Abstract
Objectives
For drugs reimbursed with limited evidence of patient benefits, confirmatory evidence of overall survival (OS) and quality of life (QoL) benefits is important. For QoL data to serve as valuable input to patients and decision-makers, it must be measured and analyzed using appropriate methods. We aimed to assess the measurement and analyses of post-reimbursement QoL data for cancer drugs introduced in Swedish healthcare with limited evidence at the time of reimbursement.
Methods
We reviewed any published post-reimbursement trial data on QoL for cancer drugs reimbursed in Sweden between 2010 and 2020 with limited evidence of improvement in QoL and OS benefits at the time of reimbursement. We extracted information on the instruments used, frequency of measurement, extent of missing data, statistical approaches, and the use of pre-registration and study protocols.
Results
Out of 22 drugs satisfying our inclusion criteria, we identified published QoL data for 12 drugs in 22 studies covering multiple cancer types. The most frequently used QoL instruments were EORTC QLQ-C30 and EQ-5D-3/5L. We identified three areas needing improvement in QoL measurement and analysis: (i) motivation for the frequency of measurements, (ii) handling of the substantial missing data problem, and (iii) inclusion and adherence to QoL analyses in clinical trial pre-registration and study protocols.
Conclusions
Our review shows that the measurements and analysis of QoL data in our sample of cancer trials covering drugs initially reimbursed without any confirmed QoL or OS evidence have significant room for improvement. The increasing use of QoL assessments must be accompanied by a stricter adherence to best-practice guidelines to provide valuable input to patients and decision-makers.
Similar content being viewed by others
Background
Alongside the objective of assessing cancer drugs in terms of Overall Survival (OS) improvements, there is an increasing focus on assessing patient-reported outcomes, such as self-assessed Quality of Life (QoL) [1,2,3,4]. Many healthcare jurisdictions also rely on cost-effectiveness analysis with Quality-Adjusted Life-Years (QALYs) as an outcome measure to inform reimbursement and coverage decisions, which requires combining QoL and OS data [5]. Valid measurements and analyses of QoL in cancer trials are fundamental for healthcare decision-makers and patients since such analyses can facilitate well-informed priority-setting and lead to better patient-centered care. Analyses of QoL endpoints in cancer drug treatments are essential in the absence of survival data or when improvements in survival are unlikely.
At the time of regulatory market authorization of cancer drugs, it is frequent that data on OS or QoL confirming clinical benefits is lacking. Market authorization decisions are instead often based on intermediate (surrogate) endpoints such as Progression-Free Survival (PFS) and Response Rates [6,7,8,9], which frequently lack validation as predictors of long-term OS and QoL benefits. Following market authorization, many healthcare systems rely on subsequent Health Technology Assessments (HTA) to decide on reimbursement and to inform pricing negotiations [10,11,12]. The HTAs usually depend on the same pivotal trial data underlying the market authorization for the clinical- and cost-effectiveness modeling, facing challenges with immature OS data and a lack of published QoL data. Therefore, it is in the interest of patients and payers to learn if OS or QoL benefits can be confirmed post-reimbursement based on updated analyses of the pivotal trials or from new controlled or pragmatic trials [13, 14]. In addition, the frequent reimbursements of high-cost cancer drugs based on uncertain surrogate endpoints [15] call for more post-reimbursement studies on patient-relevant outcomes using valid and reliable measures and analytical methods [16,17,18].
Cancer patients’ quality of life, including health-related QoL, can be measured using generic, cancer-generic, or cancer-indication-specific instruments [19, 20]. Generic instruments that can be applied across health conditions, such as the EQ-5D instrument [21], facilitate comparative analyses of patient benefits across therapeutic areas, which is relevant for decision-makers and horizontal priority setting. Cancer-specific instruments, such as the EORTC QLQ-C30 [22], or even more detailed indication-specific instruments, may capture more granular aspects of QoL, which can be particularly important for clinical decision-making and vertical priority setting. Irrespective of the instruments used, shortcomings have been identified in terms of a lack of defined hypotheses, lack of analyses to account for missing data and multiple hypothesis testing, lack of discussions of clinical significance [23,24,25,26,27], and absent or deficient study protocols [28]. There have been several calls to improve the validity and reliability of QoL assessments in cancer trials by standardization and guidelines on high-quality reporting, including recommendations from the Consolidated Standards of Reporting Trials (CONSORT-PRO) [29] and the Standards in Analyzing Patient-Reported Outcomes and Quality of Life (SISAQOL) Consortium [30]. Despite the increased attention to QoL analyses in cancer trials, no previous study has specifically addressed post-reimbursement follow-ups of drugs approved with initially limited evidence on OS and QoL.
In this study, we aim to assess measurements and analyses of QoL in post-reimbursement studies of cancer drugs to identify potential areas with room for improvement in cancer trial QoL research. Specifically, we analyze cancer drugs introduced in Swedish healthcare with limited evidence, defined as lacking randomized trial data or analysis showing any statistically significant OS or QoL benefits at the time of reimbursement. In Sweden, the Pharmaceutical and Benefits Agency (TLV) decides on the reimbursement of prescription drugs, and a large majority of all cancer drugs with European Medicines Agency (EMA) market authorization receive reimbursement [9]. In a previous study, we identified all reimbursed cancer drugs between 2010 and 2020 that had limited evidence of OS and QoL benefits at the time of reimbursement. We also analyzed how many drugs subsequently verified benefits in post-reimbursement studies [31].
The present study concerns the approaches to measurement and analysis of QoL data used to verify patient-relevant benefits, and our contributions are twofold. First, we analyze the reporting and analytical choices of QoL data specifically for cancer drugs that lacked statistically significant evidence on patient-centered outcomes (QoL and OS) at the time of reimbursement. This addresses to what extent such evidence is generated post-reimbursement to reduce the uncertainty of the benefits of these drugs, and to the best of our knowledge, this has not been the focus in the previous literature. Second, we compare the adherence of reporting and analytical choices of QoL data to what was outlined in clinical trial pre-registrations, study protocols, and statistical analysis plans, and we provide novel results regarding to what extent the analyses can be seen as confirmatory or merely exploratory. The findings from this study can be used to identify areas with room for improvement in the measurement and analysis of QoL data in cancer trials.
Methods
Identification of cancer drug indications with post-reimbursement QoL data
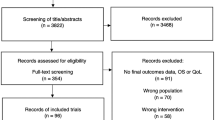
We identified prescription cancer drug indications approved by TLV between 2010 and 2020 where the producer claimed a therapeutic benefit compared to the standard of care but where the evidence base for this argument was limited. We defined limited evidence as drugs where no randomized trial data showed statistically significant (p < 0.05) OS or QoL benefits in published studies or based on the material submitted to TLV. Instead, the therapeutic benefit supposition was based on improvements in surrogate/intermediate endpoints and/or based on single-arm trials. From a total of 60 reimbursement applications, 46 drug indications were approved by TLV to be included in the Swedish Pharmaceutical Benefits Scheme. Of those 46 drug indications, 22 had limited evidence at the time of a favorable reimbursement decision.
For each of these 22 drug indications, we searched PubMed and Clinicaltrials.gov for any post-reimbursement evidence on QoL or OS from randomized controlled trials (RCT) until September 2022. We used the following search string to identify post-reimbursement studies for each of the 22 drug indications: “active substance name OR drug brand name AND cancer form AND Cochrane Highly Sensitivity Search Strategy for identifying randomized trials” [31]. We included new publications from the original pivotal trial and any other randomized (clinical or pragmatic) trials related to the specific indication for each reimbursement decision. We allowed for the inclusion of studies irrespective of whether the research objective was explicitly outlined to demonstrate clinical benefit based on an apriori hypothesis or if the objective was to conduct exploratory QoL analyses. We have previously published the full details of the search protocol and the results documenting the number of drug indications with any RCT post-reimbursement data on OS or QoL [31]. The search strategy and Flow-diagram are also presented in the Supplementary material. The work in this study was based on publicly accessible information and did not involve individual patient information. No formal ethical approval was therefore required.
Data extraction and analysis
Two authors (MS and NiJ) independently extracted information using a pre-defined data extraction template. The data extraction template covered basic study information and several established quality indicators of research design and statistical practice: the study and year, cancer type/drug indication, QoL instrument(s) used, the scoring system or tariffs used to summarize QoL data, the frequency of QoL measurements, the statistical analyses of the QoL scores, proportion of missing QoL data, if missing data adjustments were conducted, if a detailed study protocol or statistical analysis plan describing data collection and statistical analyses were published, and if all QoL instruments in the study were listed in the pre-registration on clinicaltrials.gov before the analyses were conducted (before finalizing data collection). Disagreements in the extracted data were resolved by discussion, and the data is presented using descriptive statistics.
Results
We identified post-reimbursement RCT data on QoL in 22 studies covering 12 of the 22 included drug indications (Supplementary material, Figure S1 & Table S1). Of the 22 studies, five were published between 2010 and 2016 and 17 between 2017 and 2022. The indications covered by the studies included the following cancers: lung, breast, kidney, ovarian, leukemia, and melanoma. A total of 4 of the 22 studies reported statistically significant (p < 0.05) QoL benefits (statistically significant QoL benefits for the reimbursed drug compared to the control arm). In contrast, the other 18 studies reported findings where the QoL data were not statistically significantly different between the reimbursed drug and the comparator or that the reimbursed drug had statistically significant detrimental QoL effects. Table 1 summarizes the studies in terms of the QoL measurement and analysis.
The most commonly used instruments for generic and cancer-generic QoL assessments were EQ-5D-3 L/5L and EORTC QLQ-C30 (used in 8 of 22 studies, respectively). In addition, the Karnofsky Performance Status Scale (KPSS) was used in 2 of the included studies. Of the indication-specific instruments, EORTC QLQ-LC13 was frequently used in lung cancer (4 of 6 studies). For renal cell carcinoma, the Functional Assessment of Cancer Therapy-Kidney Symptom Index was used in all identified studies (FKSI-DRS), as was the Functional Assessment of Cancer Therapy–Ovarian (FACT-O) in the identified studies on ovarian cancer. In breast cancer, EORTC-B23 was identified in 1 out of 6 studies and FACT-B in 3 out of 6 studies.
Table 1 also shows the number of studies with pre-registered trial protocols, including QoL measurement and analysis information. Nine studies had listed the QoL instruments in their pre-registration on Clinicaltrials.gov, and seven studies had published a study protocol or statistical analysis plan, including the QoL instruments and analyses (before manuscript submission).
The frequency of measurements differed to some extent between the included studies, and generally, no specific motivation was reported for the frequency of measurement. Several trials assessed QoL on the first day of each 28-day treatment cycle for the first few cycles and then less frequently in later cycles. Most studies finalized assessment at progression or treatment discontinuation, whereas a few studies continued QoL assessment in progressed states (details in Table 2).
The statistical analyses differed depending on the instrument and scoring system used. Most frequently, the analyses assessed between-group differences in the mean magnitude of change from baseline using longitudinal mixed-effect models (17 of 22 studies). The second most common analytical approach was to analyze the time to worsening with Kaplan-Meier and/or Cox regression methods (9 of 22 studies). Worsening in QLQ-C30 was consistently defined as a reduction by 10 points or more compared to the baseline score, but for other instruments, the definition of worsening varied (see Table 2). Finally, 4 of 22 studies analyzed the proportion of responders, similar to time to worsening, but typically based on assessing the proportion of patients not having worsened (i.e., maintaining or improving QoL) at a specific follow-up time. The proportion of responders analyses is thus typically modeled using binary outcome models (such as logistic regression).
Regarding missing data, some studies (6 of 22) reported the proportion missing for all measurement points (typically as supplementary material). In contrast, another set of studies reported the proportion of missing observations for a few specific time points (11 of 22), whereas five studies provided no data on the proportion of missing responses. Among the studies that reported missing data, the proportion of missing responses at 12 months follow-up (or close to 12 months) varied between 2 and 40%. The proportion of missing data was based on calculations where patients not responding at baseline and patients dropping out of the study due to disease progression were not included in the denominator. Despite the prevalent missing data problem, only two studies used a statistical approach to address the impact of missingness. The approaches to assessing the impact of missing data included pattern-mixture models and stratified analyses in sub-groups with varying missing data patterns. The full details of instruments used, frequency of assessment, and statistical analyses are shown in Table 2.
Discussion
We reviewed QoL measurements and statistical analyses applied in published RCTs after reimbursement for cancer drugs that were initially reimbursed and introduced in Swedish healthcare with a lack of evidence of QoL and OS benefits. We identified any new publications from the original pivotal trial and any other trials for the same patient indication. Considering the increasing share of reimbursements based on surrogate endpoints and single-arm trials, it is essential to assess what type of robust evidence becomes available in the post-reimbursement period to confirm claims of clinical benefit– and that any QoL data becoming available is based on valid measures and analyses of QoL [31]. Out of 22 cancer drug indications reimbursed with limited evidence, we identified and reviewed RCTs for 12 drugs in 22 published studies. EORTC QLC-C30 [15] and EQ-5D-3/5L [14] were the most frequently used instruments. Both these instruments have previously been reported as the most commonly used instruments in cancer trials [23, 27, 32], and some studies have shown success in mapping QoL scores between these instruments [33, 34]. In addition, indication-specific instruments that were used included, e.g., EORTC QLC-LC13 (in 4 out of 6 lung cancer studies), FKSI-DRS (used in 5 out of 5 studies on renal cell carcinoma), and FACT-O (used in 4 out of 4 studies on ovarian cancer), and FACT-B (used in 3 out of 6 studies on breast cancer).
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) encourage the assessment of QoL in cancer RCTs. In addition, an increasing number of payers consider QoL to be a valuable input to reimbursement and coverage decisions [35], and QoL data is a necessary input in cost-effectiveness analyses using QALYs as a health outcome measure. However, QoL data must be measured and analyzed validly and reliably to provide valuable insights to decision-makers and clinicians. The FDA has hesitated to grant QoL labeling of the product’s benefits, which may be attributed to uncertainties and a lack of quality in the measurement and analytical standards for QoL data [35, 36]. There are several calls for standardization of design, reporting, and statistical analyses of QoL data, including initiatives by the, e.g., SPIRIT-PRO [37], CONSORT-PRO [29], and SISAQOL [30] consortiums.
Our review highlights several relevant aspects with room for improvement. We found that several studies lacked information on the scoring system used, which, particularly for the EQ-5D and the range of available tariffs to predict QoL scores, can substantially impact the interpretation and comparability of results [38]. The frequency of measurements varied across studies and was rarely explicitly motivated– some studies assessed QoL every cycle (28 days), whereas other studies had three-month intervals. There may be well-motivated reasons for variations in the frequency of assessment between disease and study contexts, but this is difficult to identify when the rationale is not outlined. The approach for the timing of the last assessment also varied between studies. Some studies assessed QoL until disease progression and treatment discontinuation. In contrast, others assessed QoL until death or the last study follow-up, and in some studies, we could not identify information on the procedure for the timing of the final assessment. For QoL evidence to inform treatment decisions, capturing QoL consequences after disease progression is essential, particularly in settings where overall survival benefits have not been demonstrated [39]. For proper value assessments and cost-effectiveness analyses of drugs, it is also necessary to have valid QoL data both in the progression-free and progressed disease states.
Regarding the statistical analyses of the data, most studies used approaches that align with recommendations from, e.g., SISAQOL [30], including linear mixed-effects models for repeated measurements to assess between-treatment group differences in mean changes from baseline. However, many studies failed to report all relevant modeling choices, such as which covariates were included as control variables or whether variables (including time indicators) were treated as random or fixed effects. Besides including baseline QoL, additional covariates associated with the QoL outcome are often recommended to include to improve precision and power. Such covariates should be pre-defined in the study protocol or statistical analysis plan [40]. Based on our comparisons to clinical trial registrations and study protocols (including any statistical analysis plan referenced), only 9 of 22 studies listed all instruments analyzed in the study in the clinical trial registration before final data collection. Only seven had published their study protocol and included information for QoL analysis. Given the researcher’s degrees of freedom of choice involved in the measurement and analysis of QoL data, it is essential to have proper pre-registered documentation to increase the validity of the findings [41]. With the finding that most studies lacked appropriate pre-registration and study protocols, the QoL findings in these papers should primarily be interpreted as exploratory.
Finally, we found that many reviewed studies had substantial missing data. Among the studies that reported the share of missing data, it varied from only a few percent up to about 40%. However, the data on missingness generally excluded patients who did not complete baseline QoL assessments and those who declined follow-up in the study. Thus, the missing data shares can be considered conservative lower-bound estimates. In addition, despite the substantial missing data issue, only 2 out of 22 studies addressed the potential impact of missing data. This is a lower share than some earlier reviews of missing QoL data in cancer trials [25]. One of the studies addressing the missing data problem showed that missingness was associated with adverse QoL, implying that it is not reasonable to assume that data are missing at random [42]. The SISAQOL Consortium guidelines on handling missing data include nine items, e.g., that statistical approaches to assess missing data should be pre-specified in the protocol or statistical analysis plan and that at least two sensitivity analyses should be used [30]. Our review shows that missingness is an area where development is needed in QoL studies.
Our study has limitations that are important for interpretation and generalizability. First, our sample of included studies is based on identified published studies on cancer drugs for which there were no mature OS data or QoL data at the time of reimbursement. While our sample of studies thus reflects a practically relevant context where reimbursement decisions for costly drugs have been made based on limited evidence, the review cannot necessarily be interpreted as representative of the broader cancer drug trial literature. Second, it should be mentioned that several of the papers included in this review were published before recommendations for reporting and analysis by the SPIRIT-PRO extension (2018) and the SISAQOL Consortium Guidelines (2020). Thus, the papers included in this review should not be judged in terms of adherence to such reporting guidelines per se. Instead, we have used items from these guidelines as a proxy for what can be seen as best-practice reporting and analysis standards. Third, our study covers a subset of the criteria for best practices in the measurement and analyses of QoL data as outlined in the abovementioned guidelines. For example, this review does not assess whether the studies were explicit about a confirmatory or exploratory research objective, if any adjustments were considered for multiple hypothesis testing, or the type of potential arguments provided for the magnitude of missing data.
Conclusion
QoL data is increasingly used to inform regulatory decisions and facilitate more patient-centered care. For QoL data to serve as input to improved patient outcomes and decision-making, QoL data must be measured and analyzed using appropriate methods. We documented several deviations from high-quality measurement and analysis standards for clinical trials based on reviews of post-reimbursement studies for cancer drugs initially reimbursed in Swedish healthcare with limited evidence of QoL or OS benefits. Our results suggest areas of improvement in QoL assessments related to handling missing data, motivations for the measurement frequency, and pre-trial protocol registration and adherence. Given the increasing focus on patient-reported outcomes and the use of QoL data, future QoL assessments from cancer drug trials must be conducted with stricter adherence to best-practice guidelines to provide valuable input to patient care and decision-makers.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
EMA. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. London: European Medicines Agency; 2005.
Kluetz PG, O’Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19(5):e267–e74.
Fiero MH, Pe M, Weinstock C, King-Kallimanis BL, Komo S, Klepin HD, et al. Demystifying the estimand framework: a case study using patient-reported outcomes in oncology. Lancet Oncol. 2020;21(10):e488–e94.
Bottomley A. The cancer patient and quality of life. Oncologist. 2002;7(2):120–5.
Siverskog J, Henriksson M. On the role of cost-effectiveness thresholds in healthcare priority setting. Int J Technol Assess Health Care. 2021;37:e23.
Grössmann N, Robausch M, Rosian K, Wild C, Simon J. Monitoring evidence on overall survival benefits of anticancer drugs approved by the European Medicines Agency between 2009 and 2015. Eur J Cancer. 2019;110:1–7.
Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ. 2017;359:j4530.
Schnog J-JB, Samson MJ, Gans ROB, Duits AJ. An urgent call to raise the bar in oncology. Br J Cancer. 2021;125(11):1477–85.
Chauca Strand G, Bonander C, Jakobsson N, Johansson N, Svensson M. Assessment of the clinical and cost-effectiveness evidence in the reimbursement decisions of new cancer drugs. ESMO Open. 2022;7(5):100569.
Beletsi A, Koutrafouri V, Karampli E, Pavi E. Comparing Use of Health Technology Assessment in Pharmaceutical Policy among earlier and more recent adopters in the European Union. Value Health Reg Issues. 2018;16:81–91.
WHO. 2015 global survey on health technology assessment by national authorities. Geneva: World Health Organization; 2015. https://www.who.int/health-technology-assessment/MD_HTA_oct2015_final_web2.pdf.
Hutton J, McGrath C, Frybourg JM, Tremblay M, Bramley-Harker E, Henshall C. Framework for describing and classifying decision-making systems using technology assessment to determine the reimbursement of health technologies (fourth hurdle systems). Int J Technol Assess Health Care. 2006;22(1):10–8.
Hwang TJ, Gyawali B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int J Cancer. 2019;144(7):1746–51.
Shukuya T, Mori K, Amann JM, Bertino EM, Otterson GA, Shields PG, et al. Relationship between overall survival and response or progression-free survival in Advanced Non-small Cell Lung Cancer patients treated with Anti-PD-1/PD-L1 antibodies. J Thorac Oncol. 2016;11(11):1927–39.
Gellad WF, Kesselheim AS. Accelerated approval and expensive drugs - A challenging combination. N Engl J Med. 2017;376(21):2001–4.
Fashoyin-Aje LA, Mehta GU, Beaver JA, Pazdur R. The On- and off-ramps of Oncology Accelerated approval. N Engl J Med. 2022;387(16):1439–42.
Spreafico A, Hansen AR, Abdul Razak AR, Bedard PL, Siu LL. The future of clinical Trial Design in Oncology. Cancer Discov. 2021;11(4):822–37.
Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017;15:134.
Dawoud D, Lamb A, Moore A, Bregman C, Rupniewska E, Paling T, et al. Capturing what matters: updating NICE methods guidance on measuring and valuing health. Qual Life Res. 2022;31(7):2167–73.
Karimi M, Brazier J. Health, Health-Related Quality of Life, and quality of life: what is the difference? PharmacoEconomics. 2016;34(7):645–9.
Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–43.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Fiteni F, Anota A, Westeel V, Bonnetain F. Methodology of health-related quality of life analysis in phase III advanced non-small-cell lung cancer clinical trials: a critical review. BMC Cancer. 2016;16:122.
Brundage M, Bass B, Davidson J, Queenan J, Bezjak A, Ringash J, et al. Patterns of reporting health-related quality of life outcomes in randomized clinical trials: implications for clinicians and quality of life researchers. Qual Life Res. 2011;20(5):653–64.
Safa H, Tamil M, Spiess PE, Manley B, Pow-Sang J, Gilbert SM, et al. Patient-reported outcomes in clinical trials leading to Cancer Immunotherapy Drug approvals from 2011 to 2018: a systematic review. JNCI: J Natl Cancer Inst. 2020;113(5):532–42.
Bylicki O, Gan HK, Joly F, Maillet D, You B, Péron J. Poor patient-reported outcomes reporting according to CONSORT guidelines in randomized clinical trials evaluating systemic cancer therapy. Ann Oncol. 2015;26(1):231–7.
Hamel J-F, Saulnier P, Pe M, Zikos E, Musoro J, Coens C, et al. A systematic review of the quality of statistical methods employed for analysing quality of life data in cancer randomised controlled trials. Eur J Cancer. 2017;83:166–76.
Kyte D, Retzer A, Ahmed K, Keeley T, Armes J, Brown JM, et al. Systematic evaluation of patient-reported Outcome Protocol Content and reporting in Cancer trials. J Natl Cancer Inst. 2019;111(11):1170–8.
Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–22.
Coens C, Pe M, Dueck AC, Sloan J, Basch E, Calvert M, et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. 2020;21(2):e83–e96.
Chauca Strand G, Johansson N, Jakobsson N, Bonander C, Svensson M. Cancer drugs reimbursed with limited evidence on overall survival and quality of life: do Follow-Up studies confirm patient benefits? Clin Drug Investig. 2023.
Ciani O, Meregaglia M, De Lorenzo F, Perrone F, Pinto C. Patient-reported outcome measures in Oncology drugs approved by the European Medicines Agency, 2017–2021. JAMA Netw Open. 2023;6(1):e2251564–e.
Khan I, Morris S, Pashayan N, Matata B, Bashir Z, Maguirre J. Comparing the mapping between EQ-5D-5L, EQ-5D-3L and the EORTC-QLQ-C30 in non-small cell lung cancer patients. Health Qual Life Outcomes. 2016;14(1):60.
Gray LA, Hernandez Alava M, Wailoo AJ. Mapping the EORTC QLQ-C30 to EQ-5D-3L in patients with breast cancer. BMC Cancer. 2021;21(1):1237.
Gnanasakthy A, Barrett A, Evans E, D’Alessio D, Romano C. A review of patient-reported outcomes labeling for Oncology drugs approved by the FDA and the EMA (2012–2016). Value Health. 2019;22(2):203–9.
Ge C, Guo K, Li Y, Li G, Zhang H, Yang J, et al. Analysis of patient-reported outcomes in the approval of novel oncology drugs in the United States, 2017–2022. EClinicalMedicine. 2023;59:101953.
Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan A-W, King MT, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO Extension. JAMA. 2018;319(5):483–94.
Gerlinger C, Bamber L, Leverkus F, Schwenke C, Haberland C, Schmidt G, et al. Comparing the EQ-5D-5L utility index based on value sets of different countries: impact on the interpretation of clinical study results. BMC Res Notes. 2019;12(1):18.
Bouberhan S, Shea M, Cannistra SA. Maintenance PARP inhibitor therapy: is maintaining quality of life enough? Lancet Oncol. 2018;19(10):e504.
EMA. Guideline on adjustment for baseline covariates in clinical trials, EMA/CHMP/295050/2013. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-adjustment-baseline-covariates-clinical-trials_enpdf. 2015.
Cook BG, Wong VC, Fleming JI, Solari EJ. Pre-registration of Randomized controlled trials. Res Social Work Pract. 2022;in press0:10497315221121117.
Beaumont JL, Butt Z, Baladi J, Motzer RJ, Haas T, Hollaender N, et al. Patient-reported outcomes in a phase iii study of everolimus versus placebo in patients with metastatic carcinoma of the kidney that has progressed on vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy. Oncologist. 2011;16(5):632–40.
Pérol M, Pavlakis N, Levchenko E, Platania M, Oliveira J, Novello S, et al. Patient-reported outcomes from the randomized phase III ALEX study of alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer. Lung Cancer. 2019;138:79–87.
Li Z, Zhao J. Clinical efficacy and safety of crizotinib and alectinib in ALK-positive non-small cell lung cancer treatment and predictive value of CEA and CA125 for treatment efficacy. Am J Transl Res. 2021;13(11):13108–16.
Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–86.
Leighl NB, Karaseva N, Nakagawa K, Cho BC, Gray JE, Hovey T, et al. Patient-reported outcomes from FLAURA: Osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer. 2020;125:49–57.
Lee CK, Novello S, Rydén A, Mann H, Mok T. Patient-reported symptoms and impact of treatment with Osimertinib Versus Chemotherapy in Advanced Non-small-cell Lung Cancer: the AURA3 trial. J Clin Oncol. 2018;36(18):1853–60.
Nie K, Zhang Z, Zhang C, Geng C, Zhang L, Xu X, et al. Osimertinib compared docetaxel-bevacizumab as third-line treatment in EGFR T790M mutated non-small-cell lung cancer. Lung Cancer. 2018;121:5–11.
Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus Sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14(6):552–62.
Qin S, Bi F, Jin J, Cheng Y, Guo J, Ren X, et al. Axitinib versus Sorafenib as a second-line therapy in Asian patients with metastatic renal cell carcinoma: results from a randomized registrational study. Onco Targets Ther. 2015;8:1363–73.
Cella D, Escudier B, Tannir NM, Powles T, Donskov F, Peltola K, et al. Quality of life outcomes for cabozantinib versus everolimus in patients with metastatic renal cell carcinoma: METEOR phase III randomized trial. J Clin Oncol. 2018;36(8):757–64.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–65.
Friedlander M, Gebski V, Gibbs E, Davies L, Bloomfield R, Hilpert F, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018;19(8):1126–34.
Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA 3rd, Bidziński M, et al. Olaparib Versus Nonplatinum Chemotherapy in patients with platinum-sensitive relapsed ovarian Cancer and a germline BRCA1/2 mutation (SOLO3): a Randomized Phase III Trial. J Clin Oncol. 2020;38(11):1164–74.
Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, et al. Olaparib with or without Cediranib Versus Platinum-based chemotherapy in recurrent platinum-sensitive ovarian Cancer (NRG-GY004): a randomized, Open-Label, phase III trial. J Clin Oncol. 2022;40(19):2138–47.
Oza AM, Matulonis UA, Malander S, Hudgens S, Sehouli J, Del Campo JM, et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2018;19(8):1117–25.
Robertson JFR, Cheung K-L, Noguchi S, Shao Z, Degboe A, Lichfield J, et al. Health-related quality of life from the FALCON phase III randomised trial of fulvestrant 500 mg versus anastrozole for hormone receptor-positive advanced breast cancer. Eur J Cancer. 2018;94:206–15.
Janni W, Alba E, Bachelot T, Diab S, Gil-Gil M, Beck TJ, et al. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2- advanced breast cancer: Tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Cancer Res Treat. 2018;169(3):469–79.
Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–29.
Martin M, Zielinski C, Ruiz-Borrego M, Carrasco E, Turner N, Ciruelos EM, et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann Oncol. 2021;32(4):488–99.
Xu B, Hu X, Li W, Sun T, Shen K, Wang S, et al. Palbociclib plus Letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer: primary results from PALOMA-4. Eur J Cancer. 2022;175:236–45.
Al-Sawaf O, Gentile B, Devine J, Zhang C, Sail K, Tandon M, et al. Health-related quality of life with fixed-duration venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: results from the randomized, phase 3 CLL14 trial. Am J Hematol. 2021;96(9):1112–9.
Grob JJ, Amonkar MM, Martin-Algarra S, Demidov LV, Goodman V, Grotzinger K, et al. Patient perception of the benefit of a BRAF inhibitor in metastatic melanoma: quality-of-life analyses of the BREAK-3 study comparing dabrafenib with dacarbazine. Ann Oncol. 2014;25(7):1428–36.
Acknowledgements
Not applicable.
Funding
This work was supported by a research grant from Jan Wallanders and Tom Hedelius stiftelse & Tore Browaldhs stiftelse, grant number P21-0018.
Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
Concept and design: Svensson, Chauca Strand, Bonander, Johansson, Jakobsson. Acquisition of data: Svensson, Chauca Strand, Bonander, Johansson, Jakobsson. Analysis and interpretation of data: Svensson, Chauca Strand, Jakobsson. Drafting of the manuscript: Svensson, Jakobsson. Critical revision of the paper for important intellectual content: Svensson, Chauca Strand, Bonander, Johansson, Jakobsson. Obtaining funding: Svensson. Administrative, technical, or logistic support: Svensson. Supervision: Svensson, Bonander, Johansson, Jakobsson.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Svensson, M., Strand, G.C., Bonander, C. et al. Analyses of quality of life in cancer drug trials - a review of measurements and analytical choices in post-reimbursement studies. BMC Cancer 24, 311 (2024). https://doi.org/10.1186/s12885-024-12045-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12045-8