Abstract
Background
This study aimed to investigate the differences in the clinicopathological characteristics of younger and older patients with endometrial cancer (EC) and develop a nomogram to assess the prognosis of early onset EC in terms of overall survival.
Methods
Patients diagnosed with EC from the Surveillance, Epidemiology, and End Results (SEER) database between 2004 and 2015 were selected. Clinicopathological characteristics were compared between younger and older patients, and survival analysis was performed for both groups. Prognostic factors affecting overall survival in young patients with EC were identified using Cox regression. A nomogram was created and internal validation was performed using the consistency index, decision curve analysis, receiver operating characteristic curves, and calibration curves. External validation used data from 70 patients with early onset EC. Finally, Kaplan-Meier curves were plotted to compare survival outcomes across the risk subgroups.
Results
A total of 1042 young patients and 12,991 older patients were included in this study. Younger patients were divided into training (732) and validation (310) cohorts in a 7:3 ratio. Cox regression analysis identified age, tumorsize, grade, FIGO stage(International Federation of Gynecology and Obstetrics) and surgery as independent risk factors for overall survival, and a nomogram was constructed based on these factors. Internal and external validations demonstrated the good predictive power of the nomogram. In particular, the C-index for the overall survival nomogram was 0.832 [95% confidence interval (0.797–0.844)] in the training cohort and 0.839 (0.810–0.868) in the internal validation cohort. The differences in the Kaplan-Meier curves between the different risk subgroups were statistically significant.
Conclusions
In this study, a nomogram for predicting overall survival of patients with early onset endometrial cancer based on the SEER database was developed to help assess the prognosis of patients and guide clinical treatment.
Similar content being viewed by others
Introduction
Endometrial cancer (EC) is the sixth most common cancer affecting women worldwide. In recent years, EC has become the most common gynecological malignancy in developed countries [1], and its incidence is increasing in young women [2]. Meanwhile, the incidence of EC and associated mortality are on the rise [3]. It is estimated that there were 66,570 new cases and 12,940 deaths from this disease in the United States in 2021 [4]. Although most women diagnosed with EC have already gone through menopause, premenopausal women younger than 45 account for approximately 10% of reported cases of EC [5].
Factors associated with an increased risk of EC include age, obesity, family history, and tumor stage [6]. Previous studies have suggested age as an important factor associated with overall survival, the importance of age in assessing the prognosis of patients with EC remains controversial [7]. It has been suggested that patients with EC under 45 years of age tend to have a lower incidence of advanced disease, a higher degree of tumor differentiation, and better prognosis [8]. Early diagnosis of EC in younger patients is suggested to be lower and can easily be misdiagnosed as abnormal uterine bleeding, whereas older patients are more likely to be diagnosed at an early stage [8]. Nelson et al. [9] found no association between increasing age and the prevalence of endometrial hyperplasia, which was more common in women aged < 40 years than in those aged 40–50 years. Clinically, patients younger than 45 years were defined as having early onset EC. The FIGO staging system proposed by the International Federation of Obstetrics and Gynecology has been widely used to predict the prognosis of EC [10]. However, the FIGO staging system remains limited and does not accurately predict the prognosis. Further comprehensive analysis of the prognostic factors associated with patients with early onset EC is necessary to establish individualized treatment plans.
In this study, differences in clinicopathological characteristics affecting the prognosis of younger and older patients were explored using data from the Surveillance, Epidemiology, and End Results (SEER) database. Variables associated with the prognosis of young patients were further analyzed. A nomogram was constructed using the relevant variables to predict the prognosis of patients with early onset EC at 3-, 5-, 8-, and 10-year overall survival and to guide clinical treatment.
Materials and methods
Training and internal validation cohorts
This study used the SEER database (http://seer.cancer.gov/seerstat/), which covers 30% of the population of the United States. SEER has collected information on incidence rate, morbidity, mortality and other evidence-based drugs of cancer patients in some states and counties in the United States for decades, providing valuable information about cancer diseases for most clinical medical personnel [11, 12]. This study used data from 1975 to 2020. Data were extracted, downloaded, and analyzed using SEER*Stat 8.4.1.2. (accunt ID: 18,893,816,203@163.com). Primary sites, C54.1-9 and C55.917; site and morphology, 8380/3 (based on the International Classification of Tumor Diseases for Oncology (ICD-O), Third Edition); histology, 8000–9930 (adenomas and adenocarcinomas); FIGO stage I,II,III and IV; and therapy. This study was approved by the Ethics Committee of the Second Hospital of Lanzhou University (Ethics Approval No.2022 A-336) and strictly complied with the Declaration of Helsinki. The Ethics Committee of the Second Hospital of Lanzhou University waived the requirement for informed consent.
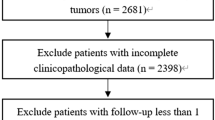
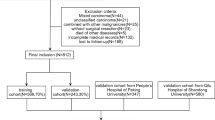
Patients diagnosed with early onset EC between 2004 and 2015 were included in this study. The exclusion criteria were as follows: (I) age > 45 years, (II) multiple primary tumors, (III) missing disease-related information (FIGO stage, grade and tumorsize), (IV) unclear cause of death, and (VI) incomplete follow-up time. The flowchart is shown in Fig. 1. In total, 1042 young patients and 12,991 older patients were selected from the SEER database. Younger patients were randomly (7:3) divided into training (n = 732) and validation (n = 310) cohorts. There were no significant differences in the variables between the two groups (all p-values < 0.05), as detailed in Table 1.
The variables included age at diagnosis, race, marital status, histological type, tumorsize, grade, FIGO stage, radiotherapy, chemotherapy, and surgery. The follow-up variables included survival status, survival time, and cause of death. The primary endpoint was overall survival, which refers to the time from diagnosis to death from any cause.Age (≤ 33 and 34–45 years) and tumorsize(≤ 4 and > 4 cm) were determined using x-tile software to obtain the optimal cutoff values. FIGO stage use 2009 version.
External validation cohort
The clinical data of 70 patients who were treated at the Department of Gynecology of the Second Hospital of Lanzhou University and pathologically diagnosed with EC between January 2011 and August 2021 were retrospectively analyzed. The inclusion criteria were as follows: (I) age < 45 years; (II) primary tumor; (III) confirmed postoperative pathological diagnosis of EC; and (V) complete clinical and postoperative follow-up data. The follow-up was conducted on August 30, 2021.
Data analysis
We first compared the basic clinical information of elderly EC patients and young EC patients, and then obtained factors that affect the prognosis of young EC patients based on Cox univariate regression. P < 0.05 was included in Cox multivariate regression for further analysis. Finally, a predictive model related to overall survival rate was constructed.The model predictive ability was evaluated using the concordance index, area under the receiver operating characteristic curve, and decision curve analysis. Calibration curves were plotted to assess the agreement between the nomogram and actual model. According to the total scores of the validation and training queues, patients are divided into low-risk and high-risk groups, respectively. Kaplan-Meier curves were used to compare overall survival between the two groups. All statistical analyses were performed using SPSS (version 26.0; SPSS Inc., Chicago, IL, USA) and R (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
We included 1042 young patients and 12,991 older patients with EC from the SEER database. In terms of race, both groups had a high proportion of white patients, 61.0% and 78.6%, respectively. The histological type of adenocarcinoma accounted for 83.7% of the younger patients. Regarding the histological grades, a higher proportion of younger patients were in grade I (58.7%), and a higher number of older patients were in grade II or above (55.7%).Many patients were located in FIGO I. The numbers of young and older patients receiving adjuvant treatment were low (Table 1). Survival analysis showed that overall survival and cancer-specific survival were higher in younger patients than in older patients (P-value < 0.001) (Fig. 2).
Younger patient characteristics
The younger group comprised 101 (13.8%) patients ≤ 33 years old and 631 (86.2%) patients 33–44 years old. In the race group, 636(61.0%) patients were white, 64(6.2%) were black, and 342 (32.8%) patients were of other ethnic groups. Further, 507 (48.7%) were identified as married and 535 (51.3%) patients were in other marital statuses. Regarding the pathological type, adenocarcinoma was the most prevalent, accounting for 83.7% of all tumors. With respect to FIGO stage, the majority of patients were classified as FIGO I (72.7%). There were 566 (54.3%) patients with tumor size (cm) ≤ 4. The treatment protocols for the patients included chemotherapy (189; 18.1%) and radiotherapy (239; 22.9%). The proportion of patients undergoing surgery was 99.1%. (Table 2)
Univariate and multivariate analysis
Univariate Cox analysis identified age, tumorsize, histology, grade, FIGO stage, radiation, chemotherapy, and surgery as risk factors significantly associated with overall survival. These risk factors were included in the multivariate analysis (P-value < 0.05). Age, grade, FIGO stage, surgery were independent risk factors for poor overall survival (Table 3).
Nomogram Construction
The different subtypes of each independent prognostic variable were projected onto a scale to obtain a score for each item. The scores corresponding to independent prognostic factors were summed to obtain the total score. The higher the total score, the worse the prognosis. For a 40 year old EC patient with a tumorsize greater than 4 cm, grade II, FIGO stage II, and no surgical treatment, adding up the scores of each prognostic factor, she scored 183 points on the overall survival chart, and estimated a 5-year overall survival rate of 59% based on the nomogram. The nomogram showed that FIGO stage had the greatest influence on prognosis (Fig. 3).
Internal validation
In the training cohort, the concordance index for the overall survival nomogram was 0.832(0.797–0.844), which was greater than that of a single independent prognostic risk factor. In the validation cohort, the concordance index for the nomogram of overall survival was 0.839 (0.810–0.868). This result was also superior to that of the independent prognostic risk factors. The AUC values for 3-, 5-, 8- and 10-year overall survival in the training cohort (0.926, 0.852, 0.830, and 0.829, respectively) were significantly higher than those for FIGO stage (0.849, 0.820, 0.781, and 0.784, respectively). The results for the validation cohort also showed significantly higher AUC values for the nomogram (0.926,0.877, 0.86, and 0.858) than for grade (0.849, 0.817, 0.814, and 0.794) (Fig. 4A, B). The calibration curves for the training and validation cohorts were close to the 45-degree diagonal, indicating that the probabilities of the predicted values were generally consistent with the actual probabilities (Fig. 4C, D). In addition, DCA curves confirmed the validity of the nomogram (Fig. 4E, F).
The area under the receiver operating characteristic curve (AUC value) was used to measure performance at 3-, 5-,8- and 10-year for the overall survival nomogram, age, tumorsize,grade, FIGO stag and surgery: (A) training cohort; (B) validation cohort; calibration curves for OS nomogram: (C) training cohort; (D) validation cohort; DCA curves for OS nomogram: (E) training cohort; (F) validation cohort
External validation
In 70 early-onset EC patients, four were aged ≤ 33 years; among FIGO stage I were 84.3% (Supplementary Table 1). The AUCs at 5-, 8-, and 10-year for the nomogram (0.802, 0.807, and 0.791, respectively) were higher than those for the FIGO stage (0.713, 0.713, and 0.659) (Supplementary Fig. 1).
Risk stratification
We divided the training cohort, the internal and external validation cohorts into high- and low-risk groups based on critical values. Comparison of overall survival between groups using Kaplan-Meier curves showed that overall survival rates were higher in all low-risk groups than in the high-risk group (P-value < 0.05) (Fig. 5).
Discussion
The nomogram can transform complex regression equations into visualized simple graphs, making the prediction results easier to read and more convenient for evaluating patient conditions; In clinical practice, it can be used for multi-indicator joint diagnosis or prediction of disease risk or prognosis. By using nomogram, an accurate digital probability of survival or risk can be provided for each patient, which can assist clinical doctors in making decisions and reflect the idea of personalized healthcare.
We first compared the differences in the clinicopathological characteristics of the prognosis of younger and older patients with EC, and the results were consistent with previous studies [13, 14]. We further analyzed the clinicopathological characteristics of patients with EC aged < 45 years and examined the variables associated with their prognosis.
Race is widely recognized as an important risk factor for tumor prognosis [15, 16]. Tarney et al. [13] found that the median age at diagnosis of black patients with plasmacytoma and carcinosarcoma was 3 years younger than that of white patients (P-value < 0.0001). Some researchers have suggested that among EC patients, the mortality rate is 2.5 times higher in black women than in white women, despite a 30% lower incidence in black patients [16, 17]. This is primarily because patients respond differently to treatments, comorbidities, and genetic mutations. Our findings showed that white patients were more likely to develop EC, accounting for over 70% of the total. Research has found that histological type is an independent risk factor affecting prognosis [18]. As previously reported, adenocarcinoma is the predominant histological type of early onset EC [19]. The results of this study showed that the histological type was a prognostic factor for patients with early onset EC, but not an independent prognostic factor based on multifactorial analysis [20, 21]. Tumor grade also significantly affects patient prognosis, with survival rates decreasing as the tumor loses differentiation. By including 1,254 patients with stage I-II EC, Haley et al. showed that tumor grade and lymphatic vascular infiltration remained independent risk factors for prognosis in both younger and older patients [22]. As shown in the nomogram, tumor grade had a significant impact on prognosis in this study. Marriage is thought to be associated with a good prognosis in most cancers in women [23]. Unmarried women with cancer are at higher risk of late diagnosis and poorer survival outcomes than married women with cancer [24, 25]. The most frequent explanation is that marital status not only affects the regulation of the patient’s hormone levels but also has affects the patient’s psychosocial well-being. Lower et al. [26] 2015suggested that both marital status and relationship type are independent prognostic factors for survival in patients with EC. Our data showed that only half of the women were married. Tumor stage is a recognized prognostic factor for EC [27]. Higher staging suggests poorer prognosis. Liang et al. [28] reported that patients with high-grade EC were older than those with low-grade EC. A retrospective analysis by Pellerin et al. [20] found that 84.2% of EC patients under 45 years of age presented with stage I. However, by comparing the clinical characteristics and outcomes of patients with EC aged 45 years and younger with those aged 45 years and older, Evans-Metcalf et al. found the same overall distribution of tumor stage and survival in older patients compared with younger patients, a finding that contradicts several previous reports [29]. The staging system is a traditional tool used for assessing tumor prognosis. Most studies have shown that stage is an independent prognostic factor in patients with EC. TNM staging is the most widely accepted tumor staging system, and we used the 6th edition of the AJCC staging [30]. In this study, T-stage was an independent risk factor for overall survival in early-onset EC, and patients in T1 stage had a relatively good prognosis in 82.2% of cases. Tumor location is also an important factor in patient prognosis, with lymph node metastases or distant tumor metastases indicating a poor prognosis. Surgery is the main treatment method for early onset EC. Total Hysterectomy combined with bilateral tubal oophorectomy is the standard treatment, which can be performed by open or minimally invasive methods. The indications for adjuvant therapy are mainly based on clinical and pathological factors, such as age, grade, histology, depth of muscle infiltration, and lymphatic space infiltration [31]. Research has found that postoperative radiotherapy can significantly reduce the risk of local recurrence in women with moderate to high-risk EC [32].
Informatics is widely recognized as a tool for clinical research. Although the impact of different factors on EC prognosis has been studied, this has been limited to small-scale studies. To the best of our knowledge, this is the first study to use the SEER database to create a nomogram of overall survival in patients with early onset EC. However, the current study has some limitations: (I) Retrospective studies may lead to selection bias. (II) The SEER database is from the US, and a large amount of Chinese data is required for further validation. (III) The time period was 2004–2015, and we could only use the 6th edition of the AJCC.
Ultimately, our findings showed that younger patients had a better prognosis than older patients with a higher incidence of early- and low-grade disease. The standard treatment for patients with early onset EC is hysterectomy and bilateral salpingo-oophorectomy, with or without lymph node dissection. However, with socio-economic development and the implementation of China’s ‘two-child’ policy, EC management can be challenging for young women whose disease is mostly confined to the endometrium, with no extra-uterine metastases, and who have a strong desire to preserve their uterus [33,34,35]. The safety and efficacy of initial fertility-preserving treatment for patients with early stage, highly differentiated EC are now well established, and a more rigorous follow-up program must be adopted.
In summary, using the SEER database, we identified the factors associated with survival in patients with early onset EC, including age, marital status, race, histology, tumor size, grade, T stage, N stage, M stage, radiotherapy, chemotherapy and surgery. The development of the nomogram and use of a combination of internal and external validations demonstrated the good clinical applicability of the nomogram. A risk stratification system was created based on the risk scores generated by the nomogram. These findings may help clinicians tailor individual treatment plans for patients with early onset EC. However, further validation of our findings is required in prospective multicenter studies. With the development of precision medicine, traditional pathological classification can no longer meet the needs of clinical diagnosis and treatment. With the emergence of molecular classification, it will promote the combination of traditional pathological classification and molecular classification, in order to comprehensively evaluate the prognosis of endometrial cancer and effectively guide clinical treatment. As the main direction for further research in the future, the development of new fertility preserving treatment strategies based on molecular typing characteristics requires more and larger clinical trials.
Data Availability
All data and materials supporting our fundings can be obtained from corresponding author upon request. Data were public for the sake of privacy and ethical restrictions. The original contributions presented in this study are included in the supplementary material, further inquiries can be directed to the corresponding authors. The website for obtaining data in this article was the SEER database (http://seer.cancer.gov/seerstat/). SEER*Stat 8.4.1.2. (account ID: 18893816203@163.com).
Abbreviations
- AJCC:
-
American Joint Committee on Cancer (AJCC)
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- SEER:
-
Surveillance, epidemiology and end results
- SPSS:
-
Statistical product and service solutions
References
Lucchini SM, Esteban A, Nigra MA, et al. Updates on Conservative management of endometrial cancer in patients younger than 45 years[J]. Gynecol Oncol. 2021;161(3):802–9.
Li J, Zhu Q, Yang B, et al. Risk factors for ovarian involvement in young and premenopausal endometrioid endometrial cancer patients[J]. Eur J Obstet Gynecol Reprod Biol. 2018;222:151–4.
Smrz SA, Calo C, Fisher JL, et al. An ecological evaluation of the increasing incidence of endometrial cancer and the obesity epidemic[J]. Am J Obstet Gynecol. 2021;224(5):501–6.
Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021[J]. CA Cancer J Clin. 2021;71(1):7–33.
Mutlu L, Manavella DD, Gullo G et al. Endometrial Cancer in Reproductive Age: Fertility-Sparing Approach and Reproductive Outcomes[J]. Cancers (Basel), 2022,14(21).
Zabuliene L, Kaceniene A, Steponaviciene L et al. Risk of Endometrial Cancer in women with Diabetes: a Population-based retrospective cohort Study[J]. J Clin Med, 2021,10(16).
Ren F, Wang S, Li F, et al. Clinical nomograms for Predicting the overall survival and Cancer-specific survival of patients with ovarian carcinosarcoma patients after primary Surgery[J]. J Cancer. 2021;12(23):7223–36.
Choi J, Holowatyj AN, Du M, et al. Distinct genomic landscapes in early-onset and late-onset endometrial Cancer[J]. JCO Precis Oncol. 2022;6:e2100401.
Nelson AL, Vasquez L, Tabatabai R, et al. The yield of endometrial aspiration in women with various risk factors and bleeding abnormalities[J]. Contracept Reprod Med. 2016;1:9.
Li X, Fan Y, Dong Y, et al. Development and validation of Nomograms Predicting the overall and the Cancer-Specific Survival in Endometrial Cancer Patients[J]. Front Med (Lausanne). 2020;7:614629.
Yang J, Li Y, Liu Q, et al. Brief introduction of medical database and data mining technology in big data era[J]. J Evid Based Med. 2020;13(1):57–69.
Wu WT, Li YJ, Feng AZ, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models[J]. Mil Med Res. 2021;8(1):44.
Tarney CM, Tian C, Wang G, et al. Impact of age at diagnosis on racial disparities in endometrial cancer patients[J]. Gynecol Oncol. 2018;149(1):12–21.
Parthasarathy S, Shen Z, Carrillo-Salinas FJ, et al. Aging modifies endometrial dendritic cell function and unconventional double negative T cells in the human genital mucosa[J]. Immun Ageing. 2023;20(1):34.
Chung YS, Woo HY, Lee JY, et al. Mismatch repair status influences response to fertility-sparing treatment of endometrial cancer[J]. Am J Obstet Gynecol. 2021;224(4):370–1.
Baskovic M, Lichtensztajn DY, Nguyen T, et al. Racial disparities in outcomes for high-grade Uterine cancer: a California cancer registry study[J]. Cancer Med. 2018;7(9):4485–95.
Clarke MA, Long BJ, Sherman ME, et al. Risk assessment of endometrial cancer and endometrial intraepithelial neoplasia in women with abnormal bleeding and implications for clinical management algorithms[J]. Am J Obstet Gynecol. 2020;223(4):541–9.
Bendifallah S, Ilenko A, Darai E. High risk endometrial cancer: clues towards a revision of the therapeutic paradigm[J]. J Gynecol Obstet Hum Reprod. 2019;48(10):863–71.
Murali R, Davidson B, Fadare O et al. High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations[J]. Int J Gynecol Pathol, 2019,38 Suppl 1(Iss 1 Suppl 1):S40-S63.
Pellerin GP, Finan MA. Endometrial cancer in women 45 years of age or younger: a clinicopathological analysis[J]. Am J Obstet Gynecol. 2005;193(5):1640–4.
Fu HC, Chen JR, Chen MY, et al. Treatment outcomes of patients with stage II pure endometrioid-type endometrial cancer: a Taiwanese Gynecologic Oncology Group (TGOG-2006) retrospective cohort study[J]. J Gynecol Oncol. 2018;29(5):e76.
Haley L, Burmeister C, Buekers T, et al. Is older Age a real adverse prognostic factor in Women with Early-Stage Endometrial Carcinoma? A matched Analysis[J]. Int J Gynecol Cancer. 2017;27(3):479–85.
Dong J, Dai Q, Zhang F. The effect of marital status on endometrial cancer-related diagnosis and prognosis: a Surveillance Epidemiology and End results database analysis[J]. Future Oncol. 2019;15(34):3963–76.
Yuan R, Zhang C, Li Q, et al. The impact of marital status on stage at diagnosis and survival of female patients with breast and gynecologic cancers: a meta-analysis[J]. Gynecol Oncol. 2021;162(3):778–87.
Chen ZH, Yang KB, Zhang YZ, et al. Assessment of Modifiable Factors for the Association of Marital Status with Cancer-Specific Survival[J]. JAMA Netw Open. 2021;4(5):e2111813.
Lowery WJ, Stany MP, Phippen NT, et al. Survival advantage of marriage in Uterine cancer patients contrasts poor outcome for widows: a Surveillance, Epidemiology and End results study[J]. Gynecol Oncol. 2015;136(2):328–35.
Zhu L, Sun X, Bai W. Nomograms for Predicting Cancer-Specific and overall survival among patients with endometrial carcinoma: a SEER based Study[J]. Front Oncol. 2020;10:269.
Liang S, Zhang Y. Clinical pathological characteristics and survival of high-grade endometrioid carcinoma[J]. J Obstet Gynaecol Res. 2021;47(10):3644–51.
Evans-Metcalf ER, Brooks SE, Reale FR, et al. Profile of women 45 years of age and younger with endometrial cancer[J]. Obstet Gynecol. 1998;91(3):349–54.
Huo X, Wang SA, Lymph Node. Count-based AJCC staging System facilitates a more accurate prediction of the prognosis of patients with endometrial Cancer[J]. Front Oncol. 2021;11:641962.
Rees M, Angioli R, Coleman RL, et al. European menopause and Andropause Society (EMAS) and International Gynecologic Cancer Society (IGCS) position statement on managing the menopause after gynecological cancer: focus on menopausal symptoms and osteoporosis[J]. Maturitas. 2020;134:56–61.
van den Heerik A, Horeweg N, de Boer SM, et al. Adjuvant therapy for endometrial cancer in the era of molecular classification: radiotherapy, chemoradiation and novel targets for therapy[J]. Int J Gynecol Cancer. 2021;31(4):594–604.
Herrera CE, Humann J, Torrejon R, et al. Chances of pregnancy and live birth among women undergoing Conservative management of early-stage endometrial cancer: a systematic review and meta-analysis[J]. Hum Reprod Update. 2022;28(2):282–95.
Gallo A, Catena U, Saccone G et al. Conservative Surgery in Endometrial Cancer[J]. J Clin Med, 2021,11(1).
Straubhar AM, Parsons MW, Francis S, et al. Refusal of Surgery and survival outcomes in endometrial cancer[J]. Int J Gynecol Cancer. 2021;31(9):1236–41.
Acknowledgements
We should provide relevant data from the Department of Gynecology and Radiology of the Second Hospital of Lanzhou University.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study. Meng Zhang: Data Collection and manuscript writing. Rui-ping Li: Visualization, Investigation; Jia-xi Zhang: Software and data processing. Yun-yun Wang and Yun-lu Wang: Project development. Yu-zhen Guo: Provided critical revision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
I confirm that all methods were performed in accordance with the relevant guidelines and regulations. All patients were treated according to the latest NCCN guidelines. We anonymized and de-identified medical records of all cases before analyses. The study was approved by the Ethics Committee of the Second Hospital of Lanzhou University (Ethics Approval No.: 2022 A-336), and strictly complied with the Helsinki Declaration. The Ethics Committee of the Second Hospital of Lanzhou University agreed to exempt patients from signing informed consent. As the clinical data in this study was collected from a publicly available database (SEER database), there were no local or national ethical issues.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, M., Li, R., Zhang, J. et al. Development and validation of a nomogram for predicting overall survival in patients with early-onset endometrial cancer. BMC Cancer 23, 1230 (2023). https://doi.org/10.1186/s12885-023-11682-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11682-9