression, which gives GSCs chemoresistance to alkylating agents such like carmustine [55].
Matrix stiffness can also promote glioma cell stemness by activating BCL9L/Wnt/β-catenin signaling. Higher matrix stiffness can enhance the stemness of glioma cells, resulting in sustained tumor growth by activating the BCL9L/Wnt/β-catenin signaling pathway [56]. CSCs promote tumorigenesis and tumor development, It has been well documented that Wnt/β-catenin signaling is a critical determinant of CSC pluripotency and self-renewal. This nest preserves tumorigenesis energy for further epigenetically recurrence.
GM-ECM facilitates tumor recurrence and progression mainly by enhancing stemness and promoting migration
After initially modified by residual glioma cells and then consequently preserves glioma cells during adjuvant therapy, GM-ECM now will promote tumor progression and recurrence at a macroscopic level, mainly by supporting cell stemness and migration. GM-ECM can promote glioma recurrence in a variety of ways, such as providing structural physical support or participating in biological regulation of multiple pathways, but its role is often concentrated in two perspectives: promoting stemness or migration (Table 2) (Fig. 3).
TNC is usually present in neural stem cell niches in the embryonic developmental stage and shortly after birth. However, it has also been found enriched in the recurrent glioma ECM, proving that the GM-ECM provides a suitable ecological niche for glioma stem cells to survive and proliferate [22]. Collagen and fibronectin enriched in the GM-ECM can provide structural support and activating critical signaling pathways such as Integrin αvβ3 Signaling, PI3K/AKT/SOX2 and CDC42/F-actin/YAP-1/Nupr1/Nestin signaling pathways, resulting in regulating cell proliferation and contributing to glioma stemness [57].
Recent studies have illuminated the critical role of thrombospondin in ECM in glioma progression. One study demonstrated that the formation of microtubes (MTs) was significantly enhanced in the resection cavity following neurosurgery in experimental models [58]. This MT formation may partly elucidate why recurrent tumors frequently arise near the resection site. In following investigation, the stimulation of GBM stem-like cell cultures with transforming growth factor-beta 1 (TGF-β1) promotes MT formation has been observed. Notably, thrombospondin-1 (TSP-1) emerged as a crucial mediator in this process, acting downstream of TGF-β1 [59]. Additionally, another study has shown that extracellular vesicles can mediate tumor progression through the thrombospondin-1 pathway, proves the pivotal role of thrombospondin-1 facilitating glioma progression [60].
Type I collagen/fibronectin (FN) in the GM-ECM can strengthen the tumorigenic potential and proliferative characteristics of glioma cells by promoting GSCs adherence through a concentration-dependent manner. Moreover, FN can drive GSCs differentiation and furtherly promote differentiated cell growth by the elevation of Ki-67 [55]. Comparing GM-FCM and normal ECM, FN seems to have a higher concentration in GM-ECM, and can promote migration of glioma cells [61].
By initially shaping GM-ECM into a stiffer environment, stiffer GM-ECM can enhance cell invasiveness and promote relapse through a feedback regulation. The stiffness of GM-ECM gradually increases in accordance with aggressiveness, from a hundred pascals of gliosis to several thousand in lower grade glioma, to tens of thousands of pascals in high grade glioma [18].
The stiffness of the peri-glioma GM-ECM creates a microenvironment that fosters aggressive behaviors, including enhanced proliferation, migration, and invasion. Previous research shows that GBM cells show higher proliferation and migration rates when cultured on stiff two-dimensional substrates [8]. On a stiffer ECM, these complexes become larger and more stable, promoting a stronger connection between the cell and the ECM. This enables glioma cells to migrate more efficiently and invade surrounding healthy brain tissue. Human GBM tissue biopsies indicated a significant correlation between the proportion of highly stiff areas within a GBM tissue (E > 1,400 Pa) and a poor patient prognosis score. By contrast, the tissues that contained a high proportion of soft ECM regions (E < 200 Pa) had the best patient prognosis score [18].
Increasing ECM stiffness increases the percentage of cells in S phase [62], representing a high level of cellular DNA synthesis and replication, in other word, increased proliferation. Increasing matrix stiffness from 0.08 kPa to 119 kPa produced a five-fold increase in phosphorylated EGFR (pEGFR) and nearly two-fold increases in phosphorylated Akt (pAkt) and total PI3K, increasing microenvironmental stiffness broadly activates EGFR signaling in GBM tumor cells to regulate proliferation [62]. Another study also indicates that increasing ECM rigidity can induce a suite of phenotypic changes in human glioma cells that includes increased cell spreading, faster motility, and enhanced proliferation [63].
Stiffness modulates expression of glioma EGFR pathway and its downstream effector Akt, to promote cell cycle progression and proliferation [62]. Research shows integrin signaling is a vital pathway in regulating stiffness of ECM [64]. Increasing matrix stiffness led to delayed U87 cell proliferation inside hydrogels, but cells form denser spheroids with extended cell protrusions. Cells cultured in stiff hydrogels also showed upregulation of HA synthase 1 and matrix metalloproteinase-1 (MMP-1) [70]. Recurrent cells grown on 0.5kPa showed higher Young’s moduli suggesting the ability of these cells to make the surrounding ECM stiffer, which further promotes glioma recurrence [9].
IDH is one of the most important molecular in glioma classification. It is widely known that IDH mutant gliomas usually have better prognosis compared to IDH wildtype ones. One of the perspectives from the GM-ECM that can explain this result is that mutant IDH1 restricts glioma aggression by reducing HIF1α-dependent TNC expression to decrease ECM stiffness and mechanosignaling. But still, recurrent IDH-mutant glioma still have a stiffer TNC-enriched ECM, which possibly due to ECM stiffness can bypass protective activity of IDH mutational status through a HIF1α and TNC mediated via a tension-dependent positive feedback loop [18].
Other than making tissue stiffer to protect glioma from treatment, integrin also helps aggression. Extensive data demonstrate that the expression of integrins, together with corresponding ECM components, facilitates the infiltration of tumor cells through normal brain tissue [71]. The integrin arsenal expressed on the cell surface is important for the cellular phenotype. Integrins constitute a large family of dimeric transmembrane receptors, which are composed of α and β subunits that initiate intracellular signaling cascades by binding to adapter proteins [65]. α3β1 has been shown to be consistently over-expressed and to be a key regulator of glioma cell migration [66]. αvβ3 (observed at the periphery of high-grade gliomas) and αvβ5 integrins (expressed more at the center of the tumor) are involved in glioma malignancy [67]. Expression of αvβ3 coincides with expression of the metalloproteinase MMP-2 in tumor cells at the invasion front.
Besides constructing a defensive shield, HA also contributes to invasiveness. HA acts as a ligand for CD44 and might thereby play a role in EMT, resulting in increased invasiveness and metastasis [68, 69]. Targeting these receptors, such as CD44 and RHAMM, has been shown to inhibit tumor invasion and migration.
Laminins is also a major glioblastoma cells production in constructing GM-ECM, other with collagen type IV and FN [23]. Laminins can be produced by GFAP positive cells during glioma cell invasion in humans [25, 26]. Laminin gamma 1 gene (LAMC1) may play an important role in glioma invasion [27], but in the meantime Laminin alpha 5 also can significantly lower the invasion of mobile U251MG cells [28].
Conclusion
In summary, this review illustrates how glioma cells engineer and remodel the ECM, and how this modified matrix assists glioma cells in resisting therapy, preserving stemness, fostering growth, increasing invasion and leads to recurrence. Despite the challenges posed by the complex glioma-ECM dynamics, as we refine our understanding of the ECM’s influence on glioma behavior, targeting key ECM elements in offers a viable strategy to postpone or even prevent glioma recurrence.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Jayaram MA, Phillips JJ. Role of the Microenvironment in Glioma Pathogenesis. Annu Rev Pathol. 2024;19:181–201.
Xu S, Tang L, Li X, Fan F, Liu Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020;476:1–12.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Wirsching H-G, Galanis E, Weller M. Glioblastoma. Handb Clin Neurol. 2016;134:381–97.
Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117.
Zhu Y, Jia J, Zhao G, Huang X, Wang L, Zhang Y, et al. Multi-responsive nanofibers composite gel for local drug delivery to inhibit recurrence of glioma after operation. J Nanobiotechnology. 2021;19:198.
Pan I-W, Ferguson SD, Lam S. Patient and treatment factors associated with survival among adult glioblastoma patients: a USA population-based study from 2000–2010. J Clin Neurosci Off J Neurosurg Soc Australas. 2015;22:1575–81.
Naumann U, Harter PN, Rubel J, Ilina E, Blank A-E, Esteban HB, et al. Glioma cell migration and invasion as potential target for novel treatment strategies. Transl Neurosci. 2013;4:314–29.
Acharekar A, Bachal K, Shirke P, Thorat R, Banerjee A, Gardi N, et al. Substrate stiffness regulates the recurrent glioblastoma cell morphology and aggressiveness. Matrix Biol J Int Soc Matrix Biol. 2023;115:107–27.
Hagemann C, Anacker J, Ernestus R-I, Vince GH. A complete compilation of matrix metalloproteinase expression in human malignant gliomas. World J Clin Oncol. 2012;3:67–79.
Oldak L, Chludzinska-Kasperuk S, Milewska P, Grubczak K, Reszec J, Gorodkiewicz E. Laminin-5, Fibronectin, and Type IV Collagen as Potential Biomarkers of Brain Glioma Malignancy. Biomedicines. 2022;10:2290.
Payne LS, Huang PH. The pathobiology of collagens in glioma. Mol Cancer Res MCR. 2013;11: https://doi.org/10.1158/1541-7786.MCR-13–0236.
Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–69.
Wu Y-H, Chou C-Y. Collagen XI Alpha 1 Chain, a Novel Therapeutic Target for Cancer Treatment. Front Oncol. 2022;12:925165.
El Ayachi I, Fernandez C, Baeza N, De Paula AM, Pesheva P, Figarella-Branger D. Spatiotemporal distribution of tenascin-R in the developing human cerebral cortex parallels neuronal migration. J Comp Neurol. 2011;519:2379–89.
Wiese S, Karus M, Faissner A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol. 2012;3:120.
Sivasankaran B, Degen M, Ghaffari A, Hegi ME, Hamou MF, Ionescu MCS, et al. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res. 2009;69:458–65.
Miroshnikova YA, Mouw JK, Barnes JM, Pickup MW, Lakins JN, Kim Y, et al. Tissue mechanics promote IDH1-dependent HIF1α–tenascin C feedback to regulate glioblastoma aggression. Nat Cell Biol. 2016;18:1336–45.
Leins A, Riva P, Lindstedt R, Davidoff MS, Mehraein P, Weis S. Expression of tenascin-C in various human brain tumors and its relevance for survival in patients with astrocytoma. Cancer. 2003;98:2430–9.
Herold-Mende C, Mueller MM, Bonsanto MM, Schmitt HP, Kunze S, Steiner H-H. Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int J Cancer. 2002;98:362–9.
Mahesparan R, Read T-A, Lund-Johansen M, Skaftnesmo KO, Bjerkvig R, Engebraaten O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol (Berl). 2003;105:49–57.
Fu Z, Zhu G, Luo C, Chen Z, Dou Z, Chen Y, et al. Matricellular protein tenascin C: Implications in glioma progression, gliomagenesis, and treatment. Front Oncol. 2022;12:971462.
Chintala SK, Sawaya R, Gokaslan ZL, Fuller G, Rao JS. Immunohistochemical localization of extracellular matrix proteins in human glioma, both in vivo and in vitro. Cancer Lett. 1996;101:107–14.
Lugano R, Vemuri K, Yu D, et al. CD93 promotes β1 integrin activation and fibronectin fibrillogenesis during tumor angiogenesis. J Clin Invest. 2018;128(8):3280–97. https://doi.org/10.1172/JCI97459.
Sinha S, Huang MS, Mikos G, Bedi Y, Soto L, Lensch S, et al. Laminin-associated integrins mediate Diffuse Intrinsic Pontine Glioma infiltration and therapy response within a neural assembloid model. Acta Neuropathol Commun. 2024;12:71.
Rabah N, Ait Mohand F-E, Kravchenko-Balasha N. Understanding Glioblastoma Signaling, Heterogeneity, Invasiveness, and Drug Delivery Barriers. Int J Mol Sci. 2023;24:14256.
Jonnakuti VS, Ji P, Gao Y, Lin A, Chu Y, Elrod N, et al. NUDT21 alters glioma migration through differential alternative polyadenylation of LAMC1. J Neurooncol. 2023;163:623–34.
Gamble JT, Reed-Harris Y, Barton CL, La Du J, Tanguay R, Greenwood JA. Quantification of glioblastoma progression in zebrafish xenografts: Adhesion to laminin alpha 5 promotes glioblastoma microtumor formation and inhibits cell invasion. Biochem Biophys Res Commun. 2018;506:833–9.
Zamecnik J. The extracellular space and matrix of gliomas. Acta Neuropathol (Berl). 2005;110:435–42.
Gladson CL. The extracellular matrix of gliomas: modulation of cell function. J Neuropathol Exp Neurol. 1999;58:1029–40.
Wintergerst ES, Rathjen FG, Schwaller B, Eggli P, Celio MR. Tenascin-R associates extracellularly with parvalbumin immunoreactive neurones but is synthesised by another neuronal population in the adult rat cerebral cortex. J Neurocytol. 2001;30:293–301.
Stewart DC, Rubiano A, Dyson K, Simmons CS. Mechanical characterization of human brain tumors from patients and comparison to potential surgical phantoms. PLoS ONE. 2017;12:e0177561.
Chauvet D, Imbault M, Capelle L, Demene C, Mossad M, Karachi C, et al. In Vivo Measurement of Brain Tumor Elasticity Using Intraoperative Shear Wave Elastography. Ultraschall Med Stuttg Ger. 1980;2016(37):584–90.
Cieśluk M, Pogoda K, Deptuła P, Werel P, Kułakowska A, Kochanowicz J, et al. Nanomechanics and Histopathology as Diagnostic Tools to Characterize Freshly Removed Human Brain Tumors. Int J Nanomedicine. 2020;15:7509–21.
Chen X, Wanggou S, Bodalia A, Zhu M, Dong W, Fan JJ, et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron. 2018;100:799-815.e7.
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6:218.
Yoshida T, Akatsuka T, Imanaka-Yoshida K. Tenascin-C and integrins in cancer. Cell Adhes Migr. 2015;9:96–104.
Grossen A, Smith K, Coulibaly N, Arbuckle B, Evans A, Wilhelm S, et al. Physical Forces in Glioblastoma Migration: A Systematic Review. Int J Mol Sci. 2022;23:4055.
Kondapaneni RV, Gurung SK, Nakod PS, Goodarzi K, Yakati V, Lenart NA, et al. Glioblastoma mechanobiology at multiple length scales. Biomater Adv. 2024;160:213860.
Zhu D, Trinh P, Li J, Grant GA, Yang F. Gradient hydrogels for screening stiffness effects on patient-derived glioblastoma xenograft cellfates in 3D. J Biomed Mater Res A. 2021;109:1027–35.
Moon LDF, Asher RA, Fawcett JW. Limited growth of severed CNS axons after treatment of adult rat brain with hyaluronidase. J Neurosci Res. 2003;71:23–37.
Eikenes L, Tari M, Tufto I, Bruland Ø, de Lange DC. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx™) in human osteosarcoma xenografts. Br J Cancer. 2005;93:81–8.
Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–20.
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGF-β attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8.
Dekker Y, Le Dévédec SE, Danen EHJ, Liu Q. Crosstalk between Hypoxia and Extracellular Matrix in the Tumor Microenvironment in Breast Cancer. Genes. 2022;13:1585.
Liu K, Jiang L, Shi Y, Liu B, He Y, Shen Q, et al. Hypoxia-induced GLT8D1 promotes glioma stem cell maintenance by inhibiting CD133 degradation through N-linked glycosylation. Cell Death Differ. 2022;29:1834–49.
Ding X-C, Wang L-L, Zhang X-D, Xu J-L, Li P-F, Liang H, et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J Hematol OncolJ Hematol Oncol. 2021;14:92.
Su X, Xie Y, Zhang J, Li M, Zhang Q, Jin G, et al. HIF-α activation by the prolyl hydroxylase inhibitor roxadustat suppresses chemoresistant glioblastoma growth by inducing ferroptosis. Cell Death Dis. 2022;13:861.
Mirzaei R, Sarkar S, Dzikowski L, Rawji KS, Khan L, Faissner A, et al. Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity. Oncoimmunology. 2018;7: e1478647.
Xia S, Lal B, Tung B, Wang S, Goodwin CR, Laterra J. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro-Oncol. 2016;18:507–17.
Ellert-Miklaszewska A, Poleszak K, Pasierbinska M, Kaminska B. Integrin Signaling in Glioma Pathogenesis: From Biology to Therapy. Int J Mol Sci. 2020;21:888.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22.
Yoon S-O, Shin S, Karreth FA, Buel GR, Jedrychowski MP, Plas DR, et al. Focal adhesion- and IGF1R-dependent survival and migratory pathways mediate tumor resistance to mTORC1/2 inhibition. Mol Cell. 2017;67:512-527.e4.
Wang JP, Hielscher A. Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. J Cancer. 2017;8:674–82.
Yu Q, Xue Y, Liu J, Xi Z, Li Z, Liu Y. Fibronectin Promotes the Malignancy of Glioma Stem-Like Cells Via Modulation of Cell Adhesion, Differentiation. Proliferation and Chemoresistance Front Mol Neurosci. 2018;11:130.
Tao B, Song Y, Wu Y, Yang X, Peng T, Peng L, et al. Matrix stiffness promotes glioma cell stemness by activating BCL9L/Wnt/β-catenin signaling. Aging. 2021;13:5284–96.
Zhong C, Tao B, Tang F, Yang X, Peng T, You J, et al. Remodeling cancer stemness by collagen/fibronectin via the AKT and CDC42 signaling pathway crosstalk in glioma. Theranostics. 2021;11:1991–2005.
Weil S, Osswald M, Solecki G, Grosch J, Jung E, Lemke D, et al. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro-Oncol. 2017;19:1316–26.
Joseph JV, Magaut CR, Storevik S, Geraldo LH, Mathivet T, Latif MA, et al. TGF-β promotes microtube formation in glioblastoma through thrombospondin 1. Neuro-Oncol. 2022;24:541–53.
Tsutsui T, Kawahara H, Kimura R, Dong Y, Jiapaer S, Sabit H, et al. Glioma-derived extracellular vesicles promote tumor progression by conveying WT1. Carcinogenesis. 2020;41:1238–45.
Jain S, Rick JW, Joshi RS, Beniwal A, Spatz J, Gill S, et al. Single-cell RNA sequencing and spatial transcriptomics reveal cancer-associated fibroblasts in glioblastoma with protumoral effects. J Clin Invest. 2023;133: e147087.
Umesh V, Rape AD, Ulrich TA, Kumar S. Microenvironmental stiffness enhances glioma cell proliferation by stimulating epidermal growth factor receptor signaling. PLoS ONE. 2014;9:e101771.
Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–74.
Kim SN, Jeibmann A, Halama K, Witte HT, Wälte M, Matzat T, et al. ECM stiffness regulates glial migration in Drosophila and mammalian glioma models. Dev Camb Engl. 2014;141:3233–42.
Winograd-Katz SE, Fässler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol. 2014;15:273–88.
Zhou P, Erfani S, Liu Z, Jia C, Chen Y, Xu B, et al. CD151-α3β1 integrin complexes are prognostic markers of glioblastoma and cooperate with EGFR to drive tumor cell motility and invasion. Oncotarget. 2015;6:29675–93.
Mittelbronn M, Warth A, Meyermann R, Goodman S, Weller M. Expression of integrins αvβ3 and αvβ5 and their ligands in primary and secondary central nervous system neoplasms. Histol Histopathol. 2013;28:749–58.
Heldin P, Basu K, Kozlova I, Porsch H. HAS2 and CD44 in breast tumorigenesis. Adv Cancer Res. 2014;123:211–29.
Melrose J. Hyaluronan hydrates and compartmentalises the CNS/PNS extracellular matrix and provides niche environments conducive to the optimisation of neuronal activity. J Neurochem. 2023;166:637–53.
Wang C, Tong X, Yang F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol Pharm. 2014;11:2115–25.
Paolillo M, Serra M, Schinelli S. Integrins in glioblastoma: Still an attractive target? Pharmacol Res. 2016;113:55–61.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
R.W. and X.L. designed the review, R.W. and J.Z. generate figures, R.W., J.Z. and B.B. write the main text and table. R.W. and J.Z. contribute equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, R., Zhou, J., Bui, B. et al. Glioma actively orchestrate a self-advantageous extracellular matrix to promote recurrence and progression. BMC Cancer 24, 974 (2024). https://doi.org/10.1186/s12885-024-12751-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12751-3