Abstract
Background
The impact of tumor-infiltrating neutrophils (TINs) on clinical outcomes has been reported in various cancer types, but their role in hepatocellular carcinoma (HCC) has not been fully evaluated. The aim of this study was to investigate the prognostic values for TINs in HCC patients undergoing curative resection.
Methods
We assessed immune markers (CD3, CD4, CD8, CD66b) using immunohistochemistry in 115 patients who underwent curative resection for HCC. We analyzed the prognostic values for tumor-infiltrating immune cells, including neutrophils, and other clinicopathological factors.
Results
In the Multivariate Cox analysis of overall survival (OS), alpha-fetoprotein (AFP) ≥ 100 ng/mL (hazard ratio (HR), 2.74, 95% confidence interval (CI), 1.17–6.44; P = 0.021) and Barcelona Clinic Liver Cancer (BCLC) B/C stage (HR, 3.98, 95% CI, 1.68–9.43; P = 0.020) were found to be independent poor prognostic factors in HCC patients undergoing resection. The presence of CD66b+TINs was observed in 66 (57.4%) patients. However, CD66b+TINs were not associated with recurrence-free survival and OS.
Conclusions
Our study identified low CD66b+TINs in resectable HCC, and CD66b+ TINs did not have a significant role for the clinical outcomes of patients undergoing curative resection. The results suggest that TINs may play a role in more advanced stages of HCC.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is one of the most common cancer and third leading cause of cancer death worldwide [1]. Although hepatic resection is the treatment of choice as a curative purpose in HCC, high recurrence rate limit the curative purpose in most patients [2]. Predicting the prognosis, including recurrence, is crucial in identifying appropriate interventions that can improve patients outcomes. Various clinical and pathological factors have been reported to predict the prognosis, such as tumor marker, tumor stage, and tumor differentiation grade. In recent years, numerous inflammatory markers have also been suggested as useful for predicting prognosis in various cancers [3,4,5].
There is growing evidence suggesting a link between inflammation and cancer [6, 7]. Tumor-infiltrating immune cells, including T cells, B cells, natural killer cells, and myeloid lineage leukocytes, are considered essential components of the host immune response against cancer, contributing to either pro-tumoral or anti-tumoral immunity. Several studies have reported the potential role of tumor-infiltrating immune cells as a prognostic factor for various malignancies [8,9,10,11,12].
Neutrophils are short-lived effector cells that play a crucial role in the immune system`s response to infections and inflammation. However, due to their short lifespan and non-proliferative characteristics, their essential role in cancer has often been underestimated. In the context of cancer, neutrophils can have both pro-tumor and anti-tumor effects, depending on the stage and type of cancer [13, 14]. They can promote tumor growth by releasing growth factors and inflammatory molecules that stimulate angiogenesis and promote tumor cell survival. On the other hand, they can also suppress the immune system`s response to the tumor, which can help the tumor evade detection and elimination by the immune system.
Previous research has shown that neutrophil to lymphocyte ratio (NLR) can be a useful biomarker for predicting cancer prognosis and response to treatment. Many studies have reported that a high NLR has been associated with poor outcomes in HCC [15]. Additionally, some studies have suggested the role of tumor-infiltrating neutrophils (TINs) in the local microenvironment [8,9,10, 12, 16, 17]. However, the prognostic role of TINs remains controversial due to the plasticity of neutrophil in the tumor microenvironment (TME). Furthermore, there has been no report regarding the correlation between the peripheral neutrophils and TINs. Therefore, in this study, we aimed to investigate the clinical significance of TINs and the correlation between the peripheral neutrophil and TINs in HCC undergone curative resection.
Patients and methods
Patients
We conducted a retrospective analysis of patients who underwent curative resection between 2009 and 2012. Curative resection was defined as removal of the tumor with a tumor-free margin and no residual tumors in the remaining liver. Patients were followed up after the hepatic resection, and laboratory measurements including alpha-fetoprotein (AFP) and dynamic computed tomography (CT) or magnetic resonance imaging were performed every 3 months for first 2–3 years and then every 6 months. Treatment for tumor recurrence followed generally accepted guidelines [18, 19]. The median follow-up duration was 101.5 months (range, 1–138 months). Recurrence free survival (RFS) was defined as the length of time from hepatic resection to recurrence or death. Overall survival (OS) was defined as the interval between the dates of hepatic resection and death.
Clinicopathological analysis
We reviewed the demographics and laboratory test findings of patients, including age, gender, hepatitis status, tumor marker (AFP), and NLR. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. We also reviewed the histopathological findings of the HCC, such as tumor size, tumor number, tumor differentiation, vascular invasion, and non-tumor liver pathology. The histologic tumor differentiation was determined according to the criteria of Edmondson and Steiner [20]. Grades I and II were considered well and moderately differentiated HCC, respectively, and grades III and IV were considered as poor-differentiated HCC.
Immunohistochemical study and quantitative analysis
Representative 4-µm sections of formalin-fixed paraffin-embedded tissues were cut on charged glass slides. Slides were placed in a 65 \(^\circ{\rm C}\) oven for 20 min to dry tissues. Immunostaining was performed using a Bond-Max (Leica, Wetzlar, Germany) or a Ventana Benchmark ULTRA (Roche Diagnostics, Basel, Switzerland) staining instrument as described by the manufacturer. Briefly, paraffin wax was removed using Bond Dewax Solution (AR9222, Novocastra, Newcastle upon Tyne, UK) or EZ prep solution (Ventana Medical Systems, Mountain View, CA, USA) at 72 \(^\circ{\rm C}\) and sections were rehydrated. Heat-induced epitope retrieval was achieved using Bond Epitope Retrieval Solution 2 (EDTA-based buffer) for 20 min at 100 \(\mathrm{^\circ{\rm C} }\) or Ultra Cell Conditioning Solution 1 (Ventana Medical Systems, Mountain View, CA, USA) for 64 min at 100 \(\mathrm{^\circ{\rm C} }\). Sections were then incubated with antibodies CD3 (A0452 polyclonal, 1:200; DAKO, Denmark) and CD66b (ab197678 polyclonal, 1:200; Abcam) for 20 min at room temperature. Monoclonal antibodies CD4 (790–4423 clone No. SP35; Roche Ventana) and CD8 (790–4460 clone No. SP57; Roche Ventana) which are prediluted ready-to-use solutions for Ventana Benchmark automated staining system were diluted 1:2 and used for Bond-Max system. Finally, 3,3′-diaminobenzidine tetrahydrochloride was used as the chromogen. The human tonsil tissue was used as a positive control.
Whole-slide images (WSIs) of CD3, CD4, CD8, and CD66b staining were acquired using the Pannoramic 250 Flash III (3DHistech Ltd., Budapest, Hungary), analyzed using the 3DHistech CaseViewer software and antibody-positive cells were counted using the 3DHistech QuantCenter module.
Statistical analysis
RFS and OS were calculated using Kaplan–Meier method, and statistical evaluation was performed using a log-rank test. Multivariate Cox proportional hazards regression model was used to evaluate the association between clinicopathological factors and survival. All statistical analyses were performed with SPSS software 21.0 (SPSS Inc., Chicago, IL, USA). P-values of less than 0.05 were considered statistically significant.
Results
Patient characteristics
This study included 115 patients with HCC who underwent curative hepatectomy. The clinicopathological factors are summarized in Table 1. The median age was 59 years (range, 31–80), and the majority of patients were male (80%). Among the patients, 80% had hepatitis B virus, and 63.5% of HCC patients had underlying liver cirrhosis. 76 patients (66.1%) had early-stage HCC (Barcelona Clinic Liver Cancer (BCLC) 0/A stage). 43.5% presented with well/moderate differentiated HCC, and vascular invasion was identified in 27.8% of patients.
Association between clinicopathological factors and prognosis
The median duration of follow-up was 101.5 months (range, 1–138 months). The 1-year, 3-year, 5-year and 8 year OS were 89.6%, 72.9%, 72.0%, and 61.5% respectively. The 1-year, 3-year, 5-year and 8 year RFS were 92.1%, 71.5%, 55.2%, and 43.7% respectively.
To identify clinically significant factors, univariate analysis was conducted on survival data. In the univariate analysis, AFP (P = 0.016) and BCLC stage (P = 0.001) were associated with OS. Subsequently, both AFP and BCLC stage, which showed significance in the univariate analysis, were included in the multivariate Cox proportional hazards regression model. In the multivariate analysis, both AFP ≥ 100 ng/mL (HR, 2.74, 95% CI, 1.17–6.44; P = 0.021) and BCLC B/C stage (HR, 3.98, 95% CI, 1.68–9.43; P = 0.020) were associated with poor OS (Table 2). However, no prognostic factors were found to be associated with RFS (Table 3).
Although we investigated the relationship between the expression of CD3, CD4, CD8 and CD66b in HCC and the clinicopathological features to further characterize the function of tumor infiltrating immune cells in HCC patients, we did not find any correlation (Table 4).
Relationship between TINs expressing CD66b and survival
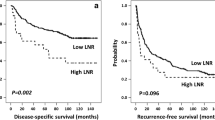
The densities of CD3, CD4, CD8 and CD66b-expressing immune cells were analyzed in the tumor tissues (Fig. 1). While CD66b+TINs was present in 66 (57.4%) patients, there were no statistically significant differences in RFS and OS according to TINs (Fig. 2). Survival analysis was also performed based on CD3, CD4 and CD8 expression; however, no significant association with survival were observed (data not shown). Additionally, there was no correlation found between the peripheral neutrophil level and TINs in patients with resectable HCC.
Discussion
Neutrophils play active roles in the TME and exhibit various prognostic effects depending on the cancer types and treatment methods [13]. In this study, we investigated the long-term prognostic value of TINs in patients undergoing curative resection for HCC. Our findings indicate that TINs were not associated with survival.
The correlation between inflammation and cancer has been widely accepted [7], as inflammation predisposes to cancer development and promotes all stages of cancer progression. Cancers develop within a complex and dynamic TME that influences tumor growth, invasion, and metastasis. The TME consists of cancer cells, as well as surrounding stromal and immune cells, which are significant components of the TME. Immune cells are highly plastic due to the reciprocal interactions within the TME, continuously changing their phenotypic and functional characteristics into either pro-tumoral or anti-tumoral immunity [21]. To date, many studies have unveiled the prognostic significance of tumor infiltrating immune cells, such as B cells, CD8+ tumor infiltrating lymphocyte, T-regulatory cells, tumor associated macrophages, natural killer cells, and myeloid derived suppressor cells [22].
Neutrophils play a role in various diseases, including infectious diseases, metabolic, and autoimmune diseases. Neutrophils, as the most dominant immune cells, also play complicated and significant roles in cancer. In various inflammatory models, they have been shown to be essential for CD8 + T cells recruitment, priming, and activation [23]. Neutrophils exert both anti-tumoral and pro-tumoral functions in the initiation, growth and metastasis of cancer, and these functions are influence by different neutrophil phenotypes. In response to cytokine stimulation, neutrophils develop the ability to polarize to an anti-tumor (N1) or pro-tumor (N2) phenotype [24, 25]. The functional plasticity of neutrophils is regulated by molecules in the TME [13, 21].
Clinical data suggests that neutrophils contribute to the tumor development, and numerous studies have reported the prognostic significance of TINs in various cancers [8,9,10, 26,27,28,29]. The pathophysiological mechanism of HCC is not completely understood, but it is thought to result from a multistep process involving molecular alteration, genomic instability, and chronic inflammation [30]. Tumor-related chronic inflammation alters the TME by promoting the infiltration of several immune cell populations, which promotes HCC development [7]. The immunological characteristics of HCC are complex and vary dynamically due to underlying chronic inflammation and cirrhosis in most HCC patients [31].
Only three studies have investigated the prognostic value of tumor-associated neutrophils in HCC, and the results are not entirely consistent. Li et al. showed that the presence of CD66b+ intratumoral neutrophils was a poor prognostic factor for HCC after resection [32], and Wang et al. reported that CD66b+intratumoral neutrophils infiltration was an independent prognostic factor for poor survival for HCC, which is consistent with the study by Li et al. [16]. Schoenberg et al. investigated the prognostic value of tumor infiltrating leukocytes in the perivascular region and found that CD66+ cells were not significant, while perivascular-infiltrating CD3+, CD8+, and CD20+ cells independently predicted OS and disease free survival [17]. However, our results did not find any prognostic value of CD66+ TINs. There could be several possible explanations for this. One possible explanation is that our sample size may not have been large enough to detect a statistically significant difference. Another possible explanation is that the patient population included in our study had less advanced HCC compared to previous studies, and TINs may be more abundant in advanced cancer compared to early-stage cancer [9, 28, 32]. In addition, TINs may interact with other factors, such as tumor infiltrating lymphocytes, cytokines, and chemokines, which could have masked any potential prognostic value of TINs. Overall, the heterogeneity of HCC and its complex immunological characteristics make it difficult to draw definitive conclusions about the prognostic value of TINs, and further research is needed to better understand their role in HCC development and progression.
This study has some limitations, including its retrospective design and small sample size, which may limit the generalizability of the findings. Further prospective studies with larger sample size are needed to confirm these results and explore the functional significance of TINs in HCC. In addition, the study only investigated the association between the level of TINs and clinical outcomes, and further mechanistic studies are needed to better understand the underlying mechanisms involved in the interactions between TINs and HCC.
In conclusion, this study suggests that TINs may not be a significant prognostic factor in HCC, highlighting the complex role of neutrophils in HCC. Further research is needed to gain a more complete understanding of the relationship between neutrophils and HCC, and to develop targeted immunotherapies that can effectively harness the power of the immune system to overcome HCC.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to protect the privacy of the patient, but are available from the corresponding author on reasonable request.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- BCLC:
-
Barcelona Clinic Liver Cancer
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hazard ratio
- NLR:
-
Neutrophil to lymphocyte ratio
- OS:
-
Overall survival
- RFS:
-
Recurrence free survival
- TINs:
-
Tumor-infiltrating neutrophils
- TME:
-
Tumor microenvironment
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359(19):2045–7.
Ishibashi Y, Tsujimoto H, Yaguchi Y, Kishi Y, Ueno H. Prognostic significance of systemic inflammatory markers in esophageal cancer: systematic review and meta-analysis. Ann Gastroenterol Surg. 2020;4(1):56–63.
Kim MR, Kim AS, Choi HI, Jung JH, Park JY, Ko HJ. Inflammatory markers for predicting overall survival in gastric cancer patients: a systematic review and meta-analysis. PLoS One. 2020;15(7):e0236445.
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99.
Wang J, Bo X, Suo T, Liu H, Ni X, Shen S, Li M, Xu J, Liu H, Wang Y. Tumor-infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer. Cancer Sci. 2018;109(7):2266–74.
Wang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G, Cheng Y. The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med. 2014;12:7.
Wang J, Liu L, Bai Q, Ou C, Xiong Y, Qu Y, Wang Z, Xia Y, Guo J, Xu J. Tumor-infiltrating neutrophils predict therapeutic benefit of tyrosine kinase inhibitors in metastatic renal cell carcinoma. Oncoimmunology. 2019;8(1):e1515611.
Knief J, Reddemann K, Petrova E, Herhahn T, Wellner U, Thorns C. High Density of Tumor-infiltrating B-Lymphocytes and Plasma Cells Signifies Prolonged Overall Survival in Adenocarcinoma of the Esophagogastric Junction. Anticancer Res. 2016;36(10):5339–45.
Schoenberg MB, Li X, Li X, Han Y, Hao J, Miksch RC, Koch D, Borner N, Beger NT, Bucher JN, et al. The predictive value of tumor infiltrating leukocytes in hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2021;47(10):2561–70.
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–46.
Galdiero MR, Varricchi G, Loffredo S, Mantovani A, Marone G. Roles of neutrophils in cancer growth and progression. J Leukoc Biol. 2018;103(3):457–64.
Lin S, Hu S, Ran Y, Wu F. Neutrophil-to-lymphocyte ratio predicts prognosis of patients with hepatocellular carcinoma: a systematic review and meta-analysis. Transl Cancer Res. 2021;10(4):1667–78.
Wang Y, Yao R, Zhang L, Xie X, Chen R, Ren Z. IDO and intra-tumoral neutrophils were independent prognostic factors for overall survival for hepatocellular carcinoma. J Clin Lab Anal. 2019;33(5):e22872.
Schoenberg MB, Hao J, Bucher JN, Miksch RC, Anger HJW, Mayer B, Mayerle J, Neumann J, Guba MO, Werner J, et al. Perivascular tumor-infiltrating leukocyte scoring for prognosis of resected hepatocellular carcinoma patients. Cancers (Basel). 2018;10(10):389.
Korean Liver Cancer Study G, National Cancer Center K. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15(3):391–423.
Bruix J, Sherman M. American Association for the Study of Liver D: Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2.
Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503.
Yuan S, Norgard RJ, Stanger BZ. Cellular Plasticity in Cancer. Cancer Discov. 2019;9(7):837–51.
Li HX, Wang SQ, Lian ZX, Deng SL, Yu K. Relationship between tumor infiltrating immune cells and tumor metastasis and its prognostic value in cancer. Cells. 2022;12(1):64.
Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8(3):125–58.
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94.
Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33(5):949–55.
Hajizadeh F, Aghebati Maleki L, Alexander M, Mikhailova MV, Masjedi A, Ahmadpour M, Hashemi V, Jadidi-Niaragh F. Tumor-associated neutrophils as new players in immunosuppressive process of the tumor microenvironment in breast cancer. Life Sci. 2021;264:118699.
Zhang WH, Wang WQ, Gao HL, Xu SS, Li S, Li TJ, Han X, Xu HX, Li H, Jiang W, et al. Tumor-infiltrating neutrophils predict poor survival of non-functional pancreatic neuroendocrine tumor. J Clin Endocrinol Metab. 2020;105(7):dgaa196.
Ishikawa R, Kadota K, Ikeda T, Yoshida C, Kimura N, Ibuki E, Go T, Yokomise H, Haba R. Prognostic impact of tumor-infiltrating lymphocytes and neutrophils in resected non-small cell lung carcinoma. Hum Pathol. 2022;125:87–96.
Yang R, Wang X, Ma C, Zhang Z, Liu N, Ma X, Zhang Y, Wang X, Liu Y. Tumor-infiltrating neutrophils and peripheral neutrophil-to-lymphocyte ratio conversely predicted the prognosis of patients with non-small cell lung cancer. Cell Immunol. 2022;379:104588.
Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas). 2019;55(9):526.
Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, Sarin SK. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer. 2013;2(3–4):367–83.
Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54(3):497–505.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: Young Mi Hong. Data curation: Young Mi Hong, Jung Hee Lee. Formal analysis: Young Mi Hong, Jung Hee Lee. Methodology: Young Mi Hong, Jung Hee Lee. Writing – original draft: Young Mi Hong. Writing – review & editing: Young Mi Hong, Jung Hee Lee.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was conducted in accordance to the ethical guidelines of the Declaration of Helsinki and was approved by the Institutional Review Boards of Pusan National University Yangsan Hospital. Requirement for informed consent was waived by the institutional review board of Pusan National Yangsan Hospital.
Consent for publication
Not applicable for our study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, J.H., Hong, Y.M. The relationship between tumor-infiltrating neutrophils and clinical outcomes in patients with resectable hepatocellular carcinoma. BMC Cancer 24, 327 (2024). https://doi.org/10.1186/s12885-024-12074-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12074-3