Abstract
Background
Lack of agreed terminology and definitions in healthcare compromises communication, patient safety, optimal management of adverse events, and research progress. The purpose of this scoping review was to understand the terminologies used to describe central venous access devices (CVADs), associated complications and reasons for premature removal in people undergoing cancer treatment. It also sought to identify the definitional sources for complications and premature removal reasons. The objective was to map language and descriptions used and to explore opportunities for standardisation.
Methods
A systematic search of MedLine, PubMed, Cochrane, CINAHL Complete and Embase databases was performed. Eligibility criteria included, but were not limited to, adult patients with cancer, and studies published between 2017 and 2022. Articles were screened and data extracted in Covidence. Data charting included study characteristics and detailed information on CVADs including terminologies and definitional sources for complications and premature removal reasons. Descriptive statistics, tables and bar graphs were used to summarise charted data.
Results
From a total of 2363 potentially eligible studies, 292 were included in the review. Most were observational studies (n = 174/60%). A total of 213 unique descriptors were used to refer to CVADs, with all reasons for premature CVAD removal defined in 84 (44%) of the 193 studies only, and complications defined in 56 (57%) of the 292 studies. Where available, definitions were author-derived and/or from national resources and/or other published studies.
Conclusion
Substantial variation in CVAD terminology and a lack of standard definitions for associated complications and premature removal reasons was identified. This scoping review demonstrates the need to standardise CVAD nomenclature to enhance communication between healthcare professionals as patients undergoing cancer treatment transition between acute and long-term care, to enhance patient safety and rigor of research protocols, and improve the capacity for data sharing.
Similar content being viewed by others
Background
Central venous access devices (CVADs) are critical for effective and efficient management of patients with malignancies because they facilitate urgent, acute or prolonged access to the bloodstream for the administration of prescribed and supportive therapies and repeated blood sampling [1]. However, they also present considerable risk of complications and many are removed prematurely before the end of prescribed therapy. Premature removal rates of up to 50% are reported in this patient cohort [1,2,3]. Complications can be related to the coagulopathic and inflammatory processes of the disease process [4], adverse effects of prescribed therapies including prolonged and profound immunosuppression [3], and adverse effects of supportive therapies such as blood products [1]. CVAD complications and premature removal may lead to delays in treatment, reduced treatment efficacy and subsequent survival due to interruptions in schedules [5], and increased morbidity from CVAD complications (e.g., infection, mortality and healthcare expenditure) [1].
Lack of standardised nomenclature in healthcare has been shown to negatively impact patient safety, patient experience and health system efficiency [6]. The lack of a common language impairs communication and interoperability between individuals and organisations [6]. The potential for complex systems such as electronic health records (EHR) to accurately capture clinical management of patients’ care and health outcomes [7] and to inform and support research is reliant on agreed nomenclature. This enables data sharing, robust data analysis, and meets the requirements of a learning health system [8]. An example of a common global language used in healthcare is the systematised nomenclature of medicine clinical terms (SNOMED CT). SNOMED CT is a comprehensive and precise medical terminology system that is coded and linked, facilitating homogenous data entry, encoding of existing data, mapping of free text, analysis of clinical data, and interoperability between systems and organisations [9].
To date, there is no consensus on CVAD terminology and no standardised definitions for CVAD associated complications and reasons for premature removal. This is imperative to advance the quality and safety of clinical assessment and management, and to drive robust, impactful research for patients undergoing cancer treatment. A scoping review fits well with reviews that map and synthesise available evidence about a given topic and identify gaps and similarities in the published literature [10]. The aim of this review was to understand the terminologies used to describe CVADs, associated complications and reasons for premature removal in people undergoing cancer treatment. It also sought to identify the definitional sources for complications and premature removal reasons. The objective was to map language and descriptions used and to explore opportunities for standardisation.
Methods
Protocol
An a priori protocol for this scoping review aligning with the five stages of Arksey and O’Malley’s scoping review framework, including identification of the research question and relevant studies, selection of studies, documentation of the data, and collating and summarising the results, was developed. Reporting was guided by the PRISMA Extension for Scoping Reviews, PRISMA-ScR [11].
Eligibility criteria
Adult patients with cancer over the age of 18 years and with any type of CVAD in situ, for example short-term centrally inserted central catheters (CICCs), or longer term CVADs, for example peripherally inserted central catheters (PICCs) or totally implantable venous access devices (TIVADs) were eligible for inclusion. In keeping with the broad aims of a scoping review, study designs included experimental, quasi-experimental, observational, systematic reviews, meta-analyses, quality improvement and surveys. Studies were limited to English and publications after the 2016 edition of the Infusion Therapy Standards of Practice [12].
Information sources
The search was executed in the MedLine, PubMed, Cochrane, CINAHL Complete and Embase databases for a comprehensive approach to the topic.
Search
Population, concept, and context
The search strategy was developed in collaboration with a medical librarian to address the question: how are reasons for premature removal and CVAD-related complications defined in the published literature? A second question was established in response to the diversity of CVAD terminologies noted during development of the search strategy: what CVAD terminology is evident in the published literature? The broader approach of a scoping review aligns with a less restrictive search strategy based on the population, concept and context (PCC) format compared to the precise research questions, and inclusion and exclusion criteria required for a systematic review [13]. The population for this review was broad, including all patients with haematological and solid tumours as this cohort requires insertion of a CVAD for the administration of prescribed therapies for treatment of their disease.
The concept in this scoping review included the various CVAD-related complications and reasons for premature removal. This was not restricted to the more commonly reported issues of infection and thrombosis and included subject headings and key terms for clinically relevant problems such as occlusion, catheter migration, skin impairment, CVAD damage or rupture, and accidental dislodgement. Categorical descriptors (e.g., equipment failure, device removal, accidental injuries, and death) were also included.
The context was patients with any type of CVAD in situ as the different CVAD types serve different functions according to the goals of treatment, and type and length of prescribed therapies. CVADs included CICCs, PICCs, tunnelled cuffed-centrally inserted central catheters, totally implantable venous access ports, and apheresis and haemodialysis catheters. Subject headings (e.g., central venous catheters or catheterization, central venous), descriptors (e.g., cuff, tunnelled, implanted), trade names commonly used in the literature (e.g., Hickman™ or Infusaport™) were included.
The search was established for the MEDLINE database (Table 1), then adapted for PUBMed – National Institutes of Health (NIH), EMBASE, CINAHL and the Cochrane Library.
Subject headings and key words were combined using Boolean operators AND/OR. The search limiters applied were publication dates before 2017, non-English language, and studies in animals (including mice, mouse, rat(s), porcine, pig(s), sheep, murine, canine or rabbit) or in vitro. Excluded study designs were qualitative studies, study protocols and study reports with limited information including conference abstracts, letters to the editor, educational, posters and case studies.
Selection of sources of evidence
The search was executed in May 2022. Studies were collated and screened for duplicates in EndNote X9 by one reviewer (KC). Eligible studies were imported into Covidence, a web-based platform that streamlines the process of systematic and other literature reviews [14], during which a further 125 duplicate records were excluded (total of 5230 duplicate studies). Paired independent review of 100% of studies at title and abstract was undertaken (KC, ET), as well as at full text level (KC, ET), reasons for exclusions were noted, and the eligible studies moved forward for data extraction.
Data charting process
Data were extracted in Covidence using an a priori template established for this review by one author (KC). Data included key study (i.e., year, title, authors, country where the study took place, study design, aims and objectives, and participant details including number and diagnoses) and device (i.e., CVAD terminologies and abbreviations, terminologies used to describe CVAD complications and definitional sources, and terminologies used to describe CVAD removal reasons and definitional sources) details. Form fields were primarily free text to accurately capture the nuances in terminologies and definitional sources for premature removals and complications.
The data charting process was undertaken independently by two authors for 20% of the studies (KC, ET). Any conflicts were discussed and resolved between the two reviewers. Level of agreement was high so individual data extraction was completed for the remainder of the studies (KC).
Synthesis of results
Study data were stratified according to whether only one or multiple reasons for premature removal, or only one or multiple complications were reported. Data from studies reporting complications that did not indicate whether the complication resulted in premature removal were reported separately.
Definitional sources for complications and removal reasons were categorised as follows: national resources or guidelines (e.g., Centers for Disease Control and Prevention-National Healthcare Safety Network (CDC-NHSN), Infectious Diseases Society of America (IDSA) guidelines), other published studies, author-derived, or a combination of the first three categories. Descriptive statistics, primarily counts and percentages, tables and bar graphs were used to summarise charted data.
Results
Selection of sources of evidence
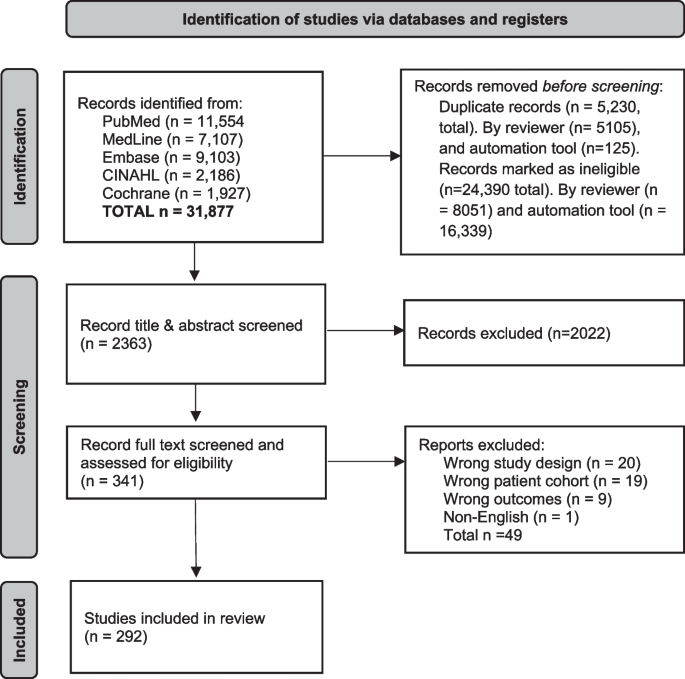
The search identified 31,877 records. After removing duplicates (n = 5230) and irrelevant studies (n = 24,390) in Endnote X9, 2363 study titles and abstracts, and then 341 full texts were screened for eligibility in Covidence. A total of 292 eligible studies were identified (Fig. 1).
Central venous access device nomenclature, and taxonomy of complications and reasons for premature removal in patients with cancer: a scoping review.
Characteristics of sources of evidence
Characteristics of the included studies are detailed in Supplement Information, Additional files 3 due to the volume of studies summarised. Of the 292 studies in this review, 193 (66%) reported on premature removal related to complications ( [2, 3, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205]. The remainder (n = 99/34%) reported on complications only [206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304] Characteristics are summarised using counts and percentages.
Synthesis of results
Samples included patients with solid tumours only (n = 93), haematological malignancies and solid tumours (n = 92), and haematological malignancies only (n = 56). The remainder were described as cancer patients (n = 51). Studies were conducted in China, (n = 61), the United States of America (USA) (n = 41), Italy (n = 25), Japan and Korea (both n = 15), and Australia, Germany and Turkey (all n = 13). Twelve were multinational. According to the Joanna Briggs Institute’s levels of evidence [13], most studies were level 4 observational, descriptive studies (n = 174). The remainder were level 3 observational, analytical designs (n = 61), level 2 quasi-experimental designs (n = 31), level 1 experimental designs (n = 24) and level 5 expert opinion, bench research (n = 2).
CVAD terminologies
A total of 213 unique descriptors were extracted from the included studies: 14 unique terms for CVADs, 104 for totally implantable venous access ports, 25 for peripherally inserted central catheters, 41 for tunnelled cuffed centrally inserted central catheters, 27 for centrally inserted central catheters, and two for femorally inserted central catheters. This did not include spelling variations, hyphenation, or use of capitals, or the use of multiple different terms for the device in the same study. The greatest variation was related to the descriptive nature of the names. For example, for totally implantable venous access ports the descriptors included combinations of totally or fully, subcutaneously or tunnelled, implanted or implantable; chest, arm, subclavian, internal jugular, brachial, groin or centrally inserted; devices, catheters, ports or systems; central venous, vascular or venous access; single or dual chamber; chemotherapy or infusion; traditional or power-injectable; PICC, peripherally inserted or peripheral central ports; variations on port, portacath, portacath and the various trade names.
Premature CVAD removal related to complications
Of the 193 studies that reported on premature removals, 128 (66%) identified multiple types of complications including catheter occlusion, malposition, dislodgement, fracture, local bleeding, infection, or skin necrosis. The remainder (n = 65, 34%) identified one complication only, most commonly infection (n = 18) or thrombosis (n = 14).
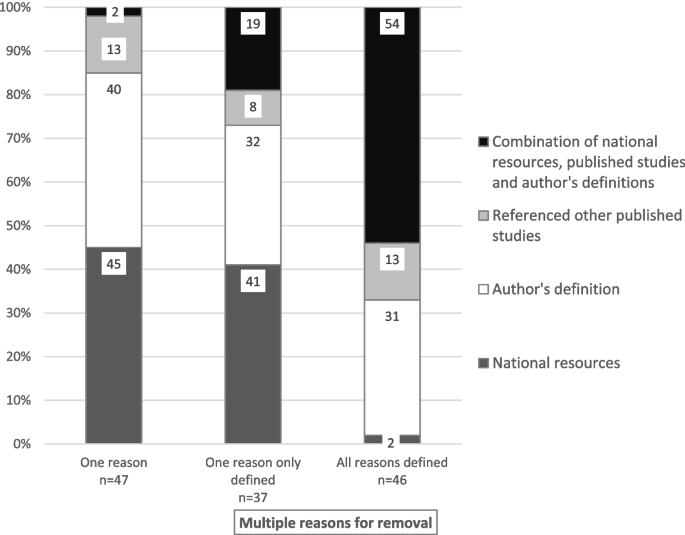
In studies reporting on multiple reasons for premature removal, definitional sources were not provided in 45 (35%) studies, for one reason only in 37 (29%) studies, and for all reasons in 46 (36%) studies. In studies that reported one premature removal reason only, the definition was provided in 47 (72%) studies, and not provided in 18 (28%) studies. The definitional sources in these studies included local national resources or guidelines in 21 (45%) studies, author-derived definitions in 19 (40%), definitions from other published studies in six (13%) and a combination of these sources in one (2%) study. The definitional sources in studies with multiple reasons for removal included a combination of national guidelines or resources, definitions from other published studies or author-derived definitions (Fig. 2).
CVAD complications
Of the 99 studies that reported CVAD-related complications, 49 (49%) reported one complication and 50 (51%) reported on multiple complications. Complication definitions were provided in 36 (73%) studies reporting one complication, and no definitions provided in 13 (27%). For studies that reported on multiple complications, all complications were defined in 20 (40%) studies, only one and not all complications in 14 (28%) studies, and no complication definitions were provided in 16 (32%) studies.
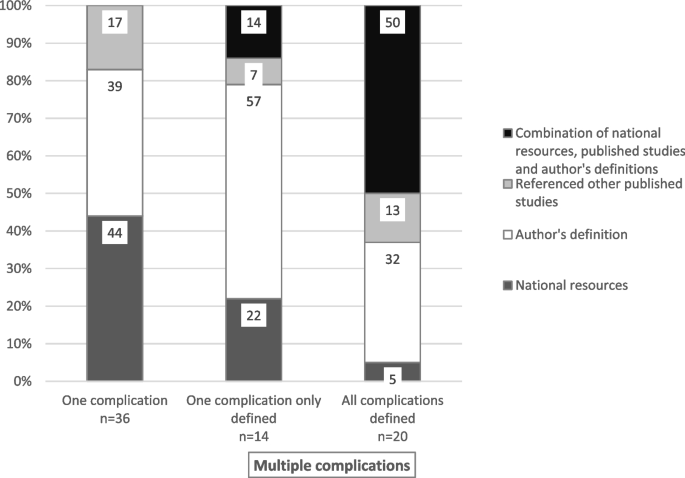
Definitional sources in studies that reported one type of complication were from national resources or guidelines in 16 (44%) studies (e.g., CDC-NHSN or IDSA), author-derived in 14 (39%), and from other published studies in six (17%) studies (Fig. 3). Comparatively, of the studies that reported on multiple complications, fewer referenced national resources (n = 2, 10%); more were author-derived (n = 10, 50%) or used a combination of sources (n = 8, 40%) when all complications were defined. Definitional sources were from national resources in three [21] studies, author-derived in eight (57%) studies, other published studies in one (7%) and a combination of sources in two (14%) studies that defined only one of the multiple complications.
Discussion
This review identified considerable variation in CVAD terminology related to reason for removal and the actual device itself. This included over 200 unique names for the different types of CVADs, with the greatest variation evident for totally implantable venous access devices or ports with over 100 unique names. In addition to inconsistency with definitions and device terminology between studies, inconsistencies were also observed within the same study, underscoring the complexity and confusion in this clinical issue.
Terminologies were used interchangeably such as central venous catheters (CVC) and central venous access devices. CVC was also used to describe the multi-lumen catheter most commonly used in critical care units. Despite the term central venous catheter being used more frequently as the term to describe all types of devices, it does not accurately describe or reflect the wide variety of implanted, cuffed or tunnelled catheters and devices, or contemporary innovations in insertion techniques; for example, tunnelling PICCs. The term central venous access device is more inclusive, intuitive, and reflective of the diversity in contemporary clinical practice [305].
Similar findings have previously been reported in other research. In a Delphi consensus study about a minimum dataset for vascular access, no standardised CVADs terms were identified [306]. The authors advocated for development of a vascular access minimum dataset to overcome lack of clarity in the literature that hampers robust data collection, analysis and interoperability within and across countries, ultimately adversely affecting patient outcomes [6, 306]. In response to their findings, Schults et al. (2020) subsequently developed a common set of descriptors (nomenclature) for commonly used vascular access devices [306]. However, these descriptors did not include CVADs commonly used in cancer care (e.g., tunnelled cuffed centrally inserted central catheters, apheresis catheters), and contemporary insertion techniques (e.g. tunnelled peripherally inserted central catheters). A more comprehensive set of descriptors need to be developed to represent CVADs used in cancer care.
Considerable variation in CVAD nomenclature evident in this review is problematic. A lack of standardised nomenclature impairs communication and interoperability between healthcare professionals and organisations locally and globally, and fractures data sharing, linkage, analysis and the evidence base from clinical practice [6, 306]. The World Health Organization states that standardised nomenclature is essential for recording and surveillance of all types of medical devices including CVADs [307], and in the systematic review of 20 papers by Gildow and Lazar (2022), standardised nomenclature was shown to be associated with reduced clinical errors and patient injury, improved communication and opportunity for standardisation of clinical care [308].
Most studies reported multiple reasons for premature device removal as opposed to a single reason for removal. Research investigating multiple reasons for removal reflects the increasing complexity of care and treatment for people with cancer, the majority of whom require CVAD support. The multiplicity of treatment and supporting therapies that commonly characterise care for a person with cancer, compounded by patient, clinician, therapy, and workplace related factors, come together to compound risk of premature CVAD removal. The interplay between one or more of these factors increases the risk of premature removal increasing morbidity and mortality, and cost of care [4, 309,310,311].
The only consistently defined premature removal reason was infection. Nearly all studies cited national sources for catheter-related blood stream infection (CRBSI) or the surveillance definition for central line-associated blood stream infection (CLABSI), with the majority citing CDC [312] or IDSA [313] from the USA. There was no consistency in definitions for any other reason for premature removal. This is an important finding with overt implications for quality and safety of care. Heterogeneity of terminology and definitions impair standardised clinical management by causing confusion and permitting an inconsistent approach for the different members of the healthcare team and clinical specialties, and consequently negatively impacts quality and safety of patients [314]. Standardised nomenclature, clinical procedures and standardisation of care have been shown to reduce errors and patient injury by improving communication and dissemination of evidence to inform clinical practices [308].
The infinite potential for utilising routinely collected patient management data and outcomes captured in EHR systems for clinical research into improving patient care and outcomes [315] cannot be realised when such variation exists. Consistency in EHR data is key to the efficient and effective collation and linkage of data required for the development of a reliable big data set [308, 315]. Clinical data, expertise and knowledge integrated with current evidence are the cornerstones of a learning health system which aims to provide informed, safer, higher quality clinical care [8]. Also, consistent data and definitions are required for meta-analyses in quantitative research [218].
Standardised nomenclature in healthcare is complex requiring a multifaceted response. Strategies require collaboration, consensus, communication, and implementation by multidisciplinary professionals including clinicians, health economists, and health service researchers, strategists, and implementation science professionals. This includes commitment by journals, national peak bodies and associations to use the standardised nomenclature as consistency at a system level is required to provide the guidance for the end users. Furthermore, regular review of nomenclature is required so it accurately reflects contemporary evidence in the literature, clinical practice, emerging technology and products.
As EHRs become increasingly prevalent across health services, they offer opportunity for standardisation of clinical nomenclature. For example, different standardised global clinical languages such as SNOMED CT or International Classification of Diseases 10th Revision are translatable and already have equivalent codes for use in EHRs. Leveraging the opportunity of EHRs will require close collaboration between EHR development teams and all end users of the EHR systems.
Limitations
There are a number of limitations of this scoping review. Limiting the patient cohort to patients with cancer may restrict the applicability to other patient cohorts. However, this was considered to have minimal impact as CVADs are used across multiple patient cohorts. The date range was five years after the 2016 edition of the Infusion Therapy Standards of Practice [12], so all descriptors and definitions may not be captured; however, it reflects contemporary practice, policy and research. The volume of studies did not allow for analysis beyond the absolute numbers of the different types of CVADs and categories of resources for definitions of CVAD complications and reasons for removal. Establishing consistent definitions for each type of premature removal or complication was not possible. The exclusion of non-English studies is important to acknowledge as a limitation when considering the results and findings of this review.
Conclusions
Standardised CVAD nomenclature and definitions for premature CVAD removal and complications do not exist. This impacts effective and accurate communication and has been shown to hamper safe, effective cancer care. It also prevents interoperability between individuals and organisations globally to inform research to reduce the incidence and impact of CVAD complications and premature removal on cancer and patients’ experience of care, health outcomes and health system costs. Collaboration, consensus, and standardisation is required to deliver quality CVAD care.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Zakhour R, Chaftari AM, Raad II. Catheter-related infections in patients with haematological malignancies: novel preventive and therapeutic strategies. Lancet Infect Dis. 2016;16(11):e241–50.
Moss JG, Wu O, Bodenham AR, Agarwal R, Menne TF, Jones BL, et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): a randomised controlled trial. Lancet. 2021;398(10298):403–15.
Mariggiò E, Iori AP, Micozzi A, Chistolini A, Latagliata R, Berneschi P, et al. Peripherally inserted central catheters in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer. 2020;28(9):4193–9.
Levi M, Sivapalaratnam S. An overview of thrombotic complications of old and new anticancer drugs. Thromb Res. 2020;191 Suppl 1:S17–21.
van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918–27.
World Health Organisation. Health products policy and standards. Nomenclature of medical devices. 2021. Retrieved from: https://www.who.int/teams/health-product-policy-and-standards/assistive-and-medical-technology/medical-devices/nomenclature. Accessed 15 Dec 2022.
Cornet R, de Keizer N. Forty years of SNOMED: a literature review. BMC Med Inf Decis Mak. 2008;8 Suppl 1(Suppl 1):S2.
Agency for Healthcare Research and Quality. About learning health systems Rockville, MD. 2019. Available from: https://www.ahrq.gov/learning-health-systems/about.html.
Gaudet-Blavignac C, Foufi V, Bjelogrlic M, Lovis C. Use of the systematized nomenclature of medicine clinical terms (SNOMED CT) for processing free text in health care: systematic scoping review. J Med Internet Res. 2021;23(1):e24594.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–473.
Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy: standards of practice. J Infus Nurs. 2016;39(1S):S1–159.
The Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2015 edition / supplement2015. Available from: https://reben.com.br/revista/wp-content/uploads/2020/10/Scoping.pdf.
Covidence. Veritas health innovation. 2023. Available from: https://www.covidence.org.
Aghamohammadi D, Fakhari S, Ataei Y, Bilehjani E, Jafari M. Totally implantable venous access port infection in northwest of Iran. Crescent J Med Biol Sci. 2017;4(3):126–30.
Anbar R, Avci D, Cetinkaya A. Port catheter complications and thrombosis issues: assessment of 114 patients with port catheter implantation by single surgeon. Biomedical Res Therapy. 2017;4(12):1898–910.
Aribas BK, Tiken R, Aribas O, Uylar T, Akdulum I, Turker I, et al. Factors on patency periods of subcutaneous central venous Port: long-term results of 1,408 patients. Iran J Radiol. 2017;14(2):1.
Bai X, Gu X, Cheng L, Yuan Q, Jing J, Jin Y, et al. Clinical diagnosis and treatment of peripherally inserted central catheter related upper extremity deep venous thrombosis. Biomedical Res (India). 2017;28(22):9707–11.
Busch JD, Vens M, Herrmann J, Adam G, Ittrich H. Material failure of silicone catheter lines: a retrospective review of partial and complete ruptures in 553 patients. AJR Am J Roentgenol. 2017;208(2):464–9.
Chaftari P, Chaftari AM, Adachi J, Hachem R, Raad S, Natividad E, et al. Improvement in the diagnosis of catheter-related bloodstream infections in a tertiary cancer center. Am J Infect Control. 2017;45(3):e34–39.
Chan RJ, Northfield S, Larsen E, Mihala G, Ullman A, Hancock P, et al. Central venous access device securement and dressing effectiveness for peripherally inserted central catheters in adult acute hospital patients (CASCADE): a pilot randomised controlled trial. Trials. 2017;18(1):458.
Chang DH, Mammadov K, Hickethier T, Borggrefe J, Hellmich M, Maintz D, et al. Fibrin sheaths in central venous port catheters: treatment with low-dose, single injection of urokinase on an outpatient basis. Ther Clin Risk Manag. 2017;13:111–5.
Chen MH, Hwang WL, Chang KH, Chiang LCJ, Teng CLJ. Application of peripherally inserted central catheter in acute myeloid leukaemia patients undergoing induction chemotherapy. Eur J Cancer Care. 2017;26(6):e12627.
Cornillon J, Martignoles JA, Tavernier-Tardy E, Gire M, Martinez P, Tranchan C, et al. Prospective evaluation of systematic use of peripherally inserted central catheters (PICC lines) for the home care after allogeneic hematopoietic stem cells transplantation. Support Care Cancer. 2017;25(9):2843–7.
Diaz JA, Rai SN, Wu X, Chao JH, Dias AL, Kloecker GH. Phase II trial on extending the maintenance flushing interval of Implanted ports. J Oncol Pract. 2017;13(1):e22–28.
Fang S, Jiang Y, Yang J, Song L, Liu Y. Comparison of three types of central venous catheters in patients with malignant tumor receiving chemotherapy. Patient Prefer Adherence. 2017;11:1197–204.
Grau D, Clarivet B, Lotthe A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;6:18.
Hashimoto Y, Fukuta T, Maruyama J, Omura H, Tanaka T. Experience of peripherally inserted central venous catheter in patients with hematologic diseases. Intern Med (Tokyo Japan). 2017;56(4):389–93.
Hyo-Cheol K, Saebeom H, Hoyong J. Malfunction of totally implantable central venous ports. Iran J Radiol. 2017;14(1):1.
Kakkos A, Bresson L, Hudry D, Cousin S, Lervat C, Bogart E, et al. Complication-related removal of totally implantable venous access port systems: does the interval between placement and first use and the neutropenia-inducing potential of chemotherapy regimens influence their incidence? A four-year prospective study of 4045 patients. Eur J Surg Oncol. 2017;43(4):689–95.
Kang J, Chen W, Sun W, Ge R, Li H, Ma E, et al. Peripherally inserted central catheter-related complications in cancer patients: a prospective study of over 50,000 catheter days. J Vasc Access. 2017;18(2):153–7.
Kang JR, Long LH, Yan SW, Wei WW, Jun HZ, Chen W. Peripherally inserted central catheter-related vein thrombosis in patients with lung cancer. Clin Appl Thromb Hemost. 2017;23(2):181–6.
Kao PF, Weng JH, Tyan YS, Yang SF, Tsao TC. The incidence of totally implantable venous access devices insertion and the associated abnormalities in patients with cancer revealed in (18)F-FDG PET-CT imaging. Acad Radiol. 2017;24(12):1588–95.
Liscynesky C, Johnston J, Haydocy KE, Stevenson KB. Prospective evaluation of peripherally inserted central catheter complications in both inpatient and outpatient settings. Am J Infect Control. 2017;45(9):1046–9.
Lo Priore E, Fliedner M, Heverhagen JT, Novak U, Marschall J. The role of a surveillance programme for intro-ducing peripherally inserted central catheters: a 2-year observational study in an academic hospital. Swiss Med Wkly. 2017;147:w14441.
Longo R, Llorens M, Goetz C, Platini C, Eid N, Sellies J, et al. Taurolidine/citrate lock therapy for primary prevention of catheter-related infections in cancer patients: results of a prospective, randomized, phase IV trial (ATAPAC). Oncology. 2017;93(2):99–105.
Luong NV, Kroll MH, Vu K. Recurrence of venous thromboembolism among adults acute leukemia patients treated at the University of Texas MD Anderson cancer center: incidence and risk factors. Thromb Res. 2017;156:14–9.
Nakamura T, Sato T, Watanabe M, Sasaki J, Asari Y, Torii S. Complications after implantation of subcutaneous central venous ports (PowerPort). Annals Med Surg. 2017;17:1.
Paquet F, Boucher LM, Valenti D, Lindsay R. Impact of arm selection on the incidence of PICC complications: results of a randomized controlled trial. J. 2017;18(5):408–14.
Rickard CM, Marsh NM, Webster J, Gavin NC, Chan RJ, McCarthy AL, et al. Peripherally InSerted CEntral catheter dressing and securement in patients with cancer: the PISCES trial. Protocol for a 2 × 2 factorial, superiority randomised controlled trial. BMJ Open. 2017;7(6):e015291.
Seo TS, Song MG, Kim JS, Choi CW, Seo JH, Oh SC, et al. Long-term clinical outcomes of the single-incision technique for implantation of implantable venous access ports via the axillary vein. J Vasc Access. 2017;18(4):345–51.
Solinas G, Platini F, Trivellato M, Rigo C, Alabiso O, Galetto AS. Port in oncology practice: 3-monthly locking with normal saline for catheter maintenance, a preliminary report. J Vasc Access. 2017;18(4):325–7.
Tabatabaie O, Kasumova GG, Eskander MF, Critchlow JF, Tawa NE, Tseng JF. Totally implantable venous access devices: a review of complications and management strategies. Am J Clin Oncol. 2017;40(1):94–105.
Verboom MC, Ouwerkerk J, Gelderblom H, Steeghs N, Kerst JM, Lutjeboer J, et al. Central venous access related adverse events after trabectedin infusions in soft tissue sarcoma patients// experience and management in a nationwide multi-center study. Clin Sarcoma Res. 2017;7(1):2.
Wang YC, Lin PL, Chou WH, Lin CP, Huang CH. Long-term outcomes of totally implantable venous access devices. Support Care Cancer. 2017;25(7):2049–54.
Webster J, Larsen E, Marsh N, Choudhury A, Harris P, Rickard CM. Chlorhexidine gluconate or polyhexamethylene biguanide disc dressing to reduce the incidence of central-line-associated bloodstream infection: a feasibility randomized controlled trial (the CLABSI trial). J Hosp Infect. 2017;Date of Publication: January 29.
Xie J, Xu L, Xu X, Huang Y. Complications of peripherally inserted central catheters in advanced cancer patients undergoing combined radiotherapy and chemotherapy. J Clin Nurs. 2017;26(23–24):4726–33.
Zerla PA, Canelli A, Cerne L, Caravella G, Gilardini A, De Luca G, et al. Evaluating safety, efficacy, and cost-effectiveness of PICC securement by subcutaneously anchored stabilization device. J Vasc Access. 2017;18(3):238–42.
Zhou H, Yang B, Wang C, Qin Y. Analysis and clinical significance of venography findings in complications associated with peripherally inserted central catheters. Biomedical Res (India). 2017;28(15):6619–25.
Alfonso Alvarez-Rodriguez J, Garcia-Suarez M, Fernandez-Garcia D, Mendez-Martinez C, Gomez-Salgado J. Analysis of peripheral central venous access ports at the forearm: an observational study. Eur J Cancer Care. 2018;27(6):e12929.
Alkindi SY, Chai-Adisaksopha C, Cheah M, Linkins L-A. Management of cancer-associated upper extremity deep vein thrombosis with and without venous catheters at a tertiary care center. Thromb Res. 2018;166:92–5.
Bouzidi H, Emirian A, Marty A, Chachaty E, Laplanche A, Gachot B, et al. Differential time to positivity of central and peripheral blood cultures is inaccurate for the diagnosis of Staphylococcus aureus long-term catheter-related sepsis. J Hosp Infect. 2018;99(2):192–9.
Burbridge B, Plewes C, Stoneham G, Szkup P, Otani R, Babyn P, et al. Randomized clinical trial evaluating complications and complication-related removal of arm-situated power-injectable and non-power-injectable totally implanted venous access devices among cancer patients. J Vasc Interv Radiol. 2018;29(5):648–656.e3.
Chaftari AM, Hachem R, Raad S, Jiang Y, Natividad E, Chaftari P, et al. Unnecessary removal of central venous catheters in Cancer patients with bloodstream infections. Infect Control Hosp Epidemiol. 2018;39(2):222–5.
Chopra V, Kaatz S, Grant P, Swaminathan L, Boldenow T, Conlon A, et al. Risk of venous thromboembolism following peripherally inserted central catheter exchange: an analysis of 23,000 hospitalized patients. Am J Med. 2018;131(6):651–60.
Davies GA, Lazo-Langner A, Gandara E, Rodger M, Tagalakis V, Louzada M, et al. A prospective study of Rivaroxaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients (catheter 2). Thromb Res. 2018;162:88–92.
Decousus H, Bourmaud A, Fournel P, Bertoletti L, Labruyère C, Presles E, et al. Cancer-associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood. 2018;132(7):707–16.
El-Balat A, Schmeil I, Karn T, Holtrich U, Mavrova-Risteska L, Rody A, et al. Catheter-related complications of subcutaneous implantable venous access devices in breast cancer patients. Vivo. 2018;32(5):1275–81.
Htun KT, Ma MJY, Lee AYY. Incidence and outcomes of catheter related thrombosis (CRT) in patients with acute leukemia using a platelet-adjusted low molecular weight heparin regimen. J Thromb Thrombolysis. 2018;46(3):386–92.
Imaoka Y, Kuranishi F, Ogawa Y. Usefulness of totally implantable central venous access devices in elderly patients: a retrospective study. Ann Nutr Metab. 2018;72(2):112–6.
Kato Y, Hagihara M, Kurumiya A, Takahashi T, Sakata M, Shibata Y, et al. Impact of mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) on central line-associated bloodstream infections (CLABSIs) in department of hematology at single university hospital in Japan. J Infect Chemotherapy: Official J Japan Soc Chemother. 2018;24(1):31–5.
Kiesow L, Mahnken AH, Keulers AR. Port Implantation in patients with severe thrombocytopenia is safe with interventional radiology. Cardiovasc Interv Radiol. 2018;41(1):80–6.
Lee YM, Lee MS, Park KH, Moon C, Kim YJ, Lee HJ. Clinical impact of delayed catheter removal for patients with central-venous-catheter-related Gram-negative bacteraemia. J Hosp Infect. 2018;99(1):106–13.
Liu K, Zhou Y, Xie W, Chen X, Wang H, Gu Z, et al. Handgrip exercise reduces peripherally-inserted central catheter-related venous thrombosis in patients with solid cancers: a randomized controlled trial. Int J Nurs Stud. 2018;86:99–106.
Madabhavi I, Patel A, Anand A, Kataria P, Kadakol N, Sarkar M. Use of tunneled-cuffed central catheters in patients with cancer: a single-center experience. JAVA - J Association Vascular Access. 2018;23(1):23–9.
Madabhavi I, Patel A, Anand A, Sarkar M, Kataria P, Kadakol N. A study of the use of peripherally inserted central catheters in cancer patients: a single-center experience. J Vascular Nursing: Official Publication Soc Peripheral Vascular Nurs. 2018;36(3):149–56.
Nucci M, Braga PR, Nouer SA, Anaissie E. Time of catheter removal in candidemia and mortality. Brazilian J Infect Diseases. 2018;22(6):455–61.
Ohtake S, Nakagawa M, Uchino Y, Miura K, Iriyama N, Hatta Y, et al. 1% chlorhexidine-alcohol for preventing central venous catheter-related infection during intensive chemotherapy for patients with haematologic malignancies. J Infect Chemother. 2018;24(7):544–8.
Raad S, Chaftari AM, Hachem RY, Shah P, Natividad E, Cleeland CS, et al. Removal and insertion of central venous catheters in cancer patients is associated with high symptom burden. Expert Rev Med Dev. 2018;15(8):591–6.
Samuelson C, Kaur H, Kritsotakis EI, Goode SD, Nield A, Partridge D. A daily topical decontamination regimen reduces catheter-related bloodstream infections in haematology patients. J Infect. 2018;76(2):132–9.
Tippit D, Ananthula A, Siegel E, Ochoa D, Hill E, Merrill A, et al. Upper-extremity deep vein thrombosis in patients with breast cancer with chest versus arm central venous port catheters. Breast Cancer. 2018;12:1178223418771909.
Vermeulin T, Lucas M, Marini H, Di Fiore F, Loeb A, Lottin M, et al. Totally implanted venous access-associated adverse events in oncology: results from a prospective 1-year surveillance programme. Bull Cancer. 2018;105(11):1003–11.
Voog E, Bourgeois H, Domont J, Denis F, Emmanuel E, Dupuis O, et al. Totally implantable venous access ports: a prospective long-term study of early and late complications in adult patients with cancer. Support Care Cancer. 2018;26(1):81–9.
Yang S-S, Ahn MS. A comparison between upper arm and chest for optimal site of totally implanted venous access ports in patients with female breast cancer. Ann Vasc Surg. 2018;50:128–34.
Yanik F, Karamustafaoglu YA, Karatas A, Yoruk Y. Experience in totally implantable venous port catheter: analysis of 3,000 patients in 12 years. Turk gogus kalp damar cerrahisi dergisi. 2018;26(3):422–8.
Zhang S, Kobayashi K, Faridnia M, Skummer P, Zhang D, Karmel MI. Clinical predictors of port infections in adult patients with hematologic malignancies. J Vascular Interventional Radiology: JVIR. 2018;29(8):1148–55.
Ahmad A, Hjerming M, Kjeldsen L, Bjerrum OW, Moser C, Classen V, et al. Hydrochloric acid prolongs the lifetime of central venous catheters in haematologic patients with bacteraemia. Dan Med J. 2019;66(5):A5544.
Ammar G, Almashaikh E, Ibdah A, Shajrawi W, Awawdeh S, Al Mousa A, et al. Impact of early dressing removal on tunneled central venous catheters: a piloting study. Asian Pac J Cancer Prev. 2019;20(9):2693–7.
Campagna S, Berchialla P, Gonella S, Dimonte V, Mussa B, Morano G, et al. Can peripherally inserted central catheters be safely placed in patients with Cancer receiving chemotherapy? A retrospective study of almost 400,000 catheter-days. Oncologist. 2019;24(9):e953–959.
Campagna S, Gonella S, Berchialla P, Rigo C, Morano G, Zerla PA, et al. A retrospective study of the safety of over 100,000 peripherally-inserted central catheters days for parenteral supportive treatments. Res Nurs Health. 2019;42(3):198–204.
Chou PL, Fu JY, Cheng CH, Chu Y, Wu CF, Ko PJ, et al. Current port maintenance strategies are insufficient: view based on actual presentations of implanted ports. Med (Baltim). 2019;98(44):e17757.
da Costa ACC, Ribeiro JM, Vasques CI, De Luca Canto G, Porporatti AL, Dos Reis PED. Interventions to obstructive long-term central venous catheter in cancer patients: a meta-analysis. Supportive care cancer: Official J Multinational Association Supportive Care Cancer. 2019;27(2):407–21.
Fares J, Khalil M, Chaftari AM, Hachem R, Jiang Y, Kantarjian HM, et al. Impact of catheter management on clinical outcome in adult cancer patients with Gram-negative bacteremia. Open Forum Infect Dis. 2019;6(10):ofz357.
Gray KL, Benson HL, Pearce CL, Steidley IG, Bachman AM, Adamski J. Implementation and 2-year outcomes of the first FDA-approved implantable apheresis vascular access device. Transfusion. 2019;59(11):3461–7.
Harrold K, Martin A, Bhuva N. A prospective audit evaluating use of urokinase in oncology patients with occluded central venous access devices. Br J Nurs. 2019;28(19):S30–36.
Hong S, Seo TS, Song MG, Seol HY, Suh SI, Ryoo IS. Clinical outcomes of totally implantable venous access port placement via the axillary vein in patients with head and neck malignancy. J Vasc Access. 2019;20(2):134–9.
Kim IJ, Shim DJ, Byeon JH, Lee JH, Kim ET, Lee HJ, et al. Impact of subcutaneous tunnels on peripherally inserted catheter placement: a multicenter retrospective study. Eur Radiol. 2019;29(5):2716–23.
Li G, Zhang Y, Ma H, Zheng J. Arm port vs chest port: a systematic review and meta-analysis. Cancer Manage Res. 2019;11:6099–112.
Nezami N, Groenwald M, Silin D, Latich I, Xing M, Kokabi N. Risk factors of infection and role of antibiotic prophylaxis in totally implantable venous access port placement: propensity score matching. Cardiovasc Interv Radiol. 2019;42(9):1302–10.
Picardi M, Della Pepa R, Cerchione C, Pugliese N, Mortaruolo C, Trastulli F, et al. A frontline approach with peripherally inserted versus centrally inserted central venous catheters for remission induction chemotherapy phase of Acute myeloid leukemia: a randomized comparison. Clin Lymphoma Myeloma Leuk. 2019;19(4):e184–194.
Ruiz-Giardin JM, Ochoa Chamorro I, Velazquez Rios L, Jaqueti Aroca J, Garcia Arata MI, SanMartin Lopez JV, et al. Blood stream infections associated with central and peripheral venous catheters. BMC Infect Dis. 2019;19(1):841.
Seckold T, Walker S, Dwyer T, Signal T. Peripherally inserted central catheter postinsertion complications: a retrospective study. J Association Vascular Access. 2019;24(1):Oct–20.
Suleman A, Jarvis V, Hadziomerovic A, Carrier M, McDiarmid S. Implanted vascular access device related deep vein thrombosis in oncology patients: a prospective cohort study. Thromb Res. 2019;177:117–21.
Balsorano P, Romagnoli S, Pinelli F, Virgili G, Villa G, De Gaudio AR, et al. Peripherally inserted central catheter-related thrombosis rate in modern vascular access era-when insertion technique matters: a systematic review and meta-analysis. J Vasc Access. 2020;21(1):45–54.
Bertoglio S, Cafiero F, Meszaros P, Varaldo E, Blondeaux E, Molinelli C, et al. PICC-PORT totally implantable vascular access device in breast cancer patients undergoing chemotherapy. J Vasc Access. 2020;21(4):460–6.
Calò F, Retamar P, Martínez Pérez-Crespo PM, Lanz-García J, Sousa A, Goikoetxea J, et al. Catheter-related bloodstream infections: predictive factors for Gram-negative bacteria aetiology and 30 day mortality in a multicentre prospective cohort. J Antimicrob Chemother. 2020;75(10):3056–61.
Carvalho Castanho LE, Nogueira dos Santos B, Salles Margatho A, Merizio Martins Braga FT, Diniz PE, de Oliveira MC, et al. Chlorhexidine gel dressing in hematopoietic stem cell transplantation. Acta Paulista de Enfermagem. 2020;33(3):1.
Chen Y, Chen H, Yang J, Jin W, Fu D, Liu M, et al. Patterns and risk factors of peripherally inserted central venous catheter-related symptomatic thrombosis events in patients with malignant tumors receiving chemotherapy. J Vascular Surg Venous Lymphatic Disorders. 2020;8(6):919–29.
Choksi A, Finnegan K, Etezadi V. Does systemic antibiotic prophylaxis prior to the placement of totally implantable venous access devices reduce early infection? A retrospective study of 1,485 cases at a large academic institution. Am J Infect Control. 2020;48(1):95–9.
Clatot F, Fontanilles M, Lefebvre L, Lequesne J, Veyret C, Alexandru C, et al. Randomized phase II trial evaluating the safety of peripherally inserted central catheters vs implanted port catheters during adjuvant chemotherapy in early breast cancer patients. Ann Oncol. 2019;30:v739.
Dai C, Li J, Li QM, Guo X, Fan YY, Qin HY. Effect of tunneled and nontunneled peripherally inserted central catheter placement: a randomized controlled trial. J Vasc Access. 2020;21(4):511–519.
de Silveira CP, Braga RC, Galvao FTMM, dos Reis CM, Ferreira PED, Clark EB. AM. Dressings for the central venous catheter to prevent infection in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Support Care in Cancer. 2020;28(2):425–438.
de la Cruz-Hernández I, Cornejo-Juárez P, Tellez-Miranda O, Barrera-Pérez L, Sandoval-Hernández S, Vilar-Compte D, et al. Microbiology and prevalence of E2SKAPE-resistant strains in catheter-related bloodstream infections in patients with cancer. Am J Infect Control. 2020;48(1):40–5.
de Mooij CEM, van der Velden WJFM, van Groningen LFJ, Blijlevens NMA, Verweij PE, Meijer C, et al. Surveillance of catheter-related bloodstream infections in haemato-oncology patients: comparison of two definitions. J Hosp Infect. 2020;105(4):686–90.
Gudiol C, Arnan M, Aguilar-Guisado M, Royo-Cebrecos C, Sanchez-Orteg I, Montero I, et al. A randomized, double-blind, placebo-controlled trial (TAURCAT study) of citrate lock solution for prevention of endoluminal central venous catheter infection in neutropenic hematological patients. Antimicrob Agents Chemother. 2020;64(2):10.
Haggstrom L, Parmar G, Brungs D. Central venous catheter thrombosis in cancer: a multi-centre retrospective study investigating risk factors and contemporary trends in management. Clin Med Insights Oncol. 2020;14:1.17955E + 15.
Heidenreich D, Hansen E, Kreil S, Nolte F, Jawhar M, Hecht de Gutierrez A, et al. Influence of the insertion site on central venous catheter-related complications in patients undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2020;26(6):1189–94.
Ince ME, Ozkan G, Ors N, Yildirim AK, Doganci S. Complications and pitfalls of central venous port catheters: experience with 782 patients with cancer. Ir J Med Sci. 2020;189(4):1371–7.
Inoue S, Yoshida T, Nishino T, Goto M, Nishioka K, Fujimoto K, et al. Safe central venous catheters for esophageal cancer treatment. J Med Investig. 2020;67(34):298–303.
Jiang M, Cui XW, Li CL, Pan CQ, Dietrich CF. Risk of venous thromboembolism associated with totally implantable venous access ports in cancer patients: a systematic review and meta-analysis. J Thromb Haemost. 2020;18(9):2253–2273.
Kao CY, Cheng YC, Chen CCC, Chai JW, Fu CH, Chen JL, et al. Outcome analysis in 270 radiologically guided implantations of totally implantable venous access ports via basilic vein. J Chin Med Association. 2020;83(3):295–301.
Kikuchi M, Sato T, Okada S, Abe N, Sato A, Suzuki Y. Maintenance antisepsis in reducing the rate of late-onset central venous catheter-related bloodstream infection: a comparison of 0.05% and 1% chlorhexidine. J Infect Chemotherapy: Official J Japan Soc Chemother. 2020;26(2):188–93.
Lingegowda D, Gehani A, Sen S, Mukhopadhyay S, Ghosh P. Centrally inserted tunnelled peripherally inserted central catheter: off-label use for venous access in oncology patients. J Vasc Access. 2020;21(5):773–7.
Low XZ, Tay KH, Leong S, Lo RHG, Zhuang KD, Chua JME, et al. Repurposing the power injectable peripherally inserted central catheter as a tunnelled, non-cuffed, centrally inserted central venous catheter in oncological patients for short- to mid-term vascular access: a pilot study. J Vasc Access. 2021;(3):457–461.
Lv L, Xu J, Bai C, Gong J, Ma W, Sun X. Cluster nursing in the prevention of PICC-related venous thrombosis and its influence on tumor patients’ coagulation functions. Int J Clin Exp Med. 2020;13(12):10005–11.
Malek AE, Raad II. Preventing catheter-related infections in cancer patients: a review of current strategies. Expert Rev Anti Infect Ther. 2020;18(6):531–8.
McParlan D, Edgar L, Gault M, Gillespie S, Menelly R, Reid M. Intravascular catheter migration: a cross-sectional and health-economic comparison of adhesive and subcutaneous engineered stabilisation devices for intravascular device securement. J Vasc Access. 2020;21(1):33–8.
Mielke D, Wittig A, Teichgraber U. Peripherally inserted central venous catheter (PICC) in outpatient and inpatient oncological treatment. Support care cancer. 2020;28(10):4753–60.
Mollee P, Abro E, Van Kuilenburg R, Joubert W, Okano S, Looke D, et al. Catheter-associated bloodstream infections in adults with cancer: a prospective randomized controlled trial. J Hosp Infect. 2020;106(2):335–42.
Park S, Moon S, Pai H, Kim B. Appropriate duration of peripherally inserted central catheter maintenance to prevent central line-associated bloodstream infection. PLoS One. 2020;15(6):e0234966.
Pu YL, Li ZS, Zhi XX, Shi YA, Meng AF, Cheng F, et al. Complications and costs of peripherally inserted central venous catheters compared with Implantable port catheters for cancer patients: a meta-analysis. Cancer Nurs. 2020;43(6):455–67.
Santacatalina-Roig E, Espinar-de Las Heras E, Ballesteros-Lizondo JM, Ibanez-Puchades I, Pescador-Marco JL. Peripherally inserted central catheter in haematopoietic stem cell transplantation. Infusion of haematopoietic cells and complications. Enfirm Clin (Engl Ed). 2020;30(5):295–301.
Tan L, Sun Y, Zhu L, Lei X, Liang D, Rao N, et al. Risk factors of catheter-related thrombosis in early-stage breast cancer patients: a single-center retrospective study. Cancer Manage Res. 2019;11:8379–89.
Tang T, Li H, Wang J, Li C, Geng C. The causes and managements of catheter misplacement in implantable vascular access devices: a retrospective analysis of 8534 patients in a single center. Int J Clin Exp Med. 2019;12(9):11864–8.
Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H, et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. 2019;122(6):734–41.
Ullman AJ, Mihala G, O’Leary K, Marsh N, Woods C, Bugden S, et al. Skin complications associated with vascular access devices: a secondary analysis of 13 studies involving 10,859 devices. Int J Nurs Stud. 2019;91:Jun–13.
Velioglu Y, Yuksel A, Sinmaz E. Complications and management strategies of totally implantable venous access port insertion through percutaneous subclavian vein. Turk Gogus kalp damar cerrahisi dergisi. 2019;27(4):499–507.
Xiong ZY, Luo Z, Chen HY. Prevalence of idle peripherally inserted central catheters in adult patients: a multicenter cross-sectional study. J Vasc Access. 2019;20(6):677–82.
Yildiz A, Albayrak M, Sahin O, Pala C, Ozturk HBA, Gunes G, et al. Incidence and risk factors of port related infections in patients with hematological malignancy. Int J Clin Exp Med. 2019;12(1):989–96.
Zabicki B, Limphaibool N, Veilemand Holstad MJ, Perkowska K. Central venous access ports in the interventional radiology suite - one-centre experience. Pol J Radiol. 2019;84:e328–334.
Zanwar S, Gokarn A, Devadas SK, Punatar S, Khurana S, Bonda A, et al. Antibiotic lock therapy for salvage of tunneled central venous catheters with catheter colonization and catheter-related bloodstream infection. Transpl Infect Disease. 2019;21(1):e13017.
Simonetti G, Sommariva A, Lusignani M, Anghileri E, Ricci CB, Eoli M, et al. Prospective observational study on the complications and tolerability of a peripherally inserted central catheter (PICC) in neuro-oncological patients. Supportive care cancer: Official J Multinational Association Supportive Care Cancer. 2020;28(6):2789–95.
Skummer P, Kobayashi K, DeRaddo JS, Blackburn T, Schoeneck M, Patel J, et al. Risk factors for early port infections in adult oncologic patients. J Vascular Interventional Radiology: JVIR. 2020;31(9):1427–36.
Slaughter E, Keogh SJ, Kynoch K, Brodribb M. Evaluating the impact of central venous catheter materials and design on thrombosis: a systematic review and meta-analysis. Worldviews evidence-based Nurs. 2020;17(5):376–84.
Song X, Lu H, Chen F, Bao Z, Li S, Li S, et al. A longitudinal observational retrospective study on risk factors and predictive model of PICC associated thrombosis in cancer patients. Sci Rep. 2020;10(1):10090.
Tang L, Kim CY, Martin JG, Pabon-Ramos WM, Sag AA, Suhocki PV, et al. Length of stay predicts risk of early infection for hospitalized patients undergoing central venous port placement. J Vascular Interventional Radiology: JVIR. 2020;31(3):454–61.
Tsuruta S, Goto Y, Miyake H, Nagai H, Yoshioka Y, Yuasa N, et al. Late complications associated with totally implantable venous access port implantation via the internal jugular vein. Supportive care cancer: Official J Multinational Association Supportive Care Cancer. 2020;28(6):2761–8.
Wang GD, Wang HZ, Shen YF, Dong J, Wang XP, Wang XZ, et al. The influence of venous characteristics on peripherally inserted central catheter-related symptomatic venous thrombosis in cancer patients. Cancer Manage Res. 2020;12:11909–20.
Yin L, Li J. Central venous catheter insertion in colorectal cancer patients, PICC or PC? Cancer Manage Res. 2020;12:5813–8.
Yin YX, Gao W, Li XY, Lu W, Deng QH, Zhao CY, et al. Randomized multicenter study on long-term complications of peripherally inserted central catheters positioned by electrocardiographic technique. Phlebology. 2020;35(8):614–622.
Akhtar N, Lee L. Utilization and complications of central venous access devices in oncology patients. Curr Oncol (Toronto Ont). 2021;28(1):367–77.
Annetta MG, Ostroff M, Marche B, Emoli A, Musarò A, Celentano D, et al. Chest-to-arm tunneling: a novel technique for medium/long term venous access devices. J Vasc Access. 2023;24(1):92–8.
Clari M, Spoto M, Franceschi G, Acuto M, Tonella S, Caristia S, et al. Short versus long timing of flushing of totally implantable venous access devices when not used routinely: a systematic review and Meta-analysis. Cancer Nurs. 2021;44(3):205–213.
Corti F, Brambilla M, Manglaviti S, Di Vico L, Pisanu MN, Facchinetti C, et al. Comparison of outcomes of central venous catheters in patients with solid and hematologic neoplasms: an Italian real-world analysis. Tumori. 2021;107(1):17–25.
Cruz-Aguilar R, Carney J, Mondaini V, Vehreschild M, Griskaitis M, Salmanton-García J, et al. A quality improvement study on the reduction of central venous catheter-associated bloodstream infections by use of self-disinfecting venous access caps (STERILE). Am J Infect Control. 2021;49(5):586–592.
D’Souza PC, Kumar S, Kakaria A, Al-Sukaiti R, Al-Baimani K, Hamid RS, et al. Complications and management of totally implantable central venous access ports in cancer patients at a University Hospital in Oman. Sultan Qaboos Univ Med J. 2021;21(1):e103–109.
Egnatios D, Gloria C. Implanted port patency: comparing heparin and normal saline. Clin J Oncol Nurs. 2021;25(2):169–73.
Kara H, Arikan AE, Dulgeroglu O, Uras C, Icten GE, Tutar B, et al. Detachment and embolization of totally implantable central venous access devices: diagnosis and management. Acta Chir Belg. 2022;122(4):240–247.
Lee YM, Ryu BH, Hong SI, Cho OH, Hong KW, Bae IG, et al. Clinical impact of early reinsertion of a central venous catheter after catheter removal in patients with catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2021;42(2):162–8.
Lichtenstein T, Rau K, Hokamp NG, Maintz D, Mammadov K, Do TD, et al. Long-term follow-up and clinical relevance of incidental findings of fibrin sheath and thrombosis on computed tomography scans of cancer patients with port catheters. Ther Clin Risk Manag. 2021;17:111–8.
Michell H, Nezami N, Morris C, Hong K. Dual-chambered venous access port as alternative access for extracorporeal apheresis therapy. J Vasc Access. 2021;22(2):173–7.
Sachs OA, Chugh P, He K, Moseley JM, Oneal PB, Whang E, Kristo G. Survival and Complications After Placement of Central Venous Access Ports for Palliative Chemotherapy: A Single-Institution Retrospective Analysis. The American Journal of Hospice and Palliative Care. 2022;39(1):34–38.
Oh SB, Park K, Kim JJ, Oh SY, Jung KS, Park BS, et al. Safety and feasibility of 3-month interval access and flushing for maintenance of totally implantable central venous port system in colorectal cancer patients after completion of curative intended treatments. Med (Baltim). 2021;100(2):e24156.
Park EJ, Park K, Kim JJ, Oh SB, Jung KS, Oh SY, et al. Safety, efficacy, and patient satisfaction with initial peripherally inserted central catheters compared with usual intravenous access in terminally Ill cancer patients: a randomized phase II study. Cancer Res Treat. 2021;53(3):881–888.
Piredda A, Radice D, Zencovich C, Cerri M, Aventino L, Naccarato F, et al. Safe use of peripherally inserted Central catheters for chemotherapy of solid malignancies in adult patients: a 1-year monocentric, prospectively-assessed, unselected cohort of 482 patients. J Vasc Access. 2020;1:E12973.
Rixecker T, Lesan V, Ahlgrimm M, Thurner L, Bewarder M, Murawski N, et al. Insertion site of central venous catheter correlates with catheter-related infectious events in patients undergoing intensive chemotherapy. Bone Marrow Transplant. 2021;56(1):195–201.
Shibata J, Hiramatsu K, Shibata Y, Aoba T, Fujii M, Arimoto A, et al. Impact of chest subcutaneous fat on the occurrence of central venous port-related infectious complications in cancer patients. Support Care Cancer. 2021;29(9):5291–5398.
Tumay LV, Guner OS. Availability of totally implantable venous access devices in cancer patients is high in the long term: a seven-year follow-up study. Support Care Cancer. 2021;29(7):3531–3538.
Xiao MF, Xiao CQ, Li J, Dai C, Fan YY, Cao HJ, et al. Subcutaneous tunneling technique to improve outcomes for patients undergoing chemotherapy with peripherally inserted central catheters: a randomized controlled trial. J Int Med Res. 2021;49(4):3.00061E + 15.
Yan W, Zhang C, Luo C, Li Z. Management of outpatient with totally implantable venous access ports during the COVID-19 epidemic. Medicine. 2021;100(7):e24720.
Sze Yong T, Vijayanathan AA, Chung E, Ng WL, Yaakup NA, Sulaiman N. Comparing catheter related bloodstream infection rate between cuffed tunnelled and non-cuffed tunnelled peripherally inserted central catheter. J Vasc Access. 2022;23(2):225–231.
Gür Ö, DonbaloĞLu MO, GÜRkan S. Comparison of clinical follow-up and complications according to cancer types in patients with permanent port catheter insertion due to Malignancy. Duzce Med J. 2018;20(3):59–62.
Ban T, Fujiwara SI, Murahashi R, Nakajima H, Ikeda T, Matsuoka S, et al. Risk factors for complications associated with peripherally inserted central catheters during induction chemotherapy for acute myeloid leukemia. Intern Med. 2022;61(7):989–95.
Baumann Kreuziger L, Gaddh M, Onadeko O, George G, Wang TF, Oo TH, et al. Treatment of catheter-related thrombosis in patients with hematologic malignancies: a venous thromboEmbolism Network U.S. retrospective cohort study. Thromb Res. 2021;202:155–61.
Bertoglio S, Annetta MG, Brescia F, Emoli A, Fabiani F, Fino M, et al. A multicenter retrospective study on 4480 implanted PICC-ports: a GAVeCeLT project. J Vasc Access. 2022;24(5):1114–1120.
Böll B, Schalk E, Buchheidt D, Hasenkamp J, Kiehl M, Kiderlen TR, et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the infectious diseases working party (AGIHO) of the German society of hematology and medical oncology (DGHO). Ann Hematol. 2021;100(1):239–59.
Brescia F, Pittiruti M, Roveredo L, Zanier C, Morabito A, Santarossa E, et al. Subcutaneously anchored securement for peripherally inserted central catheters: Immediate, early, and late complications. J Vasc Access. 2023;24(1):82–6.
Caris MG, de Jonge NA, Punt HJ, Salet DM, de Jong VMT, Lissenberg-Witte BI, et al. Indwelling time of peripherally inserted central catheters and incidence of bloodstream infections in haematology patients: a cohort study. Antimicrob Resist Infect Control. 2022;11(1):37.
Chen K, Beeraka NM, Gu Y, Li J, Sinelnikov M, Han N, et al. Totally implantable venous access port systems: implant depth-based complications in breast cancer therapy - a comparative study. Curr Pharm Des. 2021;27(46):4671–6.
Chen P, Zhu B, Wan G, Qin L. The incidence of asymptomatic thrombosis related to peripherally inserted central catheter in adults: a systematic review and meta-analysis people’s. Nurs Open. 2021;8(5):2249–61.
Cotogni P, Mussa B, Degiorgis C, De Francesco A, Pittiruti M. Comparative complication rates of 854 central venous access devices for home parenteral nutrition in cancer patients: a prospective study of over 169,000 catheter-days. JPEN J Parenter Enter Nutr. 2021;45(4):768–76.
El Boghdadly Z, Zhao Q, Koutou J, Lustberg ME, Ludwig M, Liscynesky C, et al. Evaluation of central line salvage for mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) management practices in patients with hematologic malignancies. Leuk Lymphoma. 2022;63(6):1455–63.
González S, Jiménez P, Saavedra P, Macías D, Loza A, León C, et al. Five-year outcome of peripherally inserted central catheters in adults: a separated infectious and thrombotic complications analysis. Infect Control Hosp Epidemiol. 2021;42(7):833–41.
Guan X, Yan H, Zhang J, Li Y, Zhou Y. Risk factors of infection of totally implantable venous access port: a retrospective study. J Vasc Access. 2023;24(6):1340–1348.
Hashimoto Y, Hosoda R, Omura H, Tanaka T. Catheter-related bloodstream infection associated with multiple insertions of the peripherally inserted central catheter in patients with hematological disorders. Sci Rep. 2021;11(1):12209.
Heidenreich D, Hansen E, Kreil S, Nolte F, Jawhar M, Hecht A, et al. The insertion site is the main risk factor for central venous catheter-related complications in patients with hematologic malignancies. Am J Hematol. 2022;97(3):303–10.
Huang C, Wu Z, Huang W, Zhang X, Lin X, Luo J, et al. Identifying the impact of the zone insertion method(TM) (ZIM(TM)): a randomized controlled trial. J Vasc Access. 2021;24(4):1–10. https://doi.org/10.1177/11297298211052528.
Johns J, Wahlrab L, Elefritz JL. Acutely ill hematology/oncology patients with central-line associated bloodstream infections and the impact of timing of catheter removal on outcomes. Am J Infect Control. 2022;50(7):749–54.
Kim TH, Choi YW, Ahn MS, Choi YS, Lee HW, Jeong SH, et al. Early removal of central venous catheter may not impact the in-hospital mortality in patients with acute leukemia. Ann Hematol. 2021;100(11):2825–30.
Kinoshita M, Takao S, Hiraoka J, Takechi K, Akagawa Y, Osaki K, et al. Risk factors for unsuccessful removal of central venous access ports implanted in the forearm of adult oncologic patients. Jpn J Radiol. 2022;40(4):412–8.
Krümpelmann U, Boseila A, Löhnert M, Kaup O, Clarenbach JJ, Görner M. An analysis of totally implantable central venous port system infections in an urban tertiary referral center. J Chemother. 2021;33(4):228–37.
Kumwenda MJ, Dougherty L, Jackson A, Hill S. Prospective audit to study urokinaSe use to restore Patency in occluded centRal venous caTheters in haematology and oncology patients (PASSPORT 2). J Vasc Access. 2021;22(4):568–74.
Liu B, Wu Z, Lin C, Li L, Kuang X. Applicability of TIVAP versus PICC in non-hematological malignancies patients: a meta-analysis and systematic review. PLoS One. 2021;16(8):e0255473.
Martinez J, Capela R. Infusion pump flow rates in central venous catheters: thrombus reflux and aspiration Clot. Onconews. 2021;(42):16–20.
McKeown C, Ricciuti A, Agha M, Raptis A, Hou JZ, Farah R, et al. A prospective study of the use of central venous catheters in patients newly diagnosed with acute myeloid leukemia treated with induction chemotherapy. Support Care Cancer. 2022;30(2):1673–9.
Mittal GS, Sundriyal D, Naik NB, Sehrawat A. Totally implantable venous access device (Chemoport) in Oncology: study of 168 polyurethane chemoport catheter system. South Asian J Cancer. 2021;10(4):261–4.
Moralar DG, Turkmen UA, Bilen A, Turkmen S, Feyizi H, Altan HA. Our central venous port catheter system practice - a retrospective study. J Pak Med Assoc. 2021;71(5):1442–5.
Pike S, Tan K, Burbridge B. Complications associated with totally implanted venous access devices in the arm versus the chest: a short-term retrospective study. Can Assoc Radiol J. 2022;73(3):581–8.
Pinelli F, Balsorano P, Mura B, Pittiruti M. Reconsidering the GAVeCeLT Consensus on catheter-related thrombosis, 13 years later. J Vasc Access. 2021;22(4):501–8.
Pinelli F, Pittiruti M, Van Boxtel T, Barone G, Biffi R, Capozzoli G, et al. GAVeCeLT-WoCoVA Consensus on subcutaneously anchored securement devices for the securement of venous catheters: current evidence and recommendations for future research. J Vasc Access. 2021;22(5):716–25.
Platanaki C, Zareifopoulos N, Lagadinou M, Tsiotsios K, Velissaris D. Correlation of positive blood cultures with peripherally inserted central catheter line infection in oncology patients. Cureus. 2021;13(1):e12858.
Ploton G, Brebion N, Guyomarch B, Pistorius MA, Connault J, Hersant J, et al. Predictive factors of venous recanalization in upper-extremity vein thrombosis. PLoS One. 2021;16(5):e0251269.
Ranch-Lundin M, Schedin A, Björkhem-Bergman L. Equal effect of Vancomycin lock with or without heparin in treatment of central venous catheter related blood stream infections - an observational study in palliative home care. Infect Dis (Lond). 2021;53(9):719–23.
Rejane Rabelo-Silva E, Lourenço SA, Maestri RN, Candido da Luz C, Carlos Pupin V, Bauer Cechinel R, et al. Patterns, appropriateness and outcomes of peripherally inserted central catheter use in Brazil: a multicentre study of 12 725 catheters. BMJ Qual Saf. 2022;31(9):652–61.
Rockholt MM, Thorarinsdottir HR, Lazarevic V, Rundgren M, Kander T. Central venous catheter-related complications in hematologic patients: an observational study. Acta Anaesthesiol Scand. 2022;66(4):473–82.
Sacks OA, Chugh P, He K, Moseley JM, Oneal PB, Whang E, et al. Survival and complications after placement of central venous access ports for palliative chemotherapy: a single-institution retrospective analysis. Am J Hosp Palliat Care. 2022;39(1):34–8.
Sapkota S, Sannur R, Naik R. Analysis of peripherally inserted central catheter line in cancer patients: a single-center experience. South Asian J Cancer. 2020;9(4):253–6.
Shih YH, Teng CJ, Chen TC, Chang KH, Chen MH. Dual-lumen power injectable peripherally inserted central catheters in allogeneic hematopoietic stem cell transplantation: a prospective observational study. J Clin Nurs. 2022;31(11–12):1654–61.
Snarski E, Stringer J, Mikulska M, Gil L, Tridello G, Bosman P, et al. Risk of infectious complications in adult patients after allogeneic hematopoietic stem cell transplantation depending on the site of central venous catheter insertion-multicenter prospective observational study, from the IDWP EBMT and Nurses Group of EBMT. Bone Marrow Transpl. 2021;56(12):2929–33.
Ullman AJ, Paterson RS, Schults JA, Kleidon TM, August D, O’Malley M, et al. Do antimicrobial and antithrombogenic peripherally inserted central catheter (PICC) materials prevent catheter complications? An analysis of 42,562 hospitalized medical patients. Infect Control Hosp Epidemiol. 2022;43(4):427–34.
Wan R, Gu L, Yin B, Cai S, Zhou R, Yang W. A six-year study of complications related to peripherally inserted central catheters: a multi-center retrospective cohort study in China. Perfusion. 2023;38(4):689–97.
Winkler MA, Spencer TR, Siddiqi N, Wallace JE, Gallien JZ, Elbalasi H, et al. Clinical experience with a chlorhexidine-coated PICC: a prospective, multicenter, observational study. J Vasc Access. 2021;25(1):225–231.
Yang WJ, Song MG, Seo TS, Park SJ. Effectiveness of mechanical recanalization for intraluminal occlusion of totally implantable venous access ports. J Vasc Access. 2023;24(3):430–5.
Yun WS, Yang SS. Comparison of peripherally inserted central catheters and totally implanted venous access devices as chemotherapy delivery routes in oncology patients: a retrospective cohort study. Sci Prog. 2021;104(2):368504211011871.
Zhang Y, Zhao R, Jiang N, Shi Y, Wang Q, Sheng Y. A retrospective observational study on maintenance and complications of totally implantable venous access ports in 563 patients: prolonged versus short flushing intervals. Int J Nurs Sci. 2021;8(3):252–6.
Broadhurst D, Moureau N, Ullman AJ. Management of central venous Access device-Associated skin impairment: an evidence-based Algorithm. J Wound Ostomy Cont Nurs. 2017;44(3):211–20.
Busch JD, Herrmann J, Adam G, Ittrich H, Vens M, Mahler C. Complication rates observed in silicone and polyurethane catheters of totally implanted central venous Access devices implanted in the upper arm. J Vasc Interv Radiol. 2017;28(8):1177–83.
Chong HY, Lai NM, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy of antimicrobial central venous catheters in reducing catheter-related bloodstream infections in adults: abridged cochrane systematic review and network meta-analysis. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2017;64(suppl2):S131–40.
Jones D, Wismayer K, Bozas G, Palmer J, Elliott M, Maraveyas A. The risk of venous thromboembolism associated with peripherally inserted central catheters in ambulant cancer patients. Thromb J. 2017;15:25.
Kramer RD, Mann J, Rogers MAM, Saint S, Chopra V, Conte M. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control. 2017;45(2):108–14.
Lin WY, Lin CP, Hsu CH, Lee YH, Lin YT, Hsu MC, et al. Right or left? Side selection for a totally implantable vascular access device: a randomised observational study. Br J Cancer. 2017;117(7):932–7.
Madabhavi I, Patel A, Anand A, Panchal H, Parikh S, Sarkar M. A study of Use of PORT catheter in patients with cancer: a single-center experience. Clin Med Insights: Oncol. 2017;11:1179554917691031.
McDiarmid S, Scrivens N, Carrier M, Sabri E, Toye B, Huebsch L, et al. Outcomes in a nurse-led peripherally inserted central catheter program: a retrospective cohort study. CMAJ Open. 2017;5(3):E535–539.
Milani A, Mazzocco K, Gandini S, Pravettoni G, Libutti L, Zencovich C, et al. Incidence and determinants of port occlusions in cancer outpatients. Cancer Nurs. 2017;40(2):102–7.
Patel PA, Boehm S, Zhou Y, Zhu C, Peterson KE, Grayes A, et al. Prospective observational study on central line-associated bloodstream infections and central venous catheter occlusions using a negative displacement connector with an alcohol disinfecting cap. Am J Infect Control. 2017;45(2):115–20.
Suleman A, McDiarmid S. A retrospective analysis of catheter-related upper extremity deep vein thrombosis in peripherally inserted catheters with and without a dermatotomy. JAVA - J Association Vascular Access. 2017;22(4):178–81.
Tabatabaie O, Kasumova GG, Kent TS, Eskander MF, Fadayomi AB, Ng SC, et al. Upper extremity deep venous thrombosis after port insertion: what are the risk factors? Surgery. 2017;162(2):437–44.
Takashima M, Ray-Barruel G, Ullman A, Keogh S, Rickard CM. Randomized controlled trials in central vascular access devices: a scoping review. PLoS One. 2017;12(3):e0174164.
Voor in ’t holt AF, Helder OK, Vos MC, Schafthuizen L, Sülz S, van den Hoogen A, et al. Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: a systematic review and meta-analysis. Int J Nurs Stud. 2017;69:34–40.
Wang XJ. Preventive effect of dexamethasone solution pre-treated catheter on PICC-induced phlebitis. Biomedical Res (India). 2017;28(12):5310–4.
Yu L, Zhang R, Li J, Yan X, Jin K, Li W, et al. Incidence and risk factors for peripherally inserted central catheter-related vein thrombosis in lung cancer patients. Int J Clin Exp Med. 2017;10(8):12440–6.
Zhang M, Kang L, Li Q. A comparative study on the use of different connectors in tube sealing in elderly tumor patients with PICC. Int J Clin Exp Med. 2017;10(6):9488–94.
Brito ARO, Nishinari K, Saad PF, Saad KR, Pereira MAT, Emidio SCD, et al. Comparison between saline solution containing heparin versus saline solution in the lock of totally implantable catheters. Ann Vasc Surg. 2018;47:85–9.
Hallam C, Jackson T, Rajgopal A, Russell B. Establishing catheter-related bloodstream infection surveillance to drive improvement. J Infect Prev. 2018;19(4):160–6.
Huang V. Effect of a patency bundle on central venous catheter complications among hospitalized adult patients: a best practice implementation project. JBI Database Syst Reviews Implement Rep. 2018;16(2):565–86.
Huihan Z, Yu H, Qin W, Yanping Y. Medical adhesive–related skin Injury Prevalence at the peripherally inserted central catheter insertion site: a cross-sectional, multiple-center study. J Wound Ostomy Cont Nurs. 2018;45(1):22–5.
Iftikhar R, Chaudhry QUN, Satti TM, Mahmood SK, Satti HS, Ghafoor T, et al. Noble Metal Coated Central venous catheters are not Superior to uncoated catheters in preventing infectious and non-infectious complications in immunocompromised patients. J Ayub Med Coll Abbottabad: JAMC. 2018;30(Suppl 1):S647–651.
Kim JH, Hong YS, Kim SY, Kim K-P, Choi KE, Kim TW, et al. Increased incidence of chemoport-related thrombosis in patients with colorectal cancer receiving bevacizumab: a single-institutional experience. Chin J Cancer Res. 2018;30(4):460–7.
Koo CM, Vissapragada R, Sharp R, Nguyen P, Ung T, Solanki C, et al. ABO blood group related venous thrombosis risk in patients with peripherally inserted central catheters. Br J Radiol. 2018;91(1082):20170560.
Lam PW, Volling C, Chan T, Wiggers JB, Castellani L, Wright J, et al. Impact of defaulting to single-lumen peripherally inserted central catheters on patient outcomes: an interrupted time series study. Clin Infect Dis. 2018;67(6):954–7.
Lopez-Briz E, Ruiz Garcia V, Cabello JB, Bort-Marti S, Carbonell Sanchis R, Burls A. Heparin versus 0.9% sodium chloride locking for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Reviews. 2018;2018(7):CD008462.
Lv Y, Hou Y, Yu L, Xu D, Song J, Shang H, et al. Risk associated with central catheters for malignant tumor patients: a systematic review and meta-analysis. Oncotarget. 2018;9(15):12376–88.
Mansour A, Khozouz O, Saadeh SS, Abunasser M, Abdel-Razeq N, Taqash A. Clinical course and complications of catheter and non-catheter-related upper extremity deep vein thrombosis in patients with cancer. Clin Appl Thromb Hemost. 2018;24(8):1234–40.
McDonald MK, Culos KA, Gatwood KS, Prow C, Chen H, Savani BN, et al. Defining incidence and risk factors for catheter-associated bloodstream infections in an outpatient adult hematopoietic cell transplantation program. Biology of blood and marrow transplantation: journal of the American Society for Blood and marrow transplantation. 2018;24(10):2081–7.
Rasero L, Golin L, Ditta S, Di Massimo DS, Dal Molin A, Piemonte G. Effects of prolonged flushing interval in totally implantable venous access devices (TIVADs). Br J Nurs. 2018;27(8):S4–10.
Spires SS, Rebeiro PF, Miller M, Koss K, Wright PW, Talbot TR. Medically attended catheter complications are common in patients with outpatient central venous catheters. Infect Control Hosp Epidemiol. 2018;39(4):439–44.
Wu S, Li W, Zhang Q, Li S, Wang L. Comparison of complications between peripheral arm ports and central chest ports: a meta-analysis. J Adv Nurs. 2018;74(11):2484–96.
Yu XY, Xu JL, Li D, Jiang ZF. Late complications of totally implantable venous access ports in patients with cancer: risk factors and related nursing strategies. Medicine (Baltimore). 2018;97(38):e12427.
Agrawal SK, Gautam H, Choudhary AH, Das BK, Kumar L, Kapil A. Central line-associated bloodstream infections in cancer patients: an experience from a tertiary care cancer centre. Ind J Med Microbiol. 2019;37(3):376–80.
Bademler S, Ucuncu M, Yildirim I, Karanlik H. Risk factors for complications in cancer patients with totally implantable access ports: a retrospective study and review of the literature. J Int Med Res. 2019;47(2):702–9.
Dang FP, Li HJ, Tian JH. Comparative efficacy of 13 antimicrobial dressings and different securement devices in reducing catheter-related bloodstream infections: a bayesian network meta-analysis. Medicine (Baltimore). 2019;98(14):e14940.
Dinçer M, Kocakuşak A, Hut A, Gür Ü, Çıtlak G, Akıncı M. Comparison of two different central venous Access device insertion techniques: no evil in details. Med Bull Haseki / Haseki Tip Bulteni. 2019;57(1):Sep–14.
Eldeeb H, Al-Asadi O, Almusarhed M. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single centre cohort study. Thromb J. 2019;17(1):191.
Fornaro C, Piubeni M, Tovazzi V, Cosentini D, Gelmi M, Rota G, et al. Eight-week interval in flushing and locking port-a-cath in cancer patients: a single-institution experience and systematic review. Eur J Cancer Care (Engl). 2019;28(2):e12978.
Fu X, Lu P, Wang C, Ye G. Analysis of the risk factors of peripherally inserted central catheter-associated venous thrombosis after chemotherapy in patients with lung cancer. Int J Clin Exp Med. 2019;12(5):5852–9.
Hill S, Hamblett I, Brady S, Vasileukaya S, Zuzuarregui I, Martin F. Central venous access device-related sheaths: a predictor of infective and thrombotic incidence? Br J Nurs. 2019;28(19):S10–18.
Isom C, Bream P, Gallagher K, Walia S, Ahmed R, Kauffmann R. Placement of subcutaneous central venous ports in breast Cancer patients: does side matter? J Surg Res. 2019;244:296–301.
Kagan E, Salgado CD, Banks AL, Marculescu CE, Cantey JR. Peripherally inserted central catheter-associated bloodstream infection: risk factors and the role of antibiotic-impregnated catheters for prevention. Am J Infect Control. 2019;47(2):191–5.
Lee IJ, Kim HB, Choi YJ, Lee JH, Kim ET, Shim DJ, et al. Prevalence and predictors of peripherally inserted central catheter-associated bloodstream infections in adults: a multicenter cohort study. PLoS One. 2019;14(3):e0213555.
Liu S, Cong L, Xiao Z, Song Y, Lou T, Ma Y, et al. Risk factors associated with peripherally inserted central catheter-related venous thrombosis in hospitalized patients of advanced age. J Int Med Res. 2019;48(1):300060518820744.
Liu Z, Chen J, Zan L, Ding S, Yi H, Yan C. Exploring the risk factors of thrombosis and bloodstream infections in peripherally inserted central catheter (PICC) patients. J Biomaterials Tissue Eng. 2019;9(7):929–34.
Okazaki M, Oyama K, Kinoshita J, Miyashita T, Tajima H, Takamura H, et al. Incidence of and risk factors for totally implantable vascular access device complications in patients with gastric cancer: a retrospective analysis. Mol Clin Oncol. 2019;11(4):343–8.
Russo R, Oliveira MS, Shikanai-Yasuda MA, Mendes ET, Levin AS, Costa SF, et al. Bloodstream infection in hematopoietic stem cell transplantation outpatients: risk factors for hospitalization and death. Rev Inst Med Trop Sao Paulo. 2019;61:e3.
Sengul T, Ocakci AF, Guven B, Kaya N. Connectors as a risk factor for blood-associated infections (3-way stopcock and needleless connector): a randomized-experimental study. Am J Infect Control. 2020;48(3):275–280.
Skelton Iv WP, Franke AJ, Welniak S, Bosse RC, Ayoub F, Murphy M, et al. Investigation of complications following port insertion in a cancer patient population: a retrospective analysis. Clin Med Insights: Oncol. 2019;13:N.PAG–N.PAG.
Suttle RD, Buffington HM, Madden WT, Dawson MA. Central line care: empowering patients to prevent infection and Injury Via EPIC2. Clin J Oncol Nurs. 2019;23(1):E10–16.
Ziegler M, Landsburg D, Kucharczuk C, Gorman T, Bink K, Stadtmauer EA, et al. Fluoroquinolone prophylaxis is highly effective for the prevention of central line-associated bloodstream infections in autologous stem cell transplant patients. Biol Blood Marrow Transplant. 2019;25(5):1004–10.
Bessis S, Cassir N, Meddeb L, Remacle AB, Soussan J, Vidal V, et al. Early mortality attributable to PICC-lines in 4 public hospitals of Marseille from 2010 to 2016 (revised V3). Medicine (Baltimore). 2020;99(1):e18494.
Beypinar I, Demir H, Uysal M, Araz M, Beypinar D. The comparison of central venous port catheters in gastrointestinal cancer treatment. J Oncological Sci. 2020;6(1):10–4.
Huang W, Xu J. The role of sterile chitosan-based dressing in reducing complications related to a peripherally inserted central catheter in patients with hematological tumors. Annals Palliat Med. 2020;9(4):2037–44.
Jiang M, Li CL, Pan CQ, Yu L. The risk of bloodstream infection associated with totally implantable venous access ports in cancer patient: a systematic review and meta-analysis. Support Care Cancer. 2020;28(1):361–72.
Karapanou A, Sampanis MA, Vieru A-M, Daikos GL, Samarkos M, Pantazatou A, et al. Failure of central venous catheter insertion and care bundles in a high central line-associated bloodstream infection rate, high bed occupancy hospital. Am J Infect Control. 2020;48(7):770–6.
Kitamura H, Kimura S, Kubota Y, Komukai S, Yoshida H, Kaneko Y, et al. Venue of catheter insertion does not significantly impact the event of central line-associated bloodstream infection in patients with haematological diseases. Infect Prev Pract. 2020;2(2):100050.
Kukla ME, Childs CA, Puig-Asensio M, Marra AR, Perencevich EN, Schweizer ML. Effectiveness of chlorhexidine dressings to prevent catheter-related bloodstream infections. Does one size fit all? A systematic literature review and meta-analysis. Infect Control Hosp Epidemiol. 2020;41(12):1388–1395.
Lin Y, Zeng Z, Zheng J, Lin R, Liu S, Gao X. The Caprini thrombosis risk model predicts the risk of PICC-related upper extremity venous thrombosis in cancer patients. J Vasc Surg Venous Lymphat Disord. 2021;9(5):1151–1158.
Liu Y, Li LL, Xu L, Feng DD, Cao Y, Mao XY, et al. Comparison between arm port and chest port for optimal vascular access port in patients with breast cancer: a systematic review and meta-analysis. Biomed Res Int. 2020;2020:9082924.
Qi F, Cheng H, Yuan X, Zhang L. Comparison of PICC and TIVAP in chemotherapy for patients with thyroid cancer. Oncol Lett. 2020;20(2):1657–62.
Rowe MS, Arnold K, Spencer TR. Catheter securement impact on PICC-related CLABSI: a university hospital perspective. Am J Infect Control. 2020;48(12):1497–500.
Scrivens N, Sabri E, McDiarmid S, Bredeson C. Comparison of complication rates and incidences associated with different peripherally inserted central catheters (PICC) in patients with hematological malignancies: a retrospective cohort study. Leuk Lymphoma. 2020;61(1):156–164.
Song Y, Liu S, Lou T, Ma Y, Wang N, Yong Q, et al. Risk factors associated with peripherally inserted central catheter-related venous thrombosis in hospitalized patients of advanced age. The Journal of international medical research. 2020;48(1):3.00061E + 14.
Wang G, Li Y, Wu C, Guo L, Hao L, Liao H, et al. The clinical features and related factors of PICC-related upper extremity asymptomatic venous thrombosis in cancer patients: a prospective study. Medicine. 2020;99(12):e19409.
Webber JR. Sticking it to them–tissue adhesive reduces PICC Migration and microbial contamination. Vascular Access. 2020;14(1):13–7.
Ying S, Liping Z, Yanhong D, Zhulin G, Liang G. Impact of arm choice for peripherally inserted central catheter (PICC) insertion on patients: a cross-sectional study. Contemp Nurse. 2020;56(1):80–9.
Chen XS, Wu XH, Chen LC, Zhang TT, Liu GL. Heparin versus 0.9% saline solution to maintain patency of totally implanted venous access ports in cancer patients: a systematic review and meta-analysis. Int J Nurs Pract. 2021;27:e12913.
Furuhashi S, Morita Y, Ida S, Muraki R, Kitajima R, Suzuki K, et al. Risk factors for totally implantable central venous access port-related infection in patients with malignancy. Anticancer Res. 2021;41(3):1547–53.
Kleidon TM, Horowitz J, Ratz D, Chopra V, Rickard CM, Ullman AJ, et al. Peripherally inserted central catheter thrombosis after Placement via Electrocardiography vs traditional methods. Am J Med. 2021;134(2):e79–88.
Rickard CM, Flynn J, Larsen E, Mihala G, Playford EG, Shaw J, et al. Needleless connector decontamination for prevention of central venous access device infection: a pilot randomized controlled trial. Am J Infect Control. 2021;49(2):269–73.
Schears GJ, Ferko N, Syed I, Arpino JM, Alsbrooks K. Peripherally inserted central catheters inserted with current best practices have low deep vein thrombosis and central line-associated bloodstream infection risk compared with centrally inserted central catheters: a contemporary meta-analysis. J Vasc Access. 2021;22(1):Sep–25.
Sharp R, Carr P, Childs J, Scullion A, Young M, Flynn T, et al. Catheter to vein ratio and risk of peripherally inserted central catheter (PICC)-associated thrombosis according to diagnostic group: a retrospective cohort study. BMJ Open. 2021;11(7):e045895.
Silva SRD, Reichembach MT, Pontes L, Souza G, Kusma S. Heparin solution in the prevention of occlusions in Hickman® catheters a randomized clinical trial. Rev Latinoam Enferm. 2021;29:e3385.
Tian L, Yin X, Zhu Y, Zhang X, Zhang C. Analysis of factors causing skin damage in the application of peripherally inserted central catheter in cancer patients. J Oncol. 2021;2021:6628473.
Wang G, Wang H, Shen Y, Dong J, Wang X, Wang X, et al. Association between ABO blood group and venous thrombosis related to the peripherally inserted central catheters in cancer patients. J Vasc Access. 2021;22(4):590–596.
Wu X, Zhang T, Chen L, Chen X. Prolonging the flush-lock interval of totally implantable venous access ports in patients with cancer: a systematic review and meta-analysis. J Vasc Access. 2021;22(5):814–821.
Xiong ZY, Zhou HM, Li SY. Prolonged flushing and locking interval for totally implantable vascular access device: a systematic review and meta-analysis. J Vasc Access. 2021;22(6):969–978.
Zhang Y, Chen J, Zhao R, Zhang S. Blood sampling from peripherally inserted central catheter is effective and safe for patients with head and neck cancers. J Vasc Access. 2021;22(3):424–431.
Zhong J, Wang B, Huang Q. Study on treating tumor patients with a peripherally inserted central catheter. Int J Clin Exp Med. 2021;14(1):683–9.
Zhao Y, Bian L, Yang J. Intervention efficacy of MARSI nursing management on skin injury at peripherally inserted central catheter insertion site on oncological patients. Int Wound J. 2022;19(8):2055–61.
Belloni S, Caruso R, Cattani D, Mandelli G, Donizetti D, Mazzoleni B, et al. Occurrence rate and risk factors for long-term central line-associated bloodstream infections in patients with cancer: a systematic review. Worldviews Evid Based Nurs. 2022;19(2):100–11.
Capozzi VA, Monfardini L, Sozzi G, Armano G, Butera D, Scarpelli E, et al. Peripherally inserted central venous catheters (PICC) versus totally implantable venous access device (PORT) for chemotherapy administration: a meta-analysis on gynecological cancer patients. Acta Biomed. 2021;92(5):e2021257.
Chopra V, O’Malley M, Horowitz J, Zhang Q, McLaughlin E, Saint S, et al. Improving peripherally inserted central catheter appropriateness and reducing device-related complications: a quasiexperimental study in 52 Michigan hospitals. BMJ Qual Saf. 2022;31(1):23–30.
Feng Y, Zheng R, Fu Y, Xiang Q, Yue Z, Li J, et al. Assessing the thrombosis risk of peripherally inserted central catheters in cancer patients using Caprini risk assessment model: a prospective cohort study. Support Care Cancer. 2021;29(9):5047–55.
Gilardi E, Piano A, Chellini P, Fiori B, Dolcetti L, Pittiruti M, et al. Reduction of bacterial colonization at the exit site of peripherally inserted central catheters: a comparison between chlorhexidine-releasing sponge dressings and cyano-acrylate. J Vasc Access. 2021;22(4):597–601.
He E, Ye K, Zheng H. Clinical effect and safety of venous access ports and peripherally inserted central catheters in patients receiving tumor chemotherapy: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(8):9105–13.
Hoppe A, Rupa-Matysek J, Małecki B, Dytfeld D, Hoppe K, Gil L. Risk factors for catheter-related thrombosis in multiple myeloma patients undergoing autologous stem cell transplantation. Med (Kaunas). 2021;57(10):1020.
Jabaley T, Xiong N, Conley S, Mazeika T, Johnson D, Biggins BA, et al. Transitioning from heparin to saline locks for central venous access devices in oncology: an evidence-based practice approach. Can Oncol Nurs J. 2022;32(2):286–93.
Jones M, Okano S, Looke D, Kennedy G, Pavilion G, Clouston J, et al. Catheter-associated bloodstream infection in patients with cancer: comparison of left- and right-sided insertions. J Hosp Infect. 2021;118:70–6.
Liu GD, Ma WJ, Liu HX, Tang L, Tan YH. Risk factors associated with catheter-related venous thrombosis: a meta-analysis. Public Health. 2022;205:45–54.
Liu X, Tao S, Ji H, Chen S, Gu Y, Jin X. Risk factors for peripherally inserted central catheter (PICC)-associated infections in patients receiving chemotherapy and the preventive effect of a self-efficacy intervention program: a randomized controlled trial. Ann Palliat Med. 2021;10(9):9398–405.
Peng SY, Wei T, Li XY, Yuan Z, Lin Q. A model to assess the risk of peripherally inserted central venous catheter-related thrombosis in patients with breast cancer: a retrospective cohort study. Support Care Cancer. 2022;30(2):1127–37.
Pénichoux J, Rio J, Kammoun L, Vermeulin T, Pepin LF, Camus V, et al. Retrospective analysis of the safety of peripherally inserted catheters versus implanted port catheters during first-line treatment for patients with diffuse large B-cell lymphoma. Eur J Haematol. 2022;109(1):41–9.
Perek S, Khatib A, Izhaki N, Khalaila AS, Brenner B, Horowitz NA. A prediction model for central venous catheter-related thrombosis in patients with newly-diagnosed acute myeloid leukemia: a derivation cohort analysis. Eur J Intern Med. 2022;101:68–75.
Simonetti G, Bersani A, Tramacere I, Lusignani M, Gaviani P, Silvani A. The role of body mass index in the development of thromboembolic events among cancer patients with PICCs: a systematic review. J Vasc Nurs. 2022;40(1):11–6.
Trezza C, Califano C, Iovino V, D’Ambrosio C, Grimaldi G, Pittiruti M. Incidence of fibroblastic sleeve and of catheter-related venous thrombosis in peripherally inserted central catheters: a prospective study on oncological and hematological patients. J Vasc Access. 2021;22(3):444–9.
Zhao H, He Y, Huang H, Ling Y, Zhou X, Wei Q, et al. Prevalence of medical adhesive-related skin injury at peripherally inserted central catheter insertion site in oncology patients. J Vasc Access. 2018;19(1):23–7.
Curtis K, Gavin N, Fuller F. Cancer Nurses Society of Australia, vascular access devices: evidence-based clinical practice guidelines, 2021. In: Cancer Nurses Society of Australia, editor. https://www.cnsa.org.au/practiceresources/vascular-access-guidelines2021.
Schults J, Kleidon T, Chopra V, Cooke M, Paterson R, Ullman AJ, et al. International recommendations for a vascular access minimum dataset: a Delphi consensus-building study. BMJ Qual Saf. 2021;30(9);722–730.
World Health Organization. Health products policy and standards. Nomenclature of medical devices 2021. Available from: https://www.who.int/teams/health-product-policy-and-standards/assistive-and-medical-technology/medical-devices/nomenclature.
Gildow C, Lazar B. The implementation of standard terminology in electronic health record systems and the effect on patient outcomes: a systematic literature review. Int J Acad Health Med Res. 2022;6(7):132–8.
Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol. 2001;106(1–2):33–42.
Jiang D, Lee AI. Thrombotic risk from chemotherapy and other cancer therapies. In: Soff GA, editor. Thrombosis and hemostasis in cancer. Cancer treatment and research. Springer Nature; Switzerland AG. 2019.
Levi M. Clinical characteristics of disseminated intravascular coagulation in patients with solid and hematological cancers. Thromb Res. 2018;164 Suppl 1:S77–81.
Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) patient safety component manual. 2020. Retrieved from https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current. Accessed 15 Dec 2022.
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis. 2009;49(1):1–45.
Rowley S, Clare S. Standardizing the critical clinical competency of aseptic, sterile, and clean techniques with a single international standard: Aseptic non Touch technique (ANTT®). JAVA. 2019;24(4):12–7.
Roos LL, Wall-Wieler E, Burchill C, Hamm NC, Hamad AF, Lix LM. Record linkage and big data—enhancing information and improving design. J Clin Epidemiol. 2022;150:18–24.
Acknowledgements
None.
Funding
No funding was received for the development or publication of the scoping review.
Author information
Authors and Affiliations
Contributions
KC, MK, SK and KG conceived the scoping review and participated in its design. KC and GH developed and conducted the literature search strategy. KC and ET conducted the data screening and extraction. KC and KG carried out the data analyses. KC drafted the manuscript and MK, SK, and KG revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approve and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Scoping review protocol.
Additional file 2.
Search strategy.
Additional file 3.
Included studies.
Additional file 4.
Summary of CVAD terminology.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Curtis, K., Gough, K., Krishnasamy, M. et al. Central venous access device terminologies, complications, and reason for removal in oncology: a scoping review. BMC Cancer 24, 498 (2024). https://doi.org/10.1186/s12885-024-12099-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12099-8