Abstract
Background
This study aimed to evaluate the effectiveness and safety of recombinant human endostatin (Rh-endostatin) plus programmed cell death 1 (PD-1) inhibitors and chemotherapy as first-line treatment for advanced or metastatic non-small cell lung cancer (NSCLC) in a real-world setting.
Methods
This was a retrospective study on patients with EGFR/ALK-negative, advanced or metastatic NSCLC. Patients received Rh-endostatin plus PD-1 inhibitors and chemotherapy every three weeks for 4 to 6 cycles. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were objective response rate (ORR), disease control rate (DCR), overall survival (OS), and safety.
Results
A total of 68 patients were included in this retrospective analysis. As of data cutoff (December 13, 2022), the median follow-up of 21.4 months (interquartile range [IQR], 8.3-44.4 months). The median PFS and OS was 22.0 (95% confidence interval [CI]: 16.6-27.4) and 31.0 months (95% CI: 23.4-not evaluable [NE]), respectively. The ORR was 72.06% (95% CI: 59.85-82.27%), and DCR was 95.59% (95% CI: 87.64-99.08%). Patients with stage IIIB/IIIC NSCLC had significantly longer median PFS (23.4 vs. 13.2 months), longer median OS (not reached vs. 18.0 months), and higher ORR (89.2% vs. 51.6%) than those with stage IV NSCLC (all p ≤ 0.001). The ORR was higher in patients with high PD-L1 expression (tumor proportion score [TPS] ≥ 50%) than in those with low PD-L1 expression or positive PD-L1 expression (75% vs. 50%, p = 0.025). All patients experienced treatment-related adverse events (TRAEs), and ≥ grade 3 TRAEs occurred in 16 (23.53%) patients.
Conclusions
Rh-endostatin combined with PD-1 inhibitors plus chemotherapy as first-line treatment yielded favorable effectiveness with a manageable profile in patients with advanced or metastatic NSCLC, representing a promising treatment modality.
Similar content being viewed by others
Introduction
Lung cancer is the most common cause of cancer-related deaths in China with over 760 thousand deaths in 2022 [1]. Non-small cell lung cancer (NSCLC) accounts for 85% of the cases, and the majority of cases are locally advanced or metastatic at initial diagnosis [2, 3]. However, for treatment-naive patients without a driver gene alteration, traditional platinum-based chemotherapy showed modest response rates and short progression-free survival (PFS) [4].
Recently, the treatment landscape of advanced NSCLC without driver mutations has shifted from traditional chemotherapy to immunotherapy-based treatments with or without chemotherapy [5,6,7]. The combination therapy of chemotherapy and immune checkpoint inhibitors (ICIs) as first-line treatment for NSCLC without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations has been approved by Food and Drug Administration (FDA) [8, 9]. Several phase III studies demonstrated the superiority of chemo-immunotherapy in locally advanced or metastatic NSCLC [10,11,12,13,14,15,16]. However, due to the complex immune microenvironment and the possible hyperprogression caused by genetic alteration, additional combination approaches for more effective treatments are still warranted [17, 18].
Angiogenesis plays a crucial role in tumor progression, and anti-angiogenic agents can normalize blood vessels to transform the tumor microenvironment from immunosuppressive to immune-supportive [19, 20]. The synergistic action of chemo-immunotherapy with anti-angiogenic therapy may further improve the prognosis of patients with NSCLC [21, 22]. Results from the IMpower150 trial showed that the combination of atezolizumab, bevacizumab and chemotherapy was associated with promising results in untreated advanced NSCLC, regardless of EGFR/ALK genetic alteration status [22]. Nevertheless, due to concerns about severe adverse hemoptysis by bevacizumab and requirements for better survival, more treatment modalities for patients with NSCLC should be further developed.
Previous results showed that endostatin can inhibit the tumor neovascularization by obstruction of vascular endothelial cell migration [23,24,25]. In China, recombinant human endostatin (Rh-endostatin/Endostar®) was approved by National Medical Products Administration in 2005 for patients with NSCLC [26]. However, the evidence of Rh-endostatin combined with anti-programmed cell death-1 (PD-1) antibodies and chemotherapy as first-line treatment for patients with advanced NSCLC is still limited. This study aimed to evaluate the effectiveness and safety of Rh-endostatin in combination with PD-1 blockades plus chemotherapy as first-line treatment for advanced or metastatic NSCLC.
Materials and methods
Patient eligibility
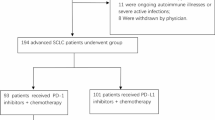
This retrospective, single-center, study aimed to evaluate the effectiveness and safety of Rh-endostatin in combination with PD-1 antibody plus chemotherapy as first-line treatment for advanced or metastatic NSCLC. Clinical data of patients with advanced or metastatic NSCLC treated at the First Medical Center of the General Hospital of the People’s Liberation Army (PLA) of China between June 2018 and December 2021 were retrospectively analyzed and reviewed. The inclusion criteria were: (1) aged 18–75 years old; (2) patients with pathologically- or cytologically-confirmed locally advanced (stage IIIB/IIIC) or metastatic NSCLC according to the American Joint Committee on Cancer tumor-node-metastasis staging system version 9.0; (3) patients with EGFR/ALK-negative status; (4) patients with no prior systemic anticancer therapy; (5) Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤ 2; (6) patients who received at least one cycle of Rh-endostatin plus chemotherapy and PD-1 antibodies in the first-line setting; (7) at least one measurable tumor lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Patients were excluded if they (1) were pregnant or lactating women; (2) were with the history of active tuberculosis; (3) were with active infection; (4) were with active bleeding; (5) Were with a history of autoimmune illness and other pathological kind. This study was approved by the ethical review board of the Chinese PLA General Hospital Ethics Committee (No.S2018-203-01) and was conducted in accordance with the Declaration of Helsinki and local applicable regulatory guidelines. Informed consent was waived due to the retrospective nature of the study.
Procedures
All included patients should receive at least 1 cycle (every 3 weeks) of Rh-endostatin (Endostar®, Simcere Biopharmaceutical Co., Ltd., Jiangsu, China) combined with PD-1 inhibitors and chemotherapy. The continuous intravenous infusion of Rh-endostatin was pumped at a rate of 10 mL/h (105 mg/m2 in 1000 mL of saline) via a mini-osmotic pump from days 1 to 5. PD-1 antibodies (pembrolizumab [MSD, Carlow, Ireland] 200 mg, sintilimab [Innovent Biopharmaceutical Co. Ltd, Suzhou, China] 200 mg, or toripalimab [Hezhong Biological Medicine Co. Ltd, Suzhou, China] 240 mg) were administered intravenously once every three weeks per cycle on day 1. All patients with NSCLC received stardand chemotherapy. Patients with lung adenocarcinoma (LUAD) received pemetrexed, whereas patients with lung squamous-cell carcinoma (LUSC) recieved paclitaxel/nab-paclitaxel orgemcitabine. Treatment was continued until disease progression or unacceptable toxicity. Patients without disease progression or unacceptable toxicity after 4 or 6 cycles of treatment received maintenance treatment of PD-1 antibodies. Dose adjustment, treatment interruption, and subsequent therapies were also recorded.
Treatment evaluation and data collection
Patient basic characteristics, tumor characteristics, tumor response, and treatment-related adverse events (TRAEs) were collected from hospital electronic medical records. Tumor evaluation was performed according to RECIST v1.1. The short-term effectiveness was evaluated at two cycles after the launch of the combined therapy.
Clinical outcomes
The primary endpoint was PFS, and the secondary endpoints were ORR, disease control rate (DCR), OS, and safety. PFS was defined as the time from initiation of treatment until progressive disease (PD) or death of any cause. OS was defined as the time from the combined treatment to death of any cause. ORR was defined as the proportion of patients with complete response (CR) and partial response (PR). Disease control rate (DCR) was defined as the proportion of patients with CR, PR, and stable disease (SD). Safety assessments included all patients. TRAEs were graded according to National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0.
Statistical analysis
Statistical analyses were conducted using SPSS software (version 25, IBM Software, Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation (SD) or median (range), while categorical variables were expressed as frequency or percentages (%). PFS and OS were calculated by the Kaplan-Meier method and compared using a stratified log-rank test. Two-sided 95% CIs for ORR and DCR were calculated via the Clopper-Pearson method, and the ORR was compared using Chi-squared or Fisher’s exact test. Subgroup analyses of ORR, PFS and OS were performed according to the baseline characteristics, including NSCLC disease stages, presence or absence of brain metastasis, programmed cell death-ligand 1 tumor proportion score (PD-L1 TPS), and others. The subgroup analyses were compared using the log-rank test. P values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
A total of 68 patients were included in the retrospective analysis, and Table 1 detailed the baseline characteristics. The mean age was 62.12 ± 8.7 years old. Male patients accounted for 61.76% (42/68). The majority of patients were younger than 65 years old (42/68, 61.76%), and had an ECOG PS of 0 or 1 (54/68, 79.41%). The disease stage at initial diagnosis was IIIB/IIIC in 54.41% (37/68) of the patients and IV in 45.59% (31/68) of the patients. Regarding histology types, 46 patients (67.65%) had squamous cell carcinoma, 18 patients (26.47%) had adenocarcinoma, and four patients (5.88%) had other carcinomas, including two poorly differentiated carcinoma, one adenosquamous carcinoma, and one sarcomatoid carcinoma. There were 14.71% (10/68) patients with brain metastasis. PD-L1 test was performed in 32 patients (47.06%), and the expression of PD-L1 scores of TPS < 1%, 1–49%, and ≥ 50% were observed in 17.65% (12/68), 17.65% (12/68), and 11.76% (8/68) of the patients, respectively.
Treatment patterns
The majority of patients (47/68, 69.12%) received ≥ 4 cycles of Rh-endostatin plus PD-1 antibody and chemotherapy. The median duration of treatment was four cycles. Among the 68 patients, 36 patients (36/68, 52.94%) received maintenance treatment following the combined treatment. The median number of maintenance treatment cycles was 8 (range, 2-24). As for the anti-PD-1 treatment, the number of patients who received pembrolizumab, toripalimab, or sintilimab was 19 (27.94%), 15 (22.06%), and 34 (50%), respectively. After receiving the combination therapy, seven patients who were initially unable to receive radical surgery had successful R0 resection, and 27 patients (39.71%) further underwent local radiotherapy.
Clinical outcomes
As of December 5, 2022, and the median follow-up was 21.4 (IQR, 8.3-44.4) months. Of 68 patients, 33 patients (48.5%) remained progression-free, and 24 patients (35.3%) died of disease progression (n = 19) or non-disease progression (n = 5), including one myocardial infarction, one heart failure, and two pulmonary infections. The median PFS was 22.0 months (95% CI: 16.6-27.4), and the median OS was 31.0 months (95% CI: 23.4-not evaluable [NE]) (Fig. 1A-B). Of all 68 patients in short-term effectiveness evaluation, three (4.41%) patients attained a CR as their best response per the RECIST v1.1 criteria for. Forty-six patients (67.65%) had PR, 16 (23.53%) had SD, and 3 (4.41%) had PD (Fig. 1C-D). The ORR and DCR were 72.06% [95CI: 59.85-82.27%], and 95.59% [95CI: 87.64-99.08%], respectively.In subgroup analyses (Table 2), disease stage (IIIB/IIIC) and presence of brain metastasis showed a significant difference in both PFS and OS. In addition, the disease stage (IIIB/IIIC) and PD-L1 TPS ≥ 50% were correlated with higher ORR. No significant differences were found between the different subgroups of age, sex, ECOG PS, histological subtype, anti-PD-1 monoclonal antibodies (mAbs), chemotherapy regimens, combined treatment cycles, or whether the patient received local chest radiotherapy in ORR, PFS, and OS (all p > 0.05).
Kaplan-Meier survival curve of PFS and OS analysis, waterfall plots, and swimming plots. (1A) Progression-free survival; (1B) Overall survival; (1C) Waterfall plot of best percentage change from baseline in size of target tumor lesions; (1D) Swimmer plot of duration of response and clinical response
CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease
Safety
As shown in Table 3, TRAEs were reported in all patients (100%). The most common AEs of any grade were leukopenia (54/68, 79.41%), anemia (52/68, 76.47%), alopecia (50/68, 73.53%), fatigue (41/68, 60.29%), neutropenia (43/68, 63.24%), and decreased appetite (45/68, 66.18%). Most TRAEs were regarded as grade 1-2 (57/68, 83.82%), and 16 patients (23.53%) had TRAEs of grade 3-4. The most fequently reported TRAEs of grade ≥ 3 were leukopenia (11/68, 16.18%), neutropenia (8/68, 11.76%), thrombocytopenia (3/68, 4.41%), and alopecia (3/68, 4.41%). The incidence of immune-related pneumonitis was 5.88% (grade –1-2, 4/68). Proteinuria (6/68, 8.82%) and epistaxis (2/68, 2.94%) were the most common TRAEs related to antiangiogenic agents. A total of 13 (19.12%) patients required dose adjustment due to TRAEs, which was performed mainly for chemotherapeutics decreasing to 70–80% of the initial dose. Three patients (4.41%) had treatment interruption, of whom two (66.67%) were unable to tolerate the treatment, and one patient refused to continue chemotherapy.
Discussion
This study retrospectively evaluated the effectiveness and safety of the combination of Rh-endostatin with PD-1 blockades plus chemotherapy in treatment-naïve patients with advanced or metastatic NSCLC who were EGFR/ALK-negative. The high ORR (72.06%) and DCR (95.59%), as well as favorable median PFS (22.0 months [95% CI: 16.6-27.4]) and OS (31.0 months [95% CI: 23.4-NE]), were observed in the present study. The results of this real-world data showed promising effectiveness in improving survival outcomes and acceptable safety profiles, indicating a promising treatment option for patients with advanced or metastatic NSCLC.
Nowadays, ICIs have revolutionized the treatment paradigm of NSCLC [27]. The efficacy of PD-1/PD-L1 inhibitors as single-modality approach or in combination with chemotherapy in treatment-naïve patients with advanced or metastatic NSCLC has been investigated in numerous large randomized phase III trials, with significantly longer median OS and median PFS than chemotherapy alone in the first-line setting [11, 13,14,15,16, 28]. Studies showed that anti-PD-1 antibodies, including sintilimab, nivolumab, camrelizumab, and tislelizumab, combined with chemotherapy significantly improved the clinical outcomes and obtained median PFS, median OS, and ORR of 7.6-8.9 months, 18.3-24.2 months, and 51.5-74.8%, respectively [11, 13,14,15,16]. The results observed in the present study (median PFS, 22.0 months; median OS, 31.0 months; ORR, 72.06%) were numerically higher than those reported in the above mentioned phase III clinical studies. Besides, emerging data suggested that anti-angiogenesis may exert positive immunomodulatory activity in the immunosuppressive tumor microenvironment and may have a synergistic effect in combination with ICIs, which can improve survival benefits in NSCLC [29,30,31], which might explain the high response and promising survival prognosis in this study. Furthermore, this study also reported numerically higher results in patients with EGFR/ALK-negative non-squamous NSCLC compared to those of atezolizumab plus bevacizumab and chemotherapy in IMpower150 (median PFS: 8.3 months; median OS: 19.5 months; ORR: 64.00%) [32, 33] and those of nivolumab combined with bevacizumab and chemotherapy in advanced non-squamous NSCLC (ONO-4538-52/TASUKI-52: median PFS: 12.1 months; median OS: 25.4 months; ORR: 61.5%) (Table 4) [34]. Therefore, the encouraging effectiveness achieved by the combination regimen in this study provided important insights into the efficacy of Rh-endostatin combined with PD-1 antibody plus for NSCLC in a real-world setting.
In subgroup analyses, patients with stage IIIB/IIIC NSCLC had significantly longer median PFS (23.4 months vs.13.2 months), longer median OS (NE vs. 18.0 months), and higher ORR (ORR: 89.19% vs. 51.61%) than those with stage IV disease (all p ≤ 0.001). In brief, results from our subgroup analyses were consistent with the subgroup analysis in ORIENT-12 trial [35]. In addition, brain metastasis was a poor prognostic factor for NSCLC [36]. In this study, patients with brain metastasis were associated with significantly poorer survival outcomes than those without brain metastasis (median PFS: 8.0 vs. 22.5 months; median OS: 13.3 vs. 31.0 months; both p < 0.05). Furthermore, patients in the PD-L1 high expression (PD-L1 TPS ≥ 50%) and the PD-L1 expression unknown group had significantly improved ORR than those in the PD-L1 low to moderate expression group (PD-L1 TPS < 1% and 1-49%) (p < 0.05), but no differences were observed regarding PFS and OS. Indeed, the PD-L1 expression-based heterogeneity in response to ICI combination chemotherapy was reported in several phase 3 studies showing evidence of favorable tumor response in patients with high PD-L1 expression, but no statistical significance was indicated in any of these studies [11, 14, 37]. Other studies also found no significant association between PD-1 expression and survival or antitumor activity [38,39,40]. Thus, whether PD-L1 expression derived more survival benefits or tumor response from the combination of Rh-endostatin with chemotherapy and PD-1 antibodies remained to be further validated by further large-scaled randomized controlled trials (RCTs).
The safety profile in this study was acceptable and the combined thrapy was tolerable for the patients. Compared to previous clinical trials of combined regimens with or without Rh-endostatin in patients with advanced or metastatic NSCLC, there were no unexpected safety events appearing in this study [10,11,12, 41]. Although all subjects experienced TRAEs, 83.82% of the patients had grade 1-2 TRAEs. Atezolizumab plus bevacizumab and chemotherapy in IMpower150 in NSCLC showed TRAEs of 14.71% vs. 55.7% [37]. Especially, no severe (grade 3 or higher) AEs related to anti-angiogenic drugs including hypertension, proteinuria, and epistaxis were observed and no treatment-relatd death was reported in this study. Gerenally, the combination of Rh-endostatin plus chemotherapy and PD-1 antibodies was well-tolerated and could be delivered safely in patients with advanced or metastatic NSCLC.
There were some limitations in this study. First, the study was retrospective in nature, and thus subjected to selection bias. Second, all patients were included from a single institute in China, which might affect the generalizability of results to a broader population. Third, the study had a limited sample size. Therefore, more multicenter RCTs with larger sample size are warranted to further validate the use of Rh-endostatin plus chemotherapy and PD-1 inhibitors as first-line treatment for advanced or metastatic NSCLC.
Conclusions
In summary, our findings revealed that the combination of Rh-endostatin with PD-1 antibodies and chemotherapy yielded promising therapeutic efficacy with safety profile as first-line treatment for patients with EGFR/ALK-negative advanced or metastatic NSCLC, indicating a potential treatment option for this population. Furthermore, more high-quality multicenter RCTs with a larger sample size are warranted to further investigate the therapeutic efficacy and safety of Rh-endostatin plus PD-1 antibodies and chemotherapy for advanced or metastatic NSCLC.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Rh-endostatin:
-
Recombinant human endostatin
- PD-1:
-
Anti-programmed cell death 1
- NSCLC:
-
Non-small cell lung cancer
- CI:
-
Confidence interval
- NE:
-
Not evaluable
- TPS:
-
Tumor proportion score
- AEs:
-
Adverse events
- PFS:
-
Progression-free survival
- ICIs:
-
Immune checkpoint inhibitors
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- EGFR:
-
Epidermal growth factor receptor
- ALK:
-
Anaplastic lymphoma kinase
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous-cell carcinoma
- TRAEs:
-
Treatment-related adverse events
- DCR:
-
Disease control rate
- PD:
-
Progressive disease
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD-L1 TPS:
-
Programmed cell death-ligand 1 tumor proportion score
- NCI-CTCAE:
-
National Cancer Institute’s Common Terminology Criteria for Adverse Events
References
Xia C, Dong X, Li H, Cao M, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–90.
Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27(8):1345–56.
Balata H, Fong KM, Hendriks LE, et al. Prevention and early detection for NSCLC: advances in thoracic oncology 2018. J Thorac Oncol. 2019;14(9):1513–27.
Griesinger F, Curigliano G, Thomas M, et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. 2022;33(11):1168–78.
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive non–small-cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced squamous-cell non–small-cell Lung Cancer. N Engl J Med. 2015;373(2):123–35.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus Docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (London England). 2017;389(10066):255–65.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Weinstock C, Khozin S, Suzman D, et al. US Food and Drug Administration approval Summary: Atezolizumab for Metastatic non–small cell lung CancerFDA approval Summary: Atezolizumab for metastatic NSCLC. Clin Cancer Res. 2017;23(16):4534–9.
Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–17.
Yang Y, Wang Z, Fang J, et al. Efficacy and safety of Sintilimab Plus Pemetrexed and Platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC: a Randomized, Double-Blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2020;15(10):1636–46.
Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of Pembrolizumab Plus Chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657–69.
Ren S, Chen J, Xu X, et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2022;17(4):544–57.
Wang J, Lu S, Yu X, et al. Tislelizumab Plus Chemotherapy vs Chemotherapy alone as first-line treatment for advanced squamous non–small-cell lung Cancer: a phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7(5):709–17.
Paz-Ares L, Ciuleanu TE, Yu X, et al. LBA3 Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as first-line (1L) treatment (tx) for advanced non-small cell lung cancer (aNSCLC): CheckMate 227 - part 2 final analysis. Ann Oncol. 2019;30:xi67–8.
Zhang L, Wang Z, Fang J, et al. Final overall survival data of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC in the phase 3 ORIENT-11 study. Lung cancer (Amsterdam Netherlands). 2022;171:56–60.
Wang S, Xie K, Liu T. Cancer immunotherapies: from efficacy to resistance mechanisms–not only checkpoint matters. Front Immunol. 2021;12:690112.
Chen Y, Hu J, Bu F, et al. Clinical characteristics of hyperprogressive disease in NSCLC after treatment with immune checkpoint inhibitor: a systematic review and meta-analysis. BMC Cancer. 2020;20:1–9.
Dewangan J, Srivastava S, Mishra S, et al. Salinomycin inhibits breast cancer progression via targeting HIF-1α/VEGF mediated tumor angiogenesis in vitro and in vivo. Biochem Pharmacol. 2019;164:326–35.
Ren S, Xiong X, You H, et al. The combination of Immune checkpoint blockade and angiogenesis inhibitors in the treatment of Advanced Non-small Cell Lung Cancer. Front Immunol. 2021;12:689132.
Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52(9):1475–85.
Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301.
Fu Y, Tang H, Huang Y, et al. Unraveling the mysteries of endostatin. IUBMB Life. 2009;61(6):613–26.
An J, Lv W. Endostar (rh-endostatin) versus placebo in combination with vinorelbine plus cisplatin chemotherapy regimen in treatment of advanced non‐small cell lung cancer: a meta‐analysis. Thorac Cancer. 2018;9(5):606–12.
Wang J, Sun Y, Qin S, Group EPIS. Results of phase IV clinical trial of combining endostar with chemotherapy for treatment of advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2010;28(15suppl):7598–7598.
Wang J, Chen J, Guo Y, et al. Strategies targeting angiogenesis in advanced non-small cell lung cancer. Oncotarget. 2017;8(32):53854–72.
Mamdani H, Matosevic S, Khalid AB, et al. Immunotherapy in Lung Cancer: current Landscape and future directions. Front Immunol. 2022;13:823618.
Meng LF, Huang JF, Luo PH, et al. The efficacy and safety of immune checkpoint inhibitor plus chemotherapy in patients with advanced non-small-cell lung cancer: a meta-analysis. Investig New Drugs. 2022;40(4):810–7.
Wang Q, Gao J, Di W, et al. Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunol Immunother. 2020;69(9):1781–99.
Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–91.
Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus Paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35.
Reck M, Socinski MA, Cappuzzo F, et al. Primary PFS and safety analyses of a randomized phase III study of carboplatin + paclitaxel+/– bevacizumab, with or without atezolizumab in 1L non-squamous metastatic NSCLC (IMPOWER150). Ann Oncol. 2017;28:xi31.
Socinski MA, Nishio M, Jotte RM, et al. IMpower150 final overall survival analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2021;16(11):1909–24.
Sugawara S, Lee J-S, Kang J-H, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32(9):1137–47.
Zhou C, Wu L, Fan Y, et al. Sintilimab Plus Platinum and Gemcitabine as First-Line treatment for Advanced or metastatic squamous NSCLC: results from a Randomized, Double-Blind, phase 3 trial (ORIENT-12). J Thorac Oncol. 2021;16(9):1501–11.
Kawabe T, Phi JH, Yamamoto M, et al. Treatment of brain metastasis from lung cancer. Curr Future Manage Brain Metastasis. 2012;25:148–55.
Reck M, Mok TS, Nishio M, et al. Atezolizumab plus Bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respiratory Med. 2019;7(5):387–401.
Antonia SJ, Goldberg SB, Balmanoukian AS et al. Phase ib study of MEDI4736, a programmed cell death ligand-1 (PD-L1) antibody, in combination with tremelimumab, a cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) antibody, in patients (pts) with advanced NSCLC. Am Soc Clin Oncol. 2015.
Schmidt LH, Kümmel A, Görlich D, et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS ONE. 2015;10(8):e0136023.
Sorensen S, Zhou W, Dolled-Filhart M, et al. Pd-L1 expression and survival among Advanced non–small cell Lung Cancer (Nsclc) patients treated with chemotherapy. Ann Oncol. 2014;25:iv467.
Wang ZQ, Wang DS, Wang FH, et al. Recombinant human endostatin plus paclitaxel/nedaplatin for recurrent or metastatic advanced esophageal squamous cell carcinoma: a prospective, single-arm, open-label, phase II study. Investig New Drugs. 2021;39:516–23.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conceptionand design. Trial design, interpretation of the results, drafting of the manuscript, and critical revision were performed by Jing Zhang, Xiao Zhao, Pei-Yuan Lv, Sheng-Jie Sun and Guo-Qing Zhang; Data analyses and the interpretation of the results were performed by Ming-Lu Liu and Lu-Peng Qiu; Patients’ selection and data collection were performed by Ming-Lu Liu and Zi-Zhong Yang. All authors reviewed and approved the fnal manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethical review board of the Chinese PLA General Hospital ethics committee (No.S2018-203-01) and was conducted in accordance with the Declaration of Helsinki and local applicable regulatory guidelines.
Consent for publication
All data published here are under the consent for publication. Written informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Lv, PY., Zhao, X. et al. Real-world effectiveness and safety of recombinant human endostatin plus PD-1 inhibitors and chemotherapy as first-line treatment for EGFR/ALK-negative, advanced or metastatic non-small cell lung cancer. BMC Cancer 24, 967 (2024). https://doi.org/10.1186/s12885-024-12708-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12708-6