Abstract
Background
Colorectal cancer (CRC) is the third most common cancer type worldwide. Colorectal cancer treatment costs vary between countries as it depends on policy factors such as treatment algorithms, availability of treatments and whether the treatment is government-funded. Hence, the objective of this systematic review is to determine the prevalence and measurements of financial toxicity (FT), including the cost of treatment, among colorectal cancer patients.
Methods
Medline via PubMed platform, Science Direct, Scopus, and CINAHL databases were searched to find studies that examined CRC FT. There was no limit on the design or setting of the study.
Results
Out of 819 papers identified through an online search, only 15 papers were included in this review. The majority (n = 12, 80%) were from high-income countries, and none from low-income countries. Few studies (n = 2) reported objective FT denoted by the prevalence of catastrophic health expenditure (CHE), 60% (9 out of 15) reported prevalence of subjective FT, which ranges from 7 to 80%, 40% (6 out of 15) included studies reported cost of CRC management– annual direct medical cost ranges from USD 2045 to 10,772 and indirect medical cost ranges from USD 551 to 795.
Conclusions
There is a lack of consensus in defining and quantifying financial toxicity hindered the comparability of the results to yield the mean cost of managing CRC. Over and beyond that, information from some low-income countries is missing, limiting global representativeness.
Similar content being viewed by others
Introduction
Colorectal cancer is the third most common cancer type worldwide; almost 2 million cases were detected in 2020. Colorectal cancer is also the second most common cause of cancer death worldwide, accounting for 1 million deaths per year [1]. Colorectal cancer treatment costs vary between countries as it depends on policy factors such as treatment algorithms, availability of treatments and whether the treatment is government-funded [2]. For underinsured patients, their out-of-pocket expenses will be higher [3]. A term that is associated with this situation is referred to as financial toxicity, which is the adverse impact of out-of-pocket healthcare costs suffered by the patients [4].
Financial toxicity can generally be divided into subjective financial distress and objective financial burden. Subjective financial distress occurs as a result of increasing cancer-related expenditures and financial difficulties, on top of the anxiety and discomfort experienced by the patient over their disease. The objective financial burden is due to the direct expenses of the cancer treatment, which will increase progressively from the first time the patient is diagnosed. As the patient spends more on cancer treatment, his income and assets will decrease over time. This financial burden is relative to the income and assets of the household of the patient with cancer, which decreases over time [5].
Costs of management for colorectal cancer patients include costs for surgery, chemotherapy, radiotherapy and palliative care. For example, the mean cost for each patient and treatment going for surgery in China range between $5,301 - $5,489 [6]. Another study in Spain revealed the cost of surgery for patients with Stage 1 to Stage 4 colorectal cancer range between $11,373 - $14,236 [7]. However, for both China and Spain, universal health care has been implemented which has benefited the population.
Studies have looked into the financial toxicity among different types of cancer patients which include prostate cancer [8, 9], breast cancer [10, 11] and lung cancer [12, 13]. However, there is a lack of studies that focus on colorectal cancer patients. It is important to determine the extent of financial toxicity among these types of patients.
Therefore, the objective of this systematic review is to determine the prevalence and measurements of financial toxicity, including the cost of treatment, among colorectal cancer patients.
Search strategy
Methods
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) [14] (supplementary file 1-PRISMA checklist). The registration number of the protocol is CRD42023399186.
Literature search strategies and study selection
The following literature databases were searched in January 2023: PubMed, Science Direct, Scopus and CINAHL databases were searched to find papers that reported FT. The primary outcome was to find the prevalence of objective and subjective FT due to CRC cancer management. The search was built on the following keywords and Medical Subject Headings which were based on the research question: population (patients), exposure (colorectal cancer), and outcome (financial toxicity/hardship/burden/stress) and their synonyms (Supplementary file 2: search strategy). Additionally, a manual search through the reference list of the eligible studies was applied. We included original quantitative research that reports the FT of CRC cost of treatment published before Jan. 2023. Papers of mixed cancer patients that reported the cost of each cancer separately were included if they involved the CRC cost. There was no limit on the design or setting of the study to minimize underreporting bias. In addition, studies that reported any cost of CRC, including direct medical, direct non-medical, and indirect costs were included. Medical research studies which include economic evaluation studies, reviews, qualitative studies, case series and case studies were excluded. This study did not assess the intangible cost as it is difficult to calculate their monetary value.
Data extraction and quality assessment
Selection and screening of titles, abstracts and full text was conducted independently by two authors with disagreements resolved via consensus or the involvement of a third author. One author performed the data extraction, and the second author checked them for completeness and accuracy. Key information extracted included the author, publication year, study type, research methods, study setting, main findings, and conclusion. The data was presented based on author date, type of cancer, study participants (sample size, socio-demographic characteristics such as age and gender), the prevalence of FT (subjective and objective), cost of illness, tools used to measure the FT, and quality scoring (Supplementary file 3). The prevalence of subjective FT is assessed using a questionnaire aimed at understanding the financial challenges individuals face due to healthcare expenses. The results were presented using a numerical description which is the proportion. However, the objective FT is measured using the prevalence of catastrophic health expenditure (CHE), which was defined as a healthcare cost-to-income ratio of more than 40% in the included studies.
The quality of all included papers was assessed using the Newcastle - Ottawa quality assessment scale (NOS) for longitudinal, cohort and cross-sectional studies (adapted for cross-sectional studies), which comprises three dimensions: selection, comfortability, and outcome. Each study was evaluated based on the NOS scale for fulfilling the established criteria in NOS for the 3 dimensions. An overall quality score was calculated by adding the number of stars for each category for a maximum total of 9. High-quality studies were defined as those with a score of 5 or higher, with higher scores suggesting a decreased likelihood of bias and higher quality [15] Disagreements between the two reviewers during full-text screening were reconciled via consensus or by the decision involving a third independent reviewer. The quality score can be found in Supplementary File 3.
Results
Description of studies
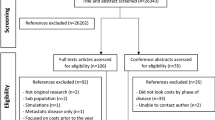
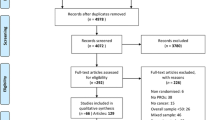
All search results were transferred to the Endnote X9 (Clarivate Analytics) which was used to manage the articles and manual searches. A total of 819 papers were identified through an online search and 2 papers through a manual search. Then, the duplicate papers were eliminated (n = 59). The titles and abstracts of the remaining 665 papers were screened and selected independently by two reviewers according to the established inclusion and exclusion criteria. Subsequently, a total of 23 papers were retained for full-text review. After a full-text review of the 23 papers, 15 were selected. The eligibility of included papers was agreed upon by all authors. The PRISMA flow chart demonstrated the screening process (Fig. 1) [16]. The studies in this review were conducted globally including Malaysia (n = 2) [17, 18], USA (n = 5) [19,20,21,22,23], Australia (n = 1) [24], China (n = 3) [25,26,27], Ireland (n = 3) [28,29,30] and Iran (n = 1) [31]. A total of 246,915 colorectal cancer patients took part in 15 studies worldwide, with samples ranging from 104 to 237,754 patients. Most studies included participants with any stage (I-IV) of cancer; however, one research included patients with stage III cancer [20] and another study included patients with stage IV cancer [21].
Measurement of objective financial toxicity
Included studies rarely focused specifically on quantifiable indicators of FT. Only 2 studies measured the objective FT in terms of the prevalence of catastrophic health expenditure (CHE), which was defined as a healthcare cost-to-income ratio of more than 40% in the included studies [18, 31] and found it to be 68.5% in Iran [30] and 47.8% in Malaysia [18].
Measurement of subjective financial toxicity
Out of the 15 research that were included in this review, 9 studies [17, 19,20,21, 24, 27,28,29] provided data on subjective FT. There was a wide variation in the measures of FT among the studies. Two of them employed the COmprehensive Score for Financial Toxicity (COST), which consists of 11 items and has a score range of 0–44 [19, 27]. Lower COST values denote greater FT. Patients with FT were categorised by Mo et al. using the median COST score, with individuals scoring fewer than 21 being classified as having experienced FT [26]. Additionally, four studies that employed a 4–7 point Likert scale to evaluate the prevalence of subjective FT reported prevalence was between 20.9% and 41% [17, 20, 25, 26, 29]. Moreover, one study utilised four questions with “yes” or “no” responses to gauge subjective FT; those who gave “yes” replies to at least one of the four questions were deemed to be suffering from financial toxicity [21]. Gordon et al. used three 3 domains to measure the FT which are: perceived prosperity, financial strain and ability to raise money ($2000) [24]. According to Edward et al. 2021, the FT included both material and psychological difficulty; the material FT was estimated to be 80% and the psychological FT was calculated using COST [19]. The details of the prevalence of subjective FT and the tools used to measure it can be found in Table 1.
Cost of cancer management
The cost of cancer management included direct medical costs, direct non-medical cost and indirect costs. It was reported in six of the included studies [17, 18, 23, 25, 26, 30] which are from China, USA, Malaysia and Ireland. The included studies were published between 1999 and 2017. All studies considered the four cancer stages except Li et al. 2016 where he considered the total cost of CRC treatment regardless of disease stage (Table 2).
Direct medical costs
Data on mean direct medical costs from different perspectives were reported in five studies in total [17, 18, 23, 25, 26, 30]. Among studies that were included, the period during which the expenditures were incurred varied widely, including annual cost [17, 18, 25, 26], colorectal cancer survival [30] and 4 years cost [23]. In regards to cost perspective, the patient perspective was employed in three research [17, 18, 30], one study considered the national health care perspective [23] and one study reported the cost from the patient perspective to calculate the non-medical cost and from the insurance plan database to find out the medical cost [25]. Detailed cost data can be found in Table 2.
Direct non-medical costs
The direct non-medical cost was included in only two studies [25, 30] where the cost in Europe was found to be €510 (USD703.8) [29] and 5588 CNY (USD901.2) in China [25] (Table 2).
Indirect cost
Two studies in total reported the indirect cost of colorectal cancer management [18, 25] where it was USD452.2 in Malaysia [18] and 6652CNY (USD1,072.9) in China [25] (Table 2).
Discussion
The present review examined the prevalence and measurement of FT among CRC patients. Fifteen studies were included; the majority (n = 12, 80%) were from high-income countries, others were from middle-income countries, and none from low-income countries. They were published between 1999 and 2023. Several main findings were identified, they were (i) few studies (n = 2) reported objective FT denoted by the prevalence of CHE, (ii) 60% (9 out of 15) reported a prevalence of subjective FT, which ranges from 7 to 80%, (iii) large variation and lacking standardized measurement tool to quantify subjective FT, (iv) 40% (6 out of 15) included studies reported cost of CRC management– annual direct medical cost ranges from USD 2045 to 10,772 and indirect medical cost ranges from USD 551 to 795.
While two studies regarded the objective FT as the prevalence of CHE, nine studies referred to subjective FT using different measurement scales. Five studies applied scaled questions (e.g., Likert-scale), two used the COST instrument, one used dichotomous questions, and another applied multidimensional dichotomous questions. Unstandardized measurement tools on subjective financial distress hindered the comparison of results between regions globally or countries by income classification, making it more challenging for global health players and state health authorities to plan an appropriate resource distribution in cancer management [32]. Future research shall consider developing a standardized instrument to measure FT using six domains– active financial spending, use of passive financial resources, psychosocial responses, support seeking, coping with care, or coping with one’s lifestyle [33].
Fifteen studies included in this review reported data from six countries that have a life expectancy of more than 75 years, and most were from high-income countries. Cancer patients living in countries with advanced medical modalities will have better survivorship provided that they have the financial assistance to receive the treatment and enough savings/assistance to support the incomeless days. Throughout the synthesis, we discovered that US data showed a high prevalence of subjective FT at more than 70%, despite having a similar proportion (17%) of people living below 50% of median income compared to Malaysia [34]. This might be because Malaysia, which has a dual-tier healthcare system, offers more affordable options in the public sector as the cost of care is heavily subsidized by the government. In contrast to the USA, where there is a reliance on private health insurance, where access to comprehensive coverage is contingent upon factors like employment status. Consequently, this review highlights the point of focusing on the financial burden attributed to CRC in unemployed ageing society to ensure healthy aging and good quality of life is one of the major determinants of healthy living among the elderly.
A paucity of studies reporting the direct non-medical [25, 30] and indirect cost [17, 18, 25], which could directly contribute to the subjective FT. The responsibility of clinicians in providing high-quality treatment is typical, however, their role in assisting to leverage financial burden and distress to the patients in the short and long term might be emphasized and supported by the national health insurers or social welfare department based on the healthcare financing system in the country [6]. Without a proper and comprehensive system to acknowledge and quantify the cost incurred to the patients, family, community and the nation, it becomes difficult to engage key stakeholders in paying attention to the public health insurance system and subsequently implement proper policy to incentivize cancer survivors [32]. Therefore, it is recommended for future researchers to obtain a situational analysis of financial burden regarding direct non-medical and indirect costs, particularly in low-income countries.
This is the first review analyzing the FT among CRC patients and the cost of CRC management. Most of the included studies recruited CRC patients from all stages, making the study population homogenous. However, a systematic review naturally presents publication bias; however, authors have attempted to minimize it by obtaining data from all available sources from the electronic databases, citations, and manual search. Secondly, a lack of consensus in defining and quantifying financial toxicity hindered the comparability of the results to yield the mean cost of managing CRC. Over and beyond that, information from some low-income countries is missing, limiting global representativeness.
Conclusion
Most of the studies included were conducted in high-income countries, with none originating from low-income nations. FT is prevalent and has emerged as a significant concern, even within publicly funded health systems with universal coverage. There is a need for additional research, particularly from low-income countries, to investigate the financial toxicity of CRC. Furthermore, it is essential to develop and validate a tool for quantifying FT in CRC patients through further research.
Data availability
All data generated or analysed during this study are included in this published article.
References
IARC. WHO; 2023. Available from: https://www.iarc.who.int/cancer-type/colorectal-cancer/#summary.
Bhimani N, Wong GY, Molloy C, Dieng M, Hugh TJ. Cost of colorectal cancer by treatment type from different health economic perspectives: a systematic review. Eur J Surg Oncol. 2022;48(10):2082–93.
Iragorri N, de Oliveira C, Fitzgerald N, Essue B. The out-of-Pocket cost Burden of Cancer Care-A systematic literature review. Curr Oncol. 2021;28(2):1216–48. https://doi.org/10.3390/curroncol28020117.
Zafar SY, Peppercorn JM, Schrag D, Taylor DH, Goetzinger AM, Zhong X, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381–90. https://doi.org/10.1634/theoncologist.2012-0279.
Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153–65. https://doi.org/10.3322/caac.21443.
Shi JF, Liu GX, Wang H, Mao A, Liu CC, Guo LW, et al. Medical expenditures for colorectal cancer diagnosis and treatment: a 10-year high-level-hospital based multicenter retrospective survey in China, 2002–2011. Chin J Cancer Res. 2019;31(5):825.
Corral J, Borras JM, Chiarello P, Garcia-Alzorriz E, Macia F, Reig A, et al. Estimation of hospital costs of colorectal cancer in Catalonia (Spain). Gac Sanit. 2015;29(6):437e44.
Stone BV, Laviana AA, Luckenbaugh AN, Huang LC, Zhao Z, Koyama T, et al. Patient-reported Financial Toxicity Associated with Contemporary Treatment for localized prostate Cancer. J Urol. 2021;205(3):761–8. https://doi.org/10.1097/ju.0000000000001423.
Imber BS, Varghese M, Ehdaie B, Gorovets D. Financial toxicity associated with treatment of localized prostate cancer. Nat Rev Urol. 2020;17(1):28–40. https://doi.org/10.1038/s41585-019-0258-3.
Politi MC, Yen RW, Elwyn G, O’Malley AJ, Saunders CH, Schubbe D, et al. Women who are Young, Non-white, and with Lower Socioeconomic Status Report Higher Financial Toxicity up to 1 year after breast Cancer surgery: a mixed-effects Regression Analysis. Oncologist. 2021;26(1):e142–e52. https://doi.org/10.1002/onco.13544.
Williams CP, Gallagher KD, Deehr K, Aswani MS, Azuero A, Daniel CL, et al. Quantifying treatment preferences and their association with financial toxicity in women with breast cancer. Cancer. 2021;127(3):449–57. https://doi.org/10.1002/cncr.33287.
Hazell SZ, Fu W, Hu C, Voong KR, Lee B, Peterson V, et al. Financial toxicity in lung cancer: an assessment of magnitude, perception, and impact on quality of life. Ann Oncol. 2020;31(1):96–102. https://doi.org/10.1016/j.annonc.2019.10.006.
Friedes C, Hazell SZ, Fu W, Hu C, Voong RK, Lee B, et al. Longitudinal trends of Financial toxicity in patients with Lung Cancer: a prospective cohort study. JCO Oncol Pract. 2021;17(8):e1094–e109. https://doi.org/10.1200/OP.20.00721.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
Kolaski K, Logan LR, Ioannidis JPA. Guidance to best tools and practices for systematic reviews. Syst Rev. 2023;12:96. https://doi.org/10.1186/s13643-023-02255-9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Azzani M, Roslani A, Su T, Roslani AC, Su TT. Financial burden of colorectal cancer treatment among patients and their families in a middle-income country. Support Care Cancer. 2016;24(10):4423–32. https://doi.org/10.1007/s00520-016-3283-2.
Azzani M, Yahya A, Roslani AC, Su TT. Catastrophic Health Expenditure among Colorectal Cancer patients and families: a case of Malaysia. Asia Pac J Public Health. 2017;29(6):485–94. https://doi.org/10.1177/1010539517732224.
Edward JS, Rayens MK, Zheng X, Vanderpool RC. The association of health insurance literacy and numeracy with financial toxicity and hardships among colorectal cancer survivors. Support Care Cancer. 2021;29(10):5673–80. https://doi.org/10.1007/s00520-021-06036-9.
Regenbogen SE, Veenstra CM, Hawley ST, Banerjee M, Ward KC, Kato I, et al. The personal financial burden of complications after colorectal cancer surgery. Cancer. 2014;120(19):3074–81. https://doi.org/10.1002/cncr.28812.
Shankaran V, Unger JM, Darke AK, Suga JM, Wade JL, Kourlas PJ, et al. S1417CD: a prospective Multicenter Cooperative Group-Led Study of Financial Hardship in Metastatic Colorectal Cancer patients. J Natl Cancer Inst. 2022;114(3):372–80. https://doi.org/10.1093/jnci/djab210.
Veenstra CM, Regenbogen SE, Hawley ST, Griggs JJ, Banerjee M, Kato I, et al. A composite measure of personal financial burden among patients with stage III colorectal cancer. Med Care. 2014;52(11):957–62. https://doi.org/10.1097/mlr.0000000000000241.
Seifeldin R, Hantsch JJ. The economic burden associated with colon cancer in the United States. Clin Ther. 1999;21(8):1370–9. https://doi.org/10.1016/s0149-2918(99)80037-x.
Gordon LG, Beesley VL, Mihala G, Koczwara B, Lynch BM. Reduced employment and financial hardship among middle-aged individuals with colorectal cancer. Eur J Cancer Care. 2017;26(5). https://doi.org/10.1111/ecc.12744.
Huang HY, Shi JF, Guo LW, Bai YN, Liao XZ, Liu GX, et al. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: a hospital-based, multicenter, cross-sectional survey. Chin J Cancer. 2017;36(1):41. https://doi.org/10.1186/s40880-017-0209-4.
Li X, Cai H, Wang C, Guo C, He Z, Ke Y. Economic burden of gastrointestinal cancer under the protection of the New Rural Cooperative Medical Scheme in a region of rural China with high incidence of oesophageal cancer: cross-sectional survey. Trop Med Int Health. 2016;21(7):907–16. https://doi.org/10.1111/tmi.12715.
Mo M, Jia P, Zhu K, Huang W, Han L, Liu C, et al. Financial toxicity following surgical treatment for colorectal cancer: a cross-sectional study. Support Care Cancer. 2023;31(2):110. https://doi.org/10.1007/s00520-022-07572-8.
Hanly P, Maguire R, Ceilleachair AO, Sharp L. Financial hardship associated with colorectal cancer survivorship: the role of asset depletion and debt accumulation. Psycho-oncology. 2018;27(9):2165–71. https://doi.org/10.1002/pon.4786.
Sharp L, O’Leary E, O’Ceilleachair A, Skally M, Hanly P. Financial Impact of Colorectal Cancer and its consequences: associations between Cancer-Related Financial stress and strain and health-related quality of life. Dis Colon Rectum. 2018;61(1):27–35. https://doi.org/10.1097/dcr.0000000000000923.
Ó Céilleachair A, Hanly P, Skally M, O’Leary E, O’Neill C, Fitzpatrick P, et al. Counting the cost of cancer: out-of-pocket payments made by colorectal cancer survivors. Support Care Cancer. 2017;25(9):2733–41. https://doi.org/10.1007/s00520-017-3683-y.
Piroozi B, Zarei B, Ghaderi B, Safari H, Moradi G, Rezaei S, et al. Catastrophic health expenditure and its determinants in households with gastrointestinal cancer patients: evidence from new health system reform in Iran. Int J Hum Rights Healthc. 2019;12(4):249–57. https://doi.org/10.1108/IJHRH-01-2019-0008.
Desai A, Gyawali B. Financial toxicity of cancer treatment: moving the discussion from acknowledgement of the problem to identifying solutions. EClinicalMedicine. (2020);20:100269DOI: https://doi.org/10.1016/j.eclinm.2020.100269.
Witte J, Mehlis K, Surmann B, Lingnau R, Damm O, Greiner W, et al. Methods for measuring financial toxicity after cancer diagnosis and treatment: a systematic review and its implications. Ann Oncol. 2019;30(7):1061–70. https://doi.org/10.1093/annonc/mdz140.
The world Bank. Available from: https://data.worldbank.org/?locations=MY-AU-US-CN-IE-IR.
Acknowledgements
Not applicable.
Funding
There is no fund in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, MA; Introduction: ZIA, methodology, ANMR.; quality assessments, EZS and SN, Results: MMA and SMA, Discussion: CXW. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Azzani, M., Azhar, Z.I., Ruzlin, A.N.M. et al. Subjective and objective financial toxicity among colorectal cancer patients: a systematic review. BMC Cancer 24, 40 (2024). https://doi.org/10.1186/s12885-023-11814-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11814-1