Abstract
Background
This study aims to explore novel microRNAs in urine for screening and predicting clinical characteristics in pancreatic cancer (PC) patients using a microRNA array-based approach.
Methods
We used the Toray® 3D-Gene microRNA array-based approach to compare urinary levels between PC patients and healthy volunteers.
Results
(1) Four oncogenic microRNAs (miR-744-5p, miR-572, miR-210-3p, and miR-575) that were highly upregulated in the urine of PC patients compared to healthy individuals were identified by comprehensive microRNA array analysis. (2) Test-scale analysis by quantitative RT-PCR for each group of 20 cases showed that miR-210-3p was significantly upregulated in the urine of PC patients compared to healthy individuals (P = 0.009). (3) Validation analysis (58 PC patients and 35 healthy individuals) confirmed that miR-210-3p was significantly upregulated in the urine of PC patients compared to healthy individuals (P < 0.001, area under the receiver operating characteristic curve = 0.79, sensitivity: 0.828, specificity: 0.743). We differentiated PC patients into invasive ductal carcinoma (IDCa) and intraductal papillary mucinous carcinoma (IPMC) groups. In addition to urinary miR-210-3p levels being upregulated in IDCa over healthy individuals (P = 0.009), urinary miR-210-3p levels were also elevated in IPMC over healthy individuals (P = 0.0018). Urinary miR-210-3p can differentiate IPMC from healthy individuals by a cutoff of 8.02 with an AUC value of 0.762, sensitivity of 94%, and specificity of 63%. (4) To test whether urinary miR210-3p levels reflected plasma miR-210-3p levels, we examined the correlation between urinary and plasma levels. Spearman’s correlation analysis showed a moderate positive correlation (ρ = 0.64, P = 0.005) between miR-210-3p expression in plasma and urine.
Conclusions
Urinary miR-210-3p is a promising, non-invasive diagnostic biomarker of PC, including IPMC.
Trial registration
Not applicable.
Similar content being viewed by others
Background
Pancreatic cancer (PC) is a highly lethal malignancy and the seventh leading cause of death worldwide [1]. Although surgical resection is essential to achieve a cure, patients without evidence of locally advanced or metastatic disease account for only 15–20% of all PC patients [2]. This is because PC is asymptomatic, locally invasive, or metastasizes to distant organs early in the clinical course [3,4,5]. Recent improvements in surgical techniques and perioperative management have improved the prognosis of resected PC, with a reported median survival time (MST) of 36.72 months in the combined preoperative and postoperative adjuvant therapy group [6]. However, the prognosis of unresectable and metastatic PC is still very poor, with an MST of 11.1 months reported for a group of patients treated with FOLFIRINOX, despite the significant improvement compared to 6.8 months in the control, gemcitabine group [7]. Therefore, PC clearly requires early detection, and curative resection can improve the prognosis.
Due to the potential to improve patient survival in cases of this fatal disease, numerous studies have attempted to identify the clinical biomarkers associated with PC [8, 9]. However, limited molecules have been validated as diagnostic and prognostic biomarkers of PC in clinical settings. Currently, serum tumor markers such as carcinoembryonic antigen and carbohydrate antigen 19 − 9 (CA19-9) have been employed to diagnose and monitor the tumor dynamics of PC [10]. Nevertheless, despite their convenience, these markers exhibit insufficient sensitivity and specificity for early detection and screening. Therefore, it is imperative to devise novel molecular biomarkers utilizing less invasive techniques, which will permit clinicians to detect PC at an early stage, monitor tumor dynamics, and predict prognosis.
MicroRNAs (miRNAs) are small non-coding RNAs that regulate the translation of specific protein-coding genes. Since their discovery in 1993 [11], miRNAs have been the subject of intensive research in the cancer field. Altered expression of miRNAs is associated with several diseases and contributes to the development of various cancers [12,13,14,15]. In recent years, several studies have demonstrated that miRNAs are detectable in plasma/serum in a remarkably stable form [13, 16,17,18,19]. Numerous studies, including our own, have reported the utility of circulating miRNAs in blood as novel biomarkers for various cancers, including PC [20,21,22]. In PC research, liquid biopsy biomarker development using cell-free nucleic acids in body fluids has been attempted, with studies reporting on nucleic acids in duodenal and pancreatic juice [23] in addition to blood. However, there are currently no liquid biopsy biomarkers in clinical use.
Urine collection is considered the ultimate non-invasive test compared to blood collection. The study for biomarkers using urinary miRNAs has been conducted in urological cancers such as prostate and bladder cancer, and their usefulness has been recognized [24, 25]. Recently, the usefulness of urinary microRNA as a biomarker has also been reported for solid tumors other than urological cancers [26, 27]. However, reports on the usefulness of urinary miRNA as a biomarker of PC are limited. In this study, we explored the usefulness of urinary miRNA as a biomarker of PC in urine, which is the non-invasive collection method.
Methods
Patients and samples
The study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine, and each individual provided signed informed consent. Between January 2010 and April 2013, a total of 58 urine samples from PC patients and 41 samples from healthy volunteers were collected. The large-scale analysis includes the samples that were subjects in the small-scale analysis. These healthy volunteers included medical personnel and patients with benign disease, such as cholecystolithiasis and inguinal hernia. Detailed clinical information on healthy volunteers is shown in Supplementary Table S1. These patients underwent medical examinations, including computed tomography and endoscopy, and were proven not to have any pancreatic or cancerous diseases. Tumor stage was assessed according to the Union for International Cancer Control classification [28].
A urine sample and peripheral blood were obtained from each patient before surgery and from the healthy volunteers. The samples were transferred into sodium heparin tubes (BD Vacutainer, Franklin Lakes, NJ). The urine was immediately subjected to the spin protocol (1500 rpm for 30 min) and the peripheral blood was subjected to the three-spin protocol (1500 rpm for 30 min, 3000 rpm for 5 min, and 4500 rpm for 5 min) to prevent contamination by cellular nucleic acids. These samples were collected and stored at -80 °C until further processing.
The resected specimens were fixed in formalin and embedded in paraffin for pathological diagnosis. Histological evaluation was performed for tissues adjacent to specimens, according to the criteria of the World Health Organization. In all cases, two pathologists agreed with the pathological observations and confirmed the diagnosis.
RNA extraction
Total RNA was extracted from 400 µl of urine and plasma using the mirVana PARIS Kit (Ambion, Austin, TX) and finally eluted into 100 µl of preheated (95 °C) elution solution according to the manufacturer’s protocol. A volume of 400 µl of plasma was used as the common denominator in each microarray analysis because the plasma miRNA analyses had no definite internal control, as shown in our previous studies [29,30,31,32,33,34,35,36].
miRNA microarray analysis
Microarray analyses of urine samples were performed using the 3D-Gene miRNA microarray platform (Toray Industries, Kamakura, Japan) [32, 34, 37, 38]. The results were compared between three different PC patients and three healthy volunteers. Each 100 µl urine sample from three PC patients, who underwent curative surgery, was equally mixed, and the total 300 µl of urine sample was used as the sample from PC patients. Similarly, each 100 µl plasma sample from three healthy volunteers was equally mixed, and the total 300 µl of urine sample was used as the healthy volunteers’ sample. This method follows the microRNA microarray approach that we have used in our plasma miRNA studies [21, 22]. RNA extraction and microarray analysis were performed according to the manufacturer’s instructions described previously [32]. Because the amount of RNA in urine is too small, 2 of 4 µl of RNA was extracted from the total 300 µl samples and used in the microarray experiments. This RNA was labeled with Hy5 using the Label IT miRNA Labeling Kit (Takara Bio, Otsu, Japan) and hybridized at 32 °C for 16 h on a 3D-Gene chip. The 3D-Gene miRNA microarray (Human_miRNA_17v1.0.0, Toray Industries) can mount > 1500 miRNAs based on the Human miRNA Version 17 of MirBase (http://microrna.sanger.ac.uk/). The microarray was scanned, and the images obtained were enumerated using a 3D-GeneH Scanner 3000 (Toray Industries). The expression level of each miRNA was globally normalized using the background-subtracted signal intensity of the entire set of miRNAs in each microarray. The obtained microarray images were analyzed using GenePix Pro TM (Molecular Devices, Sunnyvale, CA). The signal intensities calculated in this manner were compared between samples from PC patients and healthy volunteers. Subsequently, four microRNAs known for their oncogenic functions were selected as candidates in the order of their ratios.
Quantification of miRNA by qRT-PCR
The amounts of miRNAs were quantified by qRT-PCR using the human TaqMan MicroRNA Assay Kit (Applied Biosystems, Foster City, CA). The reverse transcription reaction was carried out with a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) in 5 µl of solution containing 1.67 µl of extracted RNA, 0.05 µl of 100 mM dNTPs, 0.33 µl of MultiScribe Reverse Transcriptase (50 Uµl− 1), 0.5 µl of 10× Reverse Transcription Buffer, 0.06 µl of RNase inhibitor (20 Uµl− 1), 1 µl of gene-specific primer (hsa-miR-744-5p, Assay ID:002324; hsa-miR-572, Assay ID:001614; hsa-miR-210-3p, Assay ID: 000512; hsa-miR-575, Assay ID: 001617), and 1.39 µl of nuclease-free water. To synthesize cDNA, reaction mixtures were incubated at 16 °C for 30 min, at 42 °C for 30 min, and at 85 °C for 5 min, and then were held at 4 °C. Next, 0.67 µl of cDNA was amplified using 5 µl of TaqMan 2× Universal PCR Master Mix with no AmpErase UNG (Applied Biosystems), 0.5 µl of gene-specific primers/probe, and 3.83 µl of nuclease-free water in a final volume of 10 µl. Quantitative PCR was run on a StepOnePlus PCR system (Applied Biosystems), and reaction mixtures were incubated at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Cycle threshold (Ct) values were calculated with StepOne Software v2.0 (Applied Biosystems).
As previously reported [16], we used an approach for data normalization based upon spiking the sample with a synthetic RNA oligonucleotide, cel-miR-39, which does not exist in the human genome. Caenorhabditis elegans cel-miR-39 was purchased as a custom-made RNA oligonucleotide (Qiagen, Valencia, CA). We added cel-miR-39 as a “spike-in” (known concentration of miRNA as an internal standard) to the samples before RNA extraction. The oligo used for spiking, as a mixture of 25 fmol of oligonucleotide in 5 µl total volume of water, was introduced following the addition of 2X Denaturing Solution (Ambion) to the sample to avoid degradation by endogenous RNases. As a control for each RNA sample, cel-miR-39 was used for TaqMan qRT-PCR assays (Applied Biosystems), as described earlier. We normalized the data across samples using the 2−ΔΔCt method relative to cel-miR-39. ΔCt was calculated by subtracting the Ct values of cel-miR-39 from those of the miRNAs of interest. ΔΔCt was then calculated by subtracting the mean of ΔCt of the urine of healthy volunteers from the ΔCt of the urine of PC patients. The change in gene expression was calculated using the Eq. 2−ΔΔCt [39, 40].
Statistical analysis
For miRNA array-based analyses, the signal intensity ratio and log2 ratio of each urinary miRNA were calculated by the ratio of PC patients to healthy volunteers. The Mann–Whitney U-test for unpaired data from urine samples was performed. The Kruskal–Wallis H-test was also used to compare more than two groups. The Chi-square test or Fisher’s exact probability test was used to evaluate correlations between the results of urinary miRNA levels and clinicopathological factors. Receiver operating characteristic (ROC) curves and area under the ROC curve (AUC) were used to assess the feasibility of using urinary miRNA as a diagnostic tool for detecting PC [41]. Spearman’s correlation analysis was performed to evaluate the correlation between CA19-9 and urinary miR-210-3p, and the ρ-value was calculated as the correlation coefficient. For the clinicopathologic characteristics and prognostic analysis of PC patients, the median value was used as the cutoff value for urinary miRNA-210-3p. For the analysis of survival rates, Kaplan–Meier survival curves were constructed for groups based on univariate predictors, and differences between the groups were analyzed with the log-rank test. All statistical analyses were performed using the SPSS 24.0 software program (IBM Corp, Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
Results
Study design to find novel urinary miRNA biomarkers of PC
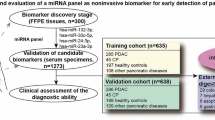
This study was designed as follows: (1) Selection of appropriate miRNA candidates based on a comparison of expression levels in urine between PC patients and healthy volunteers using the Toray® 3D-Gene microRNA array-based approach; (2) Small-scale analysis of urine samples using qRT-PCR to validate the utility of the selected miRNA candidates; (3) Large-scale analysis to validate the urinary level of miR-210-3p; (4) Analysis of the association between the urine level of miR-210-3p and clinicopathological characteristics and prognosis in PC patients; and (5) Evaluation of the correlation of miR-210-3p levels in blood and urine (Fig. 1A).
(a) Study design to find novel plasma miRNA biomarkers of PC. (b) Selection of plasma miRNA candidates from the comprehensive miRNA array-based approach. Using the miRNA array-based approach to compare urinary miRNA levels between pancreatic cancer (PC) patients and healthy volunteers, novel miRNA candidates for cancer detection were selected. Of the top 30 upregulated miRNAs in PC, four novel candidate miRNAs, which were previously reported to have an oncogenic role in cancers, were selected. Of these, miR-210-3p was selected for further analysis
Selection of urinary miRNA candidates using a comprehensive miRNA array-based approach
Using a miRNA array-based approach, we selected miRNA candidates for cancer detection based on a comparison of the urinary levels of miRNAs between PC patients and healthy volunteers (Fig. 1B). Of the 2565 candidates analyzed, the expression levels of 103 urinary miRNAs were more than two-fold higher in PC patients than in healthy volunteers (Supplementary Table S2). In order to find more sensitive biomarkers, we focused on 30 miRNAs with the highest expression levels in the urine of PC patients (Fig. 1B). Out of the 30 miRNAs, we selected four miRNAs (miR-744-5p, miR-572, miR-210-3p, and miR-575) with high ratios, taking into consideration the previously reported functions of miRNAs. High plasma levels of these miRNAs would be highly suggestive of an origin from tumor necrosis, apoptosis, and active release of secretory vesicles such as exosomes from cancer cells. Therefore, these miRNAs in urine might reflect tumor dynamics in cancer tissues [29, 30, 33, 34]. However, tumor suppressive miRNAs and functionally unknown miRNAs were excluded from this study because their origin is not well known [32].
Small-scale analysis of urinary levels of four miRNAs in PC patients and healthy volunteers
Next, we investigated the urinary levels of the four miRNAs in 20 PC patients and 20 healthy volunteers by qRT-PCR using a preliminary small-scale analysis. As indicated in the results from the miRNA array-based approach, the expression levels of miR-210-3p were validated to be significant (P = 0.009), but no significant differences could be identified for other miRNAs (Fig. 2). We, therefore, selected miR-210-3p for further analyses.
Small-scale analysis comparing urinary levels of four miRNAs between PC patients and healthy volunteers. Urinary levels of the selected four miRNAs in 20 PC patients and 20 healthy volunteers were analyzed by qRT-PCR. The expression level of each miRNA was normalized to that of cel-miR-39, as described in Materials and Methods
Large-scale analysis of the urinary level of mir-210-3p in PC patients
We then validated our observations in large-scale settings. Urinary miR-210-3p was detectable in all samples from 58 PC patients and 41 healthy volunteers. The large-scale analysis includes the samples that were subjects in the small-scale analysis. We observed that the urinary level of miR-210-3p was significantly higher in the PC patients than in the healthy volunteers (P < 0.001) (Fig. 3a). Furthermore, to detect any cut-off points that could differentiate cancer patients from healthy volunteers, we utilized the AUC with the Youden index [41] (Fig. 3b) and calculated the AUC as 0.7906. The optimal cut-off point was indicated at 8.20 in relative expression units using the miR-210-3p/cel-miR-39 ratio with a sensitivity of 82.8% and specificity of 74.3%. Our results provide evidence that the urinary level of miR-210-3p can be used to distinguish PC patients from healthy volunteers to a clinically satisfactory degree in comparison to conventional tumor markers.
Large-scale analysis of the urinary level of miR-210-3p. (a). Urinary miR-210-3p was more highly expressed in the urine of PC patients than in that of healthy volunteers. For the large-scale analysis, total RNA extracted from urine samples of 58 PC patients and 35 healthy volunteers was used to analyze the expression level of miR-210-3p using qRT-PCR. (b). Analysis of the receiver operating characteristic (ROC) curve to detect PC patients. ROC analysis showed the greatest area under the ROC curve (AUC) of 0.7906
Correlation between the urinary level of miR-210-3p and clinicopathological factors and prognosis in PC patients
The PC cohort consisted of 41 invasive ductal carcinoma (IDCa) cases (71%) and 17 intraductal papillary mucinous carcinoma (IPMC) cases (29%). Of these, 13 (22%) had non-curable factors and were not resected, whereas the remaining 45 cases (78%) were resected. Ten cases (17%) of pathological Tis (a carcinoma in situ) were included. We analyzed whether the urinary level of miR-210-3p and clinicopathological factors were correlated in PC patients (Table 1). There were no significant differences in clinicopathological characteristics according to urinary miR-210-3p level. Comparison between the high and low urinary miR-210 groups showed that the concentration of CA19-9 tended to be slightly higher in the group with low urinary miR-210, but the difference was not significant (P = 0.07). Spearman’s correlation analysis also showed no significant correlation between CA19-9 and urinary miR-210-3p (r = -0.156 P = 0.24). Although not significant, there was a trend toward more women and more IPMC in the group with higher urinary miR-210-3p than in the group with lower miR-210-3p. Therefore, we differentiated PC patients into IDCa and IPMC groups and compared the levels of urinary miR-210-3p to those in healthy individuals (Fig. 4a). In addition to urinary mi210-3p levels being upregulated in IDCa over healthy individuals (P = 0.009), urinary mi210-3p levels were also elevated in IPMC over healthy individuals (P = 0.0018). Interestingly, although not significantly different, levels of urinary mi210-3p tended to be higher in IPMC than in IDCa [15 (10–92) vs. 54 (14–177), P = 0.11]. To evaluate the diagnostic potential of urinary miR-210-3p for IPMC, ROC analysis was performed to distinguish between IDCa and intraductal papillary mucinous neoplasm (IPMN) (Fig. 4b). The results showed that urinary miR-210-3p can differentiate IPMC from healthy individuals by a cutoff of 8.02 with an AUC value of 0.762, sensitivity of 94%, and specificity of 63%.
Diagnostic ability of urinary miR-210-3p for IPMC. (a). Urinary miR-210-3p levels was upregulated in IDCa over healthy individuals (p = 0.0086). In addition, urinary miR-210-3p levels were also elevated in IPMC over healthy individuals (p = 0.0018). (b). Urinary miR-210-3p can differentiate IDCa from healthy individuals by a cutoff of 7.82 with an AUC value of 0.669, sensitivity of 80%, and specificity of 61%. Urinary miR-210-3p can differentiate IPMC from healthy individuals by a cutoff of 8.02 with an AUC value of 0.762, sensitivity of 94%, and specificity of 63%
We present a representative case of mixed-type IPMN in which urinary miR-210-3p measurement was useful in the diagnosis of IPMC (Fig. 5). The characteristics of this case were as follows: No mural nodules to be contrast enhanced; diagnosed by main pancreatic duct dilatation of more than 10 mm as high-risk stigmata. CEA was 1.5 and CA19-9 was 2.0, and no elevation of tumor markers was observed. Preoperative pancreatic fluid cytology showed class III, and pancreatic ductal brush cytology showed class IV by endoscopic retrograde cholangiopancreatography (ERCP). There was a strong suspicion of malignancy, but a definitive diagnosis of cancer was not obtained. The patient’s preoperative urinary miR-210-3p value was 126, well above the cutoff. Pancreaticoduodenectomy was performed and pathology diagnosed Tis lesion of IPMC.
A representative case in which urinary miR-210-3p measurement was useful in the diagnosis of IPMC. This is a case of mixed-type IPMN. No mural nodules to be contrast enhanced; diagnosed by main pancreatic duct dilatation of more than 10 mm as high-risk stigmata. CEA was 1.5 and CA19-9 was 2.0. No elevation of tumor markers was observed. Preoperative pancreatic fluid cytology showed class III, and pancreatic ductal abrasion cytology showed class IV by endoscopic retrograde cholangiopancreatography. There was a strong suspicion of malignancy, but a definitive diagnosis of cancer was not obtained. The patient’s preoperative urinary miR-210-3p value was 126, well above the cutoff
Finally, the prognostic value of urinary miR-210-3p in PC patients was examined. When evaluating the prognostic significance of urinary miR-210-3p in all PC patients, including non-resected and IPMC cases, no significant difference in overall survival was observed based on the level of urinary miR-230-3p (P = 0.09, Supplementary Fig. 1). A prognostic analysis was then performed on the resected IDCa and IPMC subgroups. There was no significant difference in overall survival after resection (P = 0.84, Fig. 6a) or recurrence-free survival after resection of IDCa (P = 0.54, Fig. 6b) by the level of urinary miR-230-3p. In addition, no significant difference was identified in overall survival after resection (P = 0.13, Fig. 6c) or recurrence-free survival after resection of IPMC (P = 0.19, Fig. 6d) by the level of urinary miR-230-3p.
Prognostic impact of the level of urinary miR-210-3p in PC patients. (a). Overall survival after resection for IDCa (invasive ductal carcinoma). (b). Recurrence-free survival after resection for IDCa. (c). Overall survival after resection for intraductal papillary mucinous carcinoma (IPMC). (d). Recurrence-free survival after resection for IPMC
Correlation between mir-210-3p expression in plasma and urine
To test whether urinary miR210-3p levels reflected plasma miR-210-3p levels, we examined the correlation between urinary and plasma levels. The analysis was performed on 15 PC patients for whom plasma samples were available in addition to urine. Plasma miR-210-3p was significantly upregulated in the urine of PC patients compared to healthy individuals (P = 0.03, Fig. 7a). A scatter plot of miR-210-3p level in urine and plasma is shown in Fig. 7b. The results of Spearman’s correlation analysis showed a positive correlation between these levels (ρ = 0.643, P = 0.005).
Correlation between miR-210-3p expression in plasma and urine. (a) The analysis was performed on 15 PC patients for whom plasma samples were available in addition to urine. Plasma miR-210-3p was significantly upregulated in the urine of PC patients compared to healthy individuals (p = 0.0319) (b) Scatter plot of miR-210-3p level in urine and plasma. The results of Spearman’s correlation analysis showed a positive correlation between these levels (ρ = 0.643, p = 0.005)
Discussion
Development of minimally invasive biomarker assays for the early detection and effective clinical management of PC is urgently required to reduce the high mortality associated with this lethal disease. In recent years, many miRNAs have been identified as potential biomarkers of various cancers. Several researchers, including us, have demonstrated that miRNAs circulating in the plasma/serum of PC patients are useful in detecting cancer because of a difference in their expression levels that distinguishes cancer patients from healthy individuals [31, 33, 42,43,44]. To date, however, only one report examining the diagnostic utility of urinary miRNA for PC was identified in 2015 [45], and this report included only 13 cases of PC, lacking validation by a large number of cases. Since then, no studies have reported the clinical utility of urinary miRNA in PC, and urinary miRNA still has not been used in clinical practice. In blood, contamination of cellular miRNAs of hematopoietic origin should be considered [46]. Urine is considered an ideal body fluid for biomarker exploration as an alternative to blood. This led us to investigate the clinical utility of urinary miRNA, the ultimate minimally invasive liquid biopsy biomarker that may facilitate better decision making for PC treatment.
In this study, we identified a urinary microRNA, miR-210-3p, as a novel biomarker of PC, through genome-wide miRNA profiling of the urine of PC patients using high-resolution miRNA arrays. The expression level of urinary miR-210-3p was significantly higher in PC patients than in healthy volunteers, and this finding was validated in the large-scale analysis. In fact, the sensitivity and specificity were 0.82 and 0.74, respectively, for differentiation from healthy individuals. The current cohort consists of 19% IPMC and 17% Tis (a carcinoma in situ) cases (with overlap). Urinary miR-210-3p tends to be higher in IPMC than IDCa, regardless of tumor progression. And even when IDCa and IPMC were analyzed separately, it was revealed that urinary miR-210-3p can differentiate IPMC from healthy individuals. These findings led us to expect that this urinary miR-210-3p would be useful, especially for detecting early-stage lesions and IPMC. IPMN has a multi-step carcinogenesis pattern characterized by the adenoma-carcinoma sequence and progresses to invasive carcinoma through low-grade dysplasia and high-grade dysplasia [47]. It is necessary to properly diagnose IPMC and determine the indication for surgery. However, the sensitivity of tumor markers is low, and pancreatic fluid sampling and brush cytology by ERCP also carry the risk of pancreatitis [48]. Therefore, urinary miR-210-3p could be a very useful non-invasive biomarker in clinical practice.
Using a microarray approach, we identified four microRNAs, miR-744-5p, miR-572, miR-210-3p, and miR-575, as candidate urinary biomarkers for PC. miR-744-5p has a tumor suppressive function and has been reported to be downregulated in cancer tissue [49, 50]. miRNA-572 has been reported to act as an oncogene that promotes tumor growth and invasion in various cancers, including prostate [51], ovarian [52], and renal cell carcinoma [53]. miRNA-572 has been show to be frequently upregulated in cancerous tissues compared to normal tissues, but its expression in blood and urine is not clear. miR-575 has been reported to be upregulated and to function as an oncogene in gastric cancer [54], and downregulated and to function as an tumor suppressor in thyroid cancer [55]. Its expression in blood and urine is not clear. From these three miRNAs added to miR-210-3p for a total of four candidates, miR-210-3p was selected for the present study by PCR-based test scale analysis. Expression of miR-210-3p has been previously reported to be regulated by HIF-1a and upregulated under hypoxic conditions [56]. Mir-210-3p-mediated suppression of ephrin A3 (EFNA3) promotes angiogenesis and tumor growth [57]. In addition, miR-210-3p also targets e2f transcription factor 3 (E2F3), which regulates cell cycle progression and selects more aggressive tumor cells to favor growth [58]. A meta-analysis [59] of nine published studies dealing with various cancers reported that high miR-210-3p expression was correlated with worse survival. However, the study also revealed that the prognostic role of miR-210-3p varied by cancer type. Indeed, miR-210 is an independent negative prognostic factor in breast and cervical cancer patients [60, 61]. However, ovarian cancer studies found that miR-210-3p may function as a suppressor of tumor growth [58, 62]. Validation in the Panc-1 cell line, a PC cell line, did not show any change in proliferation [63]. However, Greither et al. [64] reported that MiR-210-3p overexpression in PC tissue samples was a predictor of poor prognosis. Thus, the prognostic role of miR-210-3p in different tumors may vary. Furthermore, plasma miR-210-3p levels have also been reported to be useful in the diagnosis of PC [65, 66]. Interestingly, however, similar to the trend observed in our urinary study, a previous report on plasma miR-210-3p levels and prognosis of PC reported that high plasma miR-210-3p expression was significantly associated with favorable survival [67]. The different prognostic predictions of miR-210-3p may be explained by the hypoxic environment in which miR-210-3p functions and its different regulatory actions depending on the type of cancer and the origin, traits, and tissues of the samples. There have been no reports of the usefulness of urinary microRNA-210-3p as a biomarker. For the first time, in this study, we have demonstrated the potential diagnostic utility of urinary miR-210-3p.
There is growing evidence that circulating miRNAs in the serum and plasma of cancer patients may serve as noninvasive biomarkers. However, most reports on urinary miRNAs have been in urologic cancers, and reports in patients with other solid tumors are very limited. This study is the first to demonstrate the possibility of detecting miRNA-210-3p levels in urine. The present results showed that miR-210-3p levels are moderately correlated in plasma and urine. However, Weber et al. have shown marked differences in miRNA expression profiles in different human body fluids within individuals [68]. In their study, several miRNAs showed higher expression levels in urine compared to serum, suggesting that miRNAs are involved in specific miRNA secretory processes in the kidney and urinary epithelial compartment [68]. It has also been reported that only exosomal miRNAs are detectable in urine due to the presence of high levels of RNase in the urinary tract and the complete degradation of free RNA [69,70,71]. However, the present analysis cannot distinguish between free urinary miRNA molecules and miRNA particles that are incorporated and protected by exosomes. The miR-210-3p has been reported to be elevated in PC tissue [64] and serum [65, 66], and these findings led us to speculate that its dynamics are such that miR-210-3p elevated in PC tissue circulates in the blood and is excreted in the urine. However, the mechanism of the elevated concentration of microRNAs in urine is not clear and requires further investigation.
The main limitation of this study was its single-center design with a small sample size. The results need to be validated in a large cohort and then in an independent external cohort. Additionally, there was a lack of data on miR-210 expression in pancreatic cancer tissue. We believe that examining the correlation between tissue expression and paired samples of blood and urine will offer insights into the biological mechanisms underlying changes in urinary concentration. Based on these limitations, we are currently prospectively collecting tissue, blood, and urine samples from multiple institutions. We believe that assessing heterogeneity over time, such as pre- and post-surgery and at recurrence, will provide further insights into detailed biological mechanisms. Furthermore, in PC, other promising biomarkers for analysis besides urinary microRNAs, such as urinary metabolites [72, 73] and urinary circulating tumor DNA [74], have been reported. Since urine is noninvasive, can be collected repeatedly, and the amount of urine collected is not limited, multi-omics analysis is possible. The usefulness of urine as a biomarker may be further enhanced by combining the previously reported biomarkers and urinary miR-210, which we have reported. In the future, we plan to perform multi-omics analysis using urine. We hope to report the results of this analysis in the near future.
Conclusions
This is the first report to demonstrate the utility of urinary miR-210-3p for PC. The non-invasive nature of urine collection and the ability to diagnose IPMC in addition to IDCa suggests that urinary miR-210-3p is a promising screening marker for early detection of PC and is expected to contribute to improving the prognosis of PC.
Data availability
The datasets generated and analysed during the current study are available in the Gene Expression Omnibus repository, GSE244347
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J cancer J Int Du cancer. 2019;144(8):1941–53.
Kudo M, Izumi N, Sakamoto M, Matsuyama Y, Ichida T, Nakashima O, Matsui O, Ku Y, Kokudo N, Makuuchi M. Survival analysis over 28 years of 173,378 patients with Hepatocellular Carcinoma in Japan. Liver Cancer. 2016;5(3):190–7.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49.
Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82.
Sultana A, Tudur Smith C, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer. 2008;99(1):6–13.
Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49(2):190–4.
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–6.
Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425(6960):851–6.
Satake K, Kanazawa G, Kho I, Chung YS, Umeyama K. A clinical evaluation of carbohydrate antigen 19 – 9 and carcinoembryonic antigen in patients with pancreatic carcinoma. J Surg Oncol. 1985;29(1):15–21.
Lee RC, Feinbaum RL, Ambros V. The C. Elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54.
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–66.
He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–4.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–8.
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006.
Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–14.
Ichikawa D, Komatsu S, Konishi H, Otsuji E. Circulating microRNA in digestive tract cancers. Gastroenterology. 2012;142(5):1074–e10781071.
Imamura T, Komatsu S, Ichikawa D, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, Arita T, Konishi H, Shiozaki A, et al. Liquid biopsy in patients with pancreatic cancer: circulating tumor cells and cell-free nucleic acids. World J Gastroenterol. 2016;22(25):5627–41.
Imamura T, Komatsu S, Ichikawa D, Miyamae M, Okajima W, Ohashi T, Kiuchi J, Nishibeppu K, Konishi H, Shiozaki A, et al. Depleted tumor suppressor miR-107 in plasma relates to tumor progression and is a novel therapeutic target in pancreatic cancer. Sci Rep. 2017;7(1):5708.
Miyamae M, Komatsu S, Ichikawa D, Kawaguchi T, Hirajima S, Okajima W, Ohashi T, Imamura T, Konishi H, Shiozaki A, et al. Plasma microRNA profiles: identification of miR-744 as a novel diagnostic and prognostic biomarker in pancreatic cancer. Br J Cancer. 2015;113(10):1467–76.
Levink IJM, Nesteruk K, Visser DI, Sieuwerts AM, Fernandes CJC, Jansen M, van Driel L, Poley JW, Peppelenbosch MP, Cahen DL, et al. Optimization of pancreatic juice Collection: a first step toward Biomarker Discovery and early detection of pancreatic Cancer. Am J Gastroenterol. 2020;115(12):2103–8.
Yamada Y, Enokida H, Kojima S, Kawakami K, Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N, et al. MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: correlation with stage and grade, and comparison with urinary cytology. Cancer Sci. 2011;102(3):522–9.
Mengual L, Lozano JJ, Ingelmo-Torres M, Gazquez C, Ribal MJ, Alcaraz A. Using microRNA profiling in urine samples to develop a non-invasive test for bladder cancer. Int J Cancer. 2013;133(11):2631–41.
Erbes T, Hirschfeld M, Rücker G, Jaeger M, Boas J, Iborra S, Mayer S, Gitsch G, Stickeler E. Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker. BMC Cancer. 2015;15:193.
Záveský L, Jandáková E, Turyna R, Langmeierová L, Weinberger V, Záveská Drábková L, Hůlková M, Hořínek A, Dušková D, Feyereisl J, et al. Evaluation of cell-free urine microRNAs expression for the use in diagnosis of ovarian and endometrial cancers. A pilot study. Pathol Oncol Res. 2015;21(4):1027–35.
Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116(22):5336–9.
Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102(7):1174–9.
Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Morimura R, Nagata H, Kosuga T, Iitaka D, Konishi H, Shiozaki A, et al. Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2011;105(1):104–11.
Morimura R, Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Nagata H, Konishi H, Shiozaki A, Ikoma H, Okamoto K, et al. Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br J Cancer. 2011;105(11):1733–40.
Konishi H, Ichikawa D, Komatsu S, Shiozaki A, Tsujiura M, Takeshita H, Morimura R, Nagata H, Arita T, Kawaguchi T, et al. Detection of gastric cancer-associated microRNAs on microRNA microarray comparing pre- and post-operative plasma. Br J Cancer. 2012;106(4):740–7.
Kawaguchi T, Komatsu S, Ichikawa D, Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T, Hirajima S, et al. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer. 2013;108(2):361–9.
Komatsu S, Ichikawa D, Hirajima S, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, Arita T, Konishi H, Shiozaki A, et al. Plasma microRNA profiles: identification of miR-25 as a novel diagnostic and monitoring biomarker in oesophageal squamous cell carcinoma. Br J Cancer. 2014;111(8):1614–24.
Komatsu S, Ichikawa D, Takeshita H, Konishi H, Nagata H, Hirajima S, Kawaguchi T, Arita T, Shiozaki A, Fujiwara H, et al. Prognostic impact of circulating miR-21 and miR-375 in plasma of patients with esophageal squamous cell carcinoma. Expert Opin Biol Ther. 2012;12(Suppl 1):S53–59.
Hirajima S, Komatsu S, Ichikawa D, Takeshita H, Konishi H, Shiozaki A, Morimura R, Tsujiura M, Nagata H, Kawaguchi T, et al. Clinical impact of circulating miR-18a in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2013;108(9):1822–9.
Nagino K, Nomura O, Takii Y, Myomoto A, Ichikawa M, Nakamura F, Higasa M, Akiyama H, Nobumasa H, Shiojima S, et al. Ultrasensitive DNA chip: gene expression profile analysis without RNA amplification. J BioChem. 2006;139(4):697–703.
Giovannetti E, van der Velde A, Funel N, Vasile E, Perrone V, Leon LG, De Lio N, Avan A, Caponi S, Pollina LE, et al. High-throughput microRNA (miRNAs) arrays unravel the prognostic role of MiR-211 in pancreatic cancer. PLoS ONE. 2012;7(11):e49145.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego Calif). 2001;25(4):402–8.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45.
Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr (Oslo Norway: 1992). 2007;96(5):644–7.
Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res. 2009;2(9):807–13.
Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le Q-T, Koong AC. Circulating miR-210 as a novel hypoxia marker in pancreatic Cancer. Translational Oncol. 2010;3(2):109–13.
Liu J, Gao J, Du Y, Li Z, Ren Y, Gu J, Wang X, Gong Y, Wang W, Kong X. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int J cancer J Int Du cancer. 2012;131(3):683–91.
Debernardi S, Massat NJ, Radon TP, Sangaralingam A, Banissi A, Ennis DP, Dowe T, Chelala C, Pereira SP, Kocher HM, et al. Noninvasive urinary miRNA biomarkers for early detection of pancreatic adenocarcinoma. Am J Cancer Res. 2015;5(11):3455–66.
Duttagupta R, Jiang R, Gollub J, Getts RC, Jones KW. Impact of cellular miRNAs on circulating miRNA biomarker signatures. PLoS ONE. 2011;6(6):e20769.
Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, et al. A revised classification system and recommendations from the Baltimore Consensus Meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39(12):1730–41.
Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP Jr., Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56(5):652–6.
Kleemann M, Schneider H, Unger K, Sander P, Schneider EM, Fischer-Posovszky P, Handrick R, Otte K. MiR-744-5p inducing cell death by directly targeting HNRNPC and NFIX in ovarian cancer cells. Sci Rep. 2018;8(1):9020.
Liang H, Li L, Zhu S, Tan J, Yang B, Wang X, Wu G, Xie C, Li L, Liu Z, et al. MicroRNA-744-5p suppresses tumorigenesis and metastasis of osteosarcoma through the p38 mitogen-activated protein kinases pathway by targeting transforming growth factor-beta 1. Bioengineered. 2022;13(5):12309–25.
Zang M, Guo X, Chen M. The role of microRNA-572 in the proliferation and chemotherapeutic treatment of prostate cancer. J Int Med Res. 2021;49(5):3000605211014363.
Statement of Retraction. MicroRNA-572/ hMOF/Sirt6 regulates the progression of ovarian cancer. Cell Cycle. 2022;21(17):1895.
Pan X, Li Z, Zhao L, Quan J, Zhou L, Xu J, Xu W, Guan X, Li H, Yang S, et al. microRNA–572 functions as an oncogene and a potential biomarker for renal cell carcinoma prognosis. Oncol Rep. 2018;40(5):3092–101.
Wang YN, Xu F, Zhang P, Wang P, Wei YN, Wu C, Cheng SJ. MicroRNA-575 regulates development of gastric cancer by targeting PTEN. Biomed Pharmacother. 2019;113:108716.
Dong H, Wu YL, Zhang X, Li HL, Zheng WH. MicroRNA-575 targets Derlin 1 to regulate proliferation, migration and invasion of human thyroid cancer cells. Arch Med Sci. 2023;19(4):1108–15.
Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27(5):1859–67.
Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283(23):15878–83.
Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O’Brien-Jenkins A, Katsaros D, Weber BL, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7(2):255–64.
Li M, Ma X, Li M, Zhang B, Huang J, Liu L, Wei Y. Prognostic role of microRNA-210 in various carcinomas: a systematic review and meta-analysis. Dis Markers. 2014;2014:106197.
Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. Hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14(5):1340–8.
Gee HE, Camps C, Buffa FM, Patiar S, Winter SC, Betts G, Homer J, Corbridge R, Cox G, West CM, et al. Hsa-mir-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer. 2010;116(9):2148–58.
Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, Shimizu K, Tsujimoto G. MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem. 2011;286(1):420–8.
Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis. 2011;32(12):1881–9.
Greither T, Grochola LF, Udelnow A, Lautenschläger C, Würl P, Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2010;126(1):73–80.
Liu G, Shao C, Li A, Zhang X, Guo X, Li J. Diagnostic Value of Plasma miR-181b, miR-196a, and miR-210 Combination in Pancreatic Cancer. Gastroenterol Res Pract 2020, 2020:6073150.
Wu L, Zhou WB, Zhou J, Wei Y, Wang HM, Liu XD, Chen XC, Wang W, Ye L, Yao LC, et al. Circulating exosomal microRNAs as novel potential detection biomarkers in pancreatic cancer. Oncol Lett. 2020;20(2):1432–40.
Yu Q, Xu C, Yuan W, Wang C, Zhao P, Chen L, Ma J. Evaluation of plasma MicroRNAs as diagnostic and prognostic biomarkers in pancreatic adenocarcinoma: miR-196a and miR-210 could be negative and positive prognostic markers, respectively. Biomed Res Int. 2017;2017:6495867.
Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–41.
Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014;86(2):433–44.
Mizuta K, Awazu S, Yasuda T, Kishi K. Purification and characterization of three ribonucleases from human kidney: comparison with urine ribonucleases. Arch Biochem Biophys. 1990;281(1):144–51.
Spencer JD, Schwaderer AL, Dirosario JD, McHugh KM, McGillivary G, Justice SS, Carpenter AR, Baker PB, Harder J, Hains DS. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 2011;80(2):174–80.
Debernardi S, Blyuss O, Rycyk D, Srivastava K, Jeon CY, Cai H, Cai Q, Shu XO, Crnogorac-Jurcevic T. Urine biomarkers enable pancreatic cancer detection up to 2 years before diagnosis. Int J Cancer. 2023;152(4):769–80.
Perazzoli G, García-Valdeavero OM, Peña M, Prados J, Melguizo C, Jiménez-Luna C. Evaluating metabolite-based biomarkers for early diagnosis of pancreatic Cancer: a systematic review. Metabolites 2023, 13(7).
Terasawa H, Kinugasa H, Ako S, Hirai M, Matsushita H, Uchida D, Tomoda T, Matsumoto K, Horiguchi S, Kato H, et al. Utility of liquid biopsy using urine in patients with pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2019;20(10):1348–53.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: TI and SK (II) Administrative support: KN, JK, and EO (III) Provision of study materials or patients: YY, RM, HI, and TO (IV) Collection and assembly of data: TI, SK, KN, JK, TO, and HK (V) Data analysis and interpretation: TI, SK, and AS (VI) Manuscript writing: All authors (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine, and each individual provided signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Imamura, T., Komatsu, S., Nishibeppu, K. et al. Urinary microRNA-210-3p as a novel and non-invasive biomarker for the detection of pancreatic cancer, including intraductal papillary mucinous carcinoma. BMC Cancer 24, 907 (2024). https://doi.org/10.1186/s12885-024-12676-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12676-x