Abstract
Background
It remains uncertain whether first-line treatment with upfront brain radiotherapy (RT) in combined with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) is superior to EGFR-TKIs alone for EGFR-mutated non-small cell lung cancer with newly diagnosed brain metastases (BMs). Therefore, we performed a meta-analysis to address this issue.
Methods
We searched PubMed, Embase, Cochrane Library, and Web of Science databases for eligible studies published until February 28, 2023. The primary outcomes of interest were overall survival (OS) and intracranial progression-free survival (iPFS), reported as hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
Twenty-four retrospective studies with 3184 patients were included. First- or second-generation EGFR-TKIs were used in each study. Upfront brain RT plus EGFR-TKIs significantly prolonged OS (HR = 0.75, 95% CI: 0.64–0.88) and iPFS (HR = 0.61, 95% CI: 0.52–0.72) compared to EGFR-TKIs alone. There were no significant differences in OS and iPFS benefits from the combination therapy between asymptomatic and symptomatic patients, patients with exon 19 and 21 mutations, patients with 1–3 and > 3 BMs, and males and females, respectively (HRs interaction, P > 0.05 for each subgroup comparison).
Conclusions
First-line treatment with upfront brain RT plus EGFR-TKIs is likely to be more effective than EGFR-TKIs alone. The benefits of combination therapy did not appear to be significantly affected by BM-related symptoms, EGFR mutation subtype, number of BMs, or sex.
Similar content being viewed by others
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers [1]. Epidermal growth factor receptor (EGFR) mutations are present in approximately 40% of Asian and 10–20% of non-Asian patients [2], with a 50–70% risk of developing brain metastases (BMs) [3]. Currently, EGFR tyrosine kinase inhibitors (EGFR-TKIs) are the standard first-line treatment for advanced NSCLC with EGFR mutations. However, first- and second-generation EGFR-TKIs have low cerebrospinal fluid (CSF) penetration rates (< 6%) [4]. Although third-generation osimertinib is better able to permeate the CSF than first- and second-generation EGFR-TKIs, its concentration in the CSF is far lower than that in the plasma [5, 6].
Brain radiotherapy (RT) has been shown to damage the blood-brain barrier (BBB) and increase the concentration of EGFR-TKIs in the CSF [7]. In addition, RT can reduce EGFR-TKIs resistance [8]. Therefore, EGFR-TKIs in combination with brain RT may be more effective than EGFR-TKIs alone theoretically. However, no randomized controlled trials (RCTs) have compared the two treatment strategies, and the results from retrospective studies are inconsistent [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. It is possible that patients characteristics (such as EGFR mutation subtype, BM-related symptom, and number of BMs) or brain RT techniques affect the efficacy of the combination therapy. Although there were several published meta-analyses [34,35,36,37] of this subject have been published, the results had low statistical power as they were limited by the small number of included studies, which had small sample sizes, and few subgroup analyses.
In light of these issues, we performed a more comprehensive systematic review and meta-analysis of the currently available evidences, aiming to determine whether first-line treatment with upfront brain RT plus EGFR-TKIs was superior to EGFR-TKIs alone in patients with EGFR-mutated NSCLC with newly diagnosed BMs, and to explore the advantageous groups of the combination therapy by subgroup analyses.
Materials and methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [38], and was registered on the INPLASY international platform of registered systematic review and meta-analysis protocols (INPLASY202310013). The PRISMA checklist is shown in Table S1.
Literature search
A systematic search of PubMed, Embase, Cochrane Library, and Web of Science before February 28, 2023 was performed independently by two authors (SY and LS). Search terms mainly included: (“non-small cell lung cancer” or “non-small cell lung carcinoma”); (“brain metastases” or “brain metastasis”); (“epidermal growth factor receptor” or “EGFR”); (“tyrosine kinase inhibitor” or “TKI”); “targeted therapy”; “gefitinib”; “erlotinib”; “icotinib”; “afatinib”; “dacomitinib”; “osimertinib”; and (“irradiation” or “radiation” or “radiotherapy”). The full set of search terms and detailed strategy of each database search are listed in Table S2. The references of the relevant reviews were manually checked to obtain additional articles.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) study design: prospective or retrospective studies; (2) study population: histologically proven EGFR-mutated NSCLC, with newly diagnosed BMs identified by CT or MRI; (3) intervention: compared first-line treatment with upfront brain RT plus EGFR-TKIs with EGFR-TKIs alone; (4) outcomes: at least overall survival (OS) or intracranial progression-free survival (iPFS) reported; and (5) published in English. Upfront brain RT was defined as brain RT performed before the progression of intracranial disease to the first-line EGFR-TKIs therapy. Patients who received EGFR-TKIs prior to the diagnosis of BMs and those with ALK mutations were excluded.
Data extraction and quality assessment
Two authors (SY and LS) independently extracted the patients baseline characteristics and data on OS, iPFS, intracranial objective response rate (iORR), and intracranial disease control rate (iDCR) from each study. OS was generally measured from the start of EGFR TKI therapy or the date of BM diagnosis until death or the last follow-up, whereas iPFS was calculated from the start of EGFR TKI therapy or the date of BM diagnosis until intracranial progression or the last follow-up. The iORR and iDCR were generally assessed by brain computed tomography (CT) or magnetic resonance imaging (MRI) using the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) criteria. Responses were divided into complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). iORR was calculated as CR + PR and iDCR was calculated as CR + PR + SD.
The Newcastle-Ottawa Scale (NOS) [39] was used to assess the quality of the retrospective studies. Grading of Recommendations Assessment, Development, and Evaluations (GRADE) was used to assess the quality of the evidence [40].
Statistical analysis
Statistical analyses were performed using Review Manager Software version 5.3 (RevMan v5.3, Cochrane Collaboration, Oxford, UK). The outcomes of interest were OS, iPFS, iORR, and iDCR, presented as hazard ratios (HRs) or odds ratios (ORs) with their 95% confidence intervals (CIs). When not directly reported in the articles, HRs with 95% CIs were calculated using the Kaplan Meier curves [41, 42]. A random-effects model was used for the statistical analysis. The heterogeneity was assessed using the Chi-square (χ2) and I-square (I2) tests. Subgroup analyses of OS and iPFS were performed according to BMs related symptom (asymptomatic and symptomatic), EGFR mutation subtype (19 and 21 deletion mutations), number of BMs (1–3 and > 3), and sex (male and female). Sensitivity analysis was performed to evaluate the stability of the results. Publication bias was estimated using the funnel plots, Begg’s test [43], and Egger’s test [44].
Results
Literature search and study selection
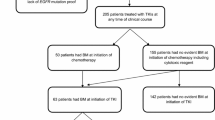
In total, 10,602 studies were included in the initial search. After removing the duplicates and screening the abstracts and/or full text, 10,581 studies were excluded. Finally, 24 retrospective studies [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] with 3184 patients were eligible for inclusion. The study selection process and reasons for exclusion are shown in Fig. 1. The majority of studies were conducted in Asia (23/24). First-generation (gefitinib, erlotinib, or icotinib) or second-generation (afatinib) EGFR-TKIs were used in all studies. The median follow up time was 22 months (interquartile range [IQR], 18–31). The median sample size was 55 participants (IQR, 38–66) in the upfront brain RT plus EGFR-TKIs group, and 60 participants (IQR, 40–88) in the EGFR-TKIs alone group. The percentage of males (34% vs. 32%), never smoking (48% vs. 51%), ECOG ≥ 2 (35% vs. 36%), exon 19 mutation (41% vs. 37%), and BM number ≤ 3 (17% vs. 22%) appeared to be similar between upfront brain RT plus EGFR-TKIs and EGFR-TKIs alone groups, while asymptomatic BMs (34% vs. 55%) appeared to be unbalanced. All the HRs used in our meta-analysis were derived without adjusting for the baseline characteristics of the two populations, except in one study [32]. OS and iPFS were calculated from the start of EGFR-TKI therapy in 9 and 14 studies, respectively; and were measured from the date of BM diagnosis in 14 and 9 studies, respectively. The patients characteristics are shown in Table 1, and the treatments and main outcomes are listed in Table 2.
Assessment of study and evidence and publication bias
All studies were judged with a score of ≥ 6 (Table S3). The GRADE assessment results for each finding are shown in Table S4. The evidence for OS, iORR, and iDCR had moderate GRADE ratings, while the evidence for iPFS had very low GRADE ratings. The evidence for all the outcomes of subgroup analyses of OS were of low and very low GRADE. Regarding the evidences for the outcomes of subgroup analyses of iPFS, asymptomatic BMs, BM number > 3, and female sex had moderate GRADE rating, and the evidence for the remaining outcomes was low or very low GRADE.
The Begg’s and Egger’s test results indicated a significant publication bias in iPFS (Begg’s test, P = 0.002; Egger’s test, P = 0.003), but not in OS (Begg’s test, P = 0.54; Egger’s test, P = 0.91). Funnel plots are shown in Fig S1.
Upfront brain RT plus EGFR-TKIs vs. EGFR-TKIs alone
There were 23 studies with 3142 patients for OS and 20 studies with 2612 patients for iPFS. Upfront brain RT plus EGFR-TKIs showed significantly longer OS (HR = 0.75, 95% CI: 0.64–0.88, I2 = 58%) and iPFS (HR = 0.61, 95% CI: 0.52–0.72, I2 = 62%) compared to EGFR-TKIs alone (Fig. 2).
There were 11 studies with 1295 patients for iORR and 9 studies with 1006 patients for iDCR. Compared to EGFR-TKIs alone, upfront brain RT plus EGFR-TKIs achieved significantly higher iORR (OR = 1.70, 95% CI: 1.26–2.29, I2 = 24%) and iDCR (OR = 2.45, 95% CI: 1.37–4.39, I2 = 49%) (Fig. 3).
Subgroup analysis for upfront brain RT plus EGFR-TKIs vs. EGFR-TKIs alone
The OS and iPFS results for each subgroup are shown in Fig. 4.
BM-related symptoms
The addition of upfront brain RT to EGFR-TKIs significantly prolonged OS (3 studies with 320 patients; HR = 0.68, 95% CI: 0.51–0.90, I2 = 0%) but iPFS (5 studies with 427 patients; HR = 0.78, 95% CI: 0.59–1.03, I2 = 35%) in patients with asymptomatic BMs. For symptomatic patients, no significant difference in iPFS was observed between EGFR-TKIs with and without upfront brain RT (4 studies with 253 patients; HR = 0.75, 95% CI: 0.30–1.88, I2 = 87%). There was no significant difference in iPFS benefit between asymptomatic and symptomatic patients (Pinteraction = 0.94).
EGFR mutation subtype
There were no significant differences in OS and iPFS between upfront brain RT plus EGFR-TKIs and EGFR-TKIs alone either in the exon 19 mutation group (2 studies with 152 patients; HR = 0.74, 95% CI: 0.41–1.34, I2 = 0% and 4 studies with 283 patients; HR = 0.82, 95% CI: 0.47–1.44, I2 = 61%) or in the exon 21 mutation group (2 studies with 199 patients; HR = 0.64, 95% CI: 0.20–2.05, I2 = 83% and 4 studies with 318 patients; HR = 0.64, 95% CI: 0.39–1.07, I2 = 65%). P-values for subgroup differences in OS and iPFS benefits were 0.83 and 0.53, respectively.
Number of BMs
Upfront brain RT plus EGFR-TKIs significantly improved OS (2 studies with 161 patients; HR = 0.58, 95% CI: 0.37–0.93, I2 = 0%) and iPFS (5 studies with 423 patients; HR = 0.58, 95% CI: 0.40–0.84, I2 = 48%) compared to EGFR-TKIs alone in patients with > 3 BMs. As for patients with 1–3 BMs, the combination therapy significantly prolonged iPFS (4 studies with 283 patients; HR = 0.61, 95% CI: 0.44–0.85, I2 = 0%) but OS (2 studies with 189 patients; HR = 0.64, 95% CI: 0.28–1.43, I2 = 45%). No significant differences in OS (Pinteraction = 0.85) or iPFS (Pinteraction = 0.81) benefits were observed between the two subgroups.
Sex
Upfront brain RT plus EGFR-TKIs was associated with significantly longer OS and iPFS compared to EGFR-TKIs alone in females (2 studies with 209 patients; HR = 0.55, 95% CI: 0.33–0.93, I2 = 0% and 4 studies with 367 patients; HR = 0.61, 95% CI: 0.40–0.93, I2 = 43%, respectively), but not in males (2 studies with 170 patients; HR = 0.83, 95% CI: 0.51–1.35, I2 = 0% and 4 studies with 267 patients; HR = 0.73, 95% CI: 0.53-1.00, I2 = 0%, respectively). However, there were no significant differences in OS (Pinteraction = 0.26) and iPFS (Pinteraction = 0.50) benefits between the two sexes.
Sensitivity analysis
When each study was omitted individually, the pooled HRs of OS or iPFS did not change markedly, suggesting a relatively stable result (Fig S2).
Discussion
This comprehensive systematic review and meta-analysis assessed the efficacy of first-line treatment with upfront brain RT plus EGFR-TKIs versus EGFR-TKIs alone in patients with EGFR-mutated NSCLC with BMs. Compared to EGFR-TKIs alone, upfront brain RT plus EGFR-TKIs achieved significantly longer OS and iPFS, and higher iORR and iDCR. Nevertheless, the current results are based on retrospective studies with significant heterogeneity, and therefore, need to be validated in large RCTs. In addition, there are still challenges for the use of upfront brain RT, such as RT techniques and advantageous groups.
Historically, whole-brain radiotherapy (WBRT) has been the mainstay of local treatment modality for BMs. However, there is a growing concern regarding its neurological toxicity. Stereotactic radiosurgery (SRS) is known to have less neurotoxicity, and has now become a more widely used brain RT technique. However, whether SRS is superior to WBRT when combined with EGFR-TKIs remains unclear. In a retrospective study of patients with NSCLC with ≤ 3 BMs and EGFR-sensitive mutation [33], SRS + EGFR-TKIs was associated with significantly longer median OS compared to WBRT + EGFR-TKIs. In another retrospective study assessing the optimal treatment strategy for EGFR-mutant NSCLC with BMs [45], EGFR-TKIs plus SRS significantly improved OS compared to EGFR-TKI without SRS for patients with Lung-mol graded prognostic assessment (GPA) ≥ 3 but not for those with Lung-mol GPA < 3. In addition, no significant difference in OS was observed between EGFR-TKI with and without WBRT. These results suggest that first-line SRS plus EGFR-TKIs is more effective than WBRT plus EGFR-TKIs. Nevertheless, SRS is likely limited by the number of intracranial lesions [33] or Lung-mol GPA score [45]. For patients with more BM lesions or low Lung-mol GPA scores, the superiority of SRS requires further evaluation.
In terms of the number of BMs, Zhu et al. [12] and He et al. [28] found that upfront brain RT prolonged iPFS in patients with > 3 BMs, but not in patients with 1–3 BMs. However, Liu et al. [30] reported the better iPFS with upfront brain RT regardless of the number of BMs. In our study, although the upfront brain significantly improved iPFS both in the 1–3 and > 3 BMs groups, only patients with > 3 BMs had significantly longer OS. Nevertheless, no differences in OS (HR = 0.55 vs. 0.83, P = 0.26) and iPFS (HR = 0.58 vs. 0.73, P = 0.31) benefits were observed between the two groups. In addition, many patients with 1–3 BMs in brain RT group received WBRT in this study. As mentioned previously, SRS appears to be more effective than WBRT for patients with limited BM lesions. Thus, the number of BMs is unlikely to be an independent factor associated with the efficacy of upfront brain RT.
The necessity of upfront brain RT in patients with asymptomatic BM remains controversial. The current National Comprehensive Cancer Network (NCCN) guidelines for central nervous system cancers (Version 2.2022) recommend that first-line EGFR-TKIs alone should be considered in asymptomatic patients [46]. However, although EGFR-TKIs alone can prevent brain RT toxicities, they still pose a higher risk of subsequent intracranial progression. In our meta-analysis, upfront brain RT plus EGFR-TKIs significantly improved OS compared with EGFR-TKIs alone in patients with asymptomatic BMs. In addition, iPFS benefit was not significantly different between asymptomatic and symptomatic patients (HRs: 0.78 vs. 0.75; P = 0.94). These findings highlight the value of upfront brain RT for patients with asymptomatic BMs and suggest that first-line treatment with EGFR-TKIs monotherapy may be insufficient for this patients population.
EGFR-TKIs have demonstrated superior OS and PFS in patients with exon 19-deletion mutated NSCLC than compared to those with exon 21-deletion mutated NSCLC [47]. However, compared to low plasma concentration, a high plasma concentration of gefitinib was found to be associated with longer PFS in patients harboring exon 21 mutations but not in those harboring exon 19 mutations [48]. Combined brain RT can disrupt the BBB, leading to increased concentration of EGFR-TKIs in the CSF. Thus, patients with exon 21 mutations may benefit more from the addition of brain RT to EGFR-TKIs than patients with exon 19 mutations. However, we did not find significant difference in OS and iPFS benefits between the two mutation subtypes in this meta-analysis (HRs of OS: 0.74 vs. 0.64, P = 0.83; HRs of iPFS: 0.82 vs. 0.64, P = 0.53). Mutation subtype differences in the benefits of the combination therapy require further investigation in future RCTs.
Sex differences in the efficacy of EGFR-TKIs for NSCLC have also been investigated in clinical studies. A more recent review [49] summarized that women tended to benefit more from first-generation EGFR-TKIs than men in terms of PFS. However, whether sex affects the efficacy of the combination of EGFR-TKIs and brain RT in patients with NSCLC with BMs remains unclear. In our study, although upfront brain RT plus EGFR-TKIs was associated with significantly improved OS and iPFS in females but not in males, the difference in either OS or iPFS benefit between the two sexes did not reach statistical significance (HR of OS: 0.55 vs. 0.83, P = 0.26; HR of iPFS: 0.61 vs. 0.73, P = 0.31). Thus, it remains difficult to conclude whether females have a better response to upfront brain RT than males.
It should be noted that the results of this meta-analysis were based on the first- and second-generation TKIs. Third-generation osimertinib has demonstrated a better BBB penetration [5, 6], and higher iORR and iPFS [50] than first- and second-generation TKIs. These findings raise the question of whether upfront brain RT can be omitted in patients treated with first-line osimertinib therapy? In contrast, osimertinib has been reported to enhance radiosensitivity by reducing NSCLC cell cycle arrest in the G2/M phase, and blocking the repair of RT-induced DNA double-strand breaks [51], suggesting greater efficacy of osimertinib combined with RT. Currently, only a few clinical studies have been conducted on this topic. In a retrospective study by Zhao et al. [32], upfront SRS and/or surgery plus osimertinib was associated with improved OS compared with osimertinib alone. However, the addition of brain RT to osimertinib treatment failed to prolong PFS and OS in another retrospective study [52]. In addition, Cheng et al. [45] found that patients receiving first-line first- or secondgeneration EGFR-TKIs with sequential osimertinib had significantly longer survival than those without sequential osimertinib, regardless of the use of brain RT. Collectively, the value of upfront brain RT in the era of first-line osimertinib treatment requires further exploration.
Compared with the previous meta-analyses [34,35,36,37], our study included more studies with larger sample sizes. In addition, subgroup analyses of OS and iPFS were performed to identify advantageous groups. Moreover, our meta-analysis revealed some new findings, including that patients with asymptomatic BMs could also benefit from the upfront brain RT; and the benefits from the combination therapy did not appear to be influenced by BM-related symptoms, mutation subtype, number of BMs, or sex. These findings will be helpful in determining first-line treatment strategies for patients with NSCLC with EGFR mutations and newly diagnosed BMs.
Nevertheless, our meta-analysis had some limitations. First, all data were extracted from retrospective studies, which might have selection bias. In addition, clinical characteristics were unbalanced between groups in some studies. Moreover, the number of studies included in subgroup analyses was relatively small. All these factors may have resulted in lower reliability of our results. Second, there was significant heterogeneity and publication bias for OS and/or PFS. The results of the subgroup analyses indicated that BMs related symptoms, EGFR mutation subtype, number of BMs, and sex might be associated with the heterogeneity. In addition, the patients’ ECOG performance status and extracranial metastases were inconsistent among studies, which might also account for some heterogeneity. Third, most of the included studies were conducted in Asia, and generalizing the findings to other regions should be done with caution. Fourth, OS and iPFS were measured from the start of EGFR-TKI therapy or from the date of BM diagnosis. The inconsistent starting time points for OS and iPFS used in the included studies might have resulted in an immortal time bias. Fifth, some HRs were not directly reported in the texts, and were calculated using the Kaplan-Meier curves. This may also result in bias. Finally, treatment-related adverse events were not assessed in our study because of a lack of data.
Conclusions
First-line treatment with upfront brain RT and EGFR-TKIs is likely to be more effective than EGFR-TKIs alone. The benefits of the combination therapy did not appear to be significantly affected by BM-related symptoms, EGFR mutation subtype, number of BMs, or sex. Nevertheless, these findings need to be confirmed in future RCTs. Whether these results can be extrapolated to third-generation EGFR-TKIs requires further investigation.
Data availability
All datasets generated for this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Abbreviations
- RT:
-
Radiotherapy
- EGFR-TKIs:
-
Epidermal growth factor receptor tyrosine kinase inhibitors
- BMs:
-
Brain metastases
- OS:
-
Overall survival
- iPFS:
-
Intracranial progression-free survival
- HRs:
-
Hazard ratios
- CIs:
-
Confidence intervals
- WBRT:
-
Whole-brain radiotherapy
- SRS:
-
Stereotactic radiosurgery
- NSCLC:
-
Non-small cell lung cancer
- EGFR:
-
Epidermal growth factor receptor
- CSF:
-
Cerebrospinal fluid
- BBB:
-
Blood-brain barrier
- RCTs:
-
Randomized controlled trials
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analysis
- iORR:
-
Intracranial objective response rate
- iDCR:
-
Intracranial disease control rate
- NOS:
-
Newcastle-ottawa scale
- ORs:
-
Odds ratios
- IQR:
-
Interquartile range
References
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311.
Martínez P, Mak RH, Oxnard GR. Targeted therapy as an alternative to whole-brain Radiotherapy in EGFR-Mutant or ALK-Positive non-small-cell Lung Cancer with Brain metastases. JAMA Oncol. 2017;3(9):1274–5.
Ahluwalia MS, Becker K, Levy BP. Epidermal growth factor receptor tyrosine kinase inhibitors for Central Nervous System metastases from Non-small Cell Lung Cancer. Oncologist. 2018;23(10):1199–209.
Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of Osimertinib with other EGFR-TKIs in EGFR-Mutant NSCLC Brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–40.
Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC, Ahn JS, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell Lung Cancer and Leptomeningeal metastases: the BLOOM Study. J Clin Oncol. 2020;38(6):538–47.
Khalifa J, Amini A, Popat S, Gaspar LE, Faivre-Finn C, International Association for the Study of Lung Cancer Advanced Radiation Technology Committee. Brain metastases from NSCLC: Radiation Therapy in the era of targeted therapies. J Thorac Oncol. 2016;11(10):1627–43.
Zhang J, Yu J, Sun X, Meng X. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of central nerve system metastases from non-small cell Lung cancer. Cancer Lett. 2014;351(1):6–12.
Byeon S, Ham JS, Sun JM, Lee SH, Ahn JS, Park K, et al. Analysis of the benefit of sequential cranial radiotherapy in patients with EGFR mutant non-small cell Lung cancer and brain Metastasis. Med Oncol. 2016;33(8):97.
Jiang T, Su C, Li X, Zhao C, Zhou F, Ren S, et al. EGFR TKIs plus WBRT demonstrated no Survival Benefit Other Than that of TKIs alone in patients with NSCLC and EGFR Mutation and Brain metastases. J Thorac Oncol. 2016;11(10):1718–28.
Chen Y, Yang J, Li X, Hao D, Wu X, Yang Y, et al. First-line epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor alone or with whole-brain radiotherapy for brain metastases in patients with EGFR-mutated lung adenocarcinoma. Cancer Sci. 2016;107(12):1800–5.
Zhu Q, Sun Y, Cui Y, Ye K, Yang C, Yang D, et al. Clinical outcome of tyrosine kinase inhibitors alone or combined with radiotherapy for brain metastases from epidermal growth factor receptor (EGFR) mutant non small cell Lung cancer (NSCLC). Oncotarget. 2017;8(8):13304–11.
Liu Y, Deng L, Zhou X, Gong Y, Xu Y, Zhou L, et al. Concurrent brain radiotherapy and EGFR-TKI may improve intracranial metastases control in non-small cell Lung cancer and have survival benefit in patients with low DS-GPA score. Oncotarget. 2017;8(67):111309–17.
Fan Y, Xu Y, Gong L, Fang L, Lu H, Qin J, et al. Effects of icotinib with and without radiation therapy on patients with EGFR mutant non-small cell Lung cancer and brain metastases. Sci Rep. 2017;7:45193.
Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase Inhibitor-Naïve epidermal growth factor receptor-mutant non-small-cell Lung Cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35(10):1070–7.
Wang W, Song Z, Zhang Y. Efficacy of brain radiotherapy plus EGFR-TKI for EGFR-mutated non-small cell Lung cancer patients who develop brain Metastasis. Arch Med Sci. 2018;14(6):1298–307.
Sung S, Lee SW, Kwak YK, Kang JH, Hong SH, Kim YS. Intracranial control and survival outcome of tyrosine kinase inhibitor (TKI) alone versus TKI plus radiotherapy for brain Metastasis of epidermal growth factor receptor-mutant non-small cell Lung cancer. J Neurooncol. 2018;139(1):205–13.
Li SH, Liu CY, Hsu PC, Fang YF, Wang CC, Kao KC, et al. Response to afatinib in treatment-naïve patients with advanced mutant epidermal growth factor receptor lung adenocarcinoma with brain metastases. Expert Rev Anticancer Ther. 2018;18(1):81–9.
Chen H, Wu A, Tao H, Yang D, Luo Y, Li S, et al. Concurrent versus sequential whole brain radiotherapy and TKI in EGFR-mutated NSCLC patients with brain Metastasis: a single institution retrospective analysis. Med (Baltim). 2018;97(44):e13014.
Ke SB, Qiu H, Chen JM, Shi W, Chen YS. Therapeutic effect of first-line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR-TKI) combined with whole brain radiotherapy on patients with EGFR mutation-positive lung adenocarcinoma and brain metastases. Curr Med Sci. 2018;38(6):1062–8.
Yu M, Zhao Q, Li Y, Zhang S, Xu Y, Gong Y, et al. Progression of Central Nervous System metastases in Advanced Nonsmall Cell Lung Cancer patients effectively treated with first-generation epidermal growth factor receptor-tyrosine kinase inhibitor. Cancer Biother Radiopharm. 2018;33(10):421–6.
Wang C, Lu X, Zhou Z, Wang J, Hui Z, Liang J, et al. The efficacy of Upfront Intracranial Radiation with TKI compared to TKI alone in the NSCLC patients Harboring EGFR Mutation and Brain metastases. J Cancer. 2019;10(9):1985–90.
Lee JH, Chen HY, Hsu FM, Chen JS, Liao WY, Shih JY, et al. Cranial irradiation for patients with Epidermal Growth Factor Receptor (EGFR) Mutant Lung Cancer who have brain metastases in the era of a New Generation of EGFR inhibitors. Oncologist. 2019;24(12):e1417–25.
Chen Y, Wei J, Cai J, Liu A. Combination therapy of brain radiotherapy and EGFR-TKIs is more effective than TKIs alone for EGFR-mutant lung adenocarcinoma patients with asymptomatic brain Metastasis. BMC Cancer. 2019;19(1):793.
An N, Wang H, Li J, Zhai X, Jing W, Jia W, et al. Therapeutic effect of First-Line EGFR-TKIs Combined with Concurrent Cranial Radiotherapy on NSCLC patients with EGFR activating mutation and brain Metastasis: a retrospective study. Onco Targets Ther. 2019;12:8311–8.
Saida Y, Watanabe S, Abe T, Shoji S, Nozaki K, Ichikawa K, et al. Efficacy of EGFR-TKIs with or without upfront brain radiotherapy for EGFR-mutant NSCLC patients with central nervous system metastases. Thorac Cancer. 2019;10(11):2106–16.
Saruwatari K, Ikeda T, Saeki S, Shingu N, Imamura K, Komatu T, et al. Upfront cranial Radiotherapy followed by Erlotinib positively affects clinical outcomes of epidermal growth factor receptor-mutant non-small cell Lung Cancer with Brain metastases. Anticancer Res. 2019;39(2):923–31.
He ZY, Li MF, Lin JH, Lin D, Lin RJ. Comparing the efficacy of concurrent EGFR-TKI and whole-brain radiotherapy vs EGFR-TKI alone as a first-line therapy for advanced EGFR-mutated non-small-cell Lung cancer with brain metastases: a retrospective cohort study. Cancer Manag Res. 2019;11:2129–38.
Hyun DG, Choi CM, Lee DH, Kim SW, Yoon S, Kim WS, et al. Outcomes according to initial and subsequent therapies following intracranial progression in patients with EGFR-mutant Lung cancer and brain Metastasis. PLoS ONE. 2020;15(4):e0231546.
Liu Y, Wang J, Wu J, Yang Q, Zeng Y, Wu D, Tian C, Hu Y, Gu F, Li C, Zhang K, Liu L. The efficacy of First-Generation EGFR-TKI Combined with Brain Radiotherapy as the First-Line treatment for lung adenocarcinoma patients with brain metastases and EGFR sensitive mutations: a retrospective study. Technol Cancer Res Treat. 2021;20:1533033821997819.
Gu Y, Xu Y, Zhuang H, Jiang W, Zhang H, Li X, et al. Value and significance of brain radiation therapy during first-line EGFR-TKI treatment in lung adenocarcinoma with EGFR sensitive mutation and synchronous brain Metastasis: appropriate timing and technique. Thorac Cancer. 2021;12(23):3157–68.
Zhao Y, Li S, Yang X, Chu L, Wang S, Tong T, et al. Overall survival benefit of osimertinib and clinical value of upfront cranial local therapy in untreated EGFR-mutant nonsmall cell Lung cancer with brain Metastasis. Int J Cancer. 2022;150(8):1318–28.
Jia F, Cheng X, Zeng H, Miao J, Hou M. Clinical research on stereotactic radiosurgery combined with epithermal growth factor tyrosine kinase inhibitors in the treatment of brain Metastasis of non-small cell Lung cancer. J BUON. 2019;24(2):578–84.
Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of up-front radiotherapy and TKI with TKI alone for NSCLC with brain metastases and EGFR mutation: a meta-analysis. Lung Cancer. 2018;122:94–9.
Du XJ, Pan SM, Lai SZ, Xu XN, Deng ML, Wang XH, et al. Upfront cranial radiotherapy vs. EGFR tyrosine kinase inhibitors alone for the treatment of Brain metastases from non-small-cell Lung Cancer: a Meta-analysis of 1465 patients. Front Oncol. 2018;8:603.
Wang X, Xu Y, Tang W, Liu L. Efficacy and safety of Radiotherapy Plus EGFR-TKIs in NSCLC patients with brain metastases: a Meta-analysis of published data. Transl Oncol. 2018;11(5):1119–27.
Dong K, Liang W, Zhao S, Guo M, He Q, Li C, et al. EGFR-TKI plus brain radiotherapy versus EGFR-TKI alone in the management of EGFR-mutated NSCLC patients with brain metastases. Transl Lung Cancer Res. 2019;8(3):268–79.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Cheng WC, Shen YC, Chien CR, Liao WC, Chen CH, Hsia TC, et al. The optimal therapy strategy for epidermal growth factor receptor-mutated non-small cell Lung cancer patients with brain Metastasis: a real-world study from Taiwan. Thorac Cancer. 2022;13(10):1505–12.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology for central nervous system cancers. Version 2.2022. http://www.nccn.org/patients.
Hayashi H, Nadal E, Gray JE, Ardizzoni A, Caria N, Puri T, et al. Overall treatment strategy for patients with metastatic NSCLC with activating EGFR mutations. Clin Lung Cancer. 2022;23(1):e69–e82.
Okuda Y, Sato K, Sudo K, Hasegawa Y, Asano M, Miura H, et al. Low plasma concentration of gefitinib in patients with EGFR exon 21 L858R point mutations shortens progression-free survival. Cancer Chemother Pharmacol. 2017;79(5):1013–20.
Huang Y, Cho HJ, Stranger BE, Huang RS. Sex dimorphism in response to targeted therapy and immunotherapy in non-small cell Lung cancer patients: a narrative review. Transl Lung Cancer Res. 2022;11(5):920–34.
Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A et al. CNS response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in patients with untreated EGFR-Mutated Advanced Non-small-cell Lung Cancer. J Clin Oncol. 2018:JCO2018783118.
Wang N, Wang L, Meng X, Wang J, Zhu L, Liu C, et al. Osimertinib (AZD9291) increases radio-sensitivity in EGFR T790M non–small cell Lung cancer. Oncol Rep. 2019;41(1):77–86.
Xie L, Nagpal S, Wakelee HA, Li G, Soltys SG, Neal JW. Osimertinib for EGFR-Mutant Lung Cancer with Brain metastases: results from a single-Center Retrospective Study. Oncologist. 2019;24(6):836–43.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception and design: DJ. Collection and assembly of data: SY and LS. Data analysis and interpretation: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
PRISMA Checklist. Table S2 Search strategy. Table S3 Quality assessment of retrospective studies using the Newcastle-Ottawa scale. Table S4 GRADE assessment. Fig S1 Funnel plots of publication bias. OS, overall survival; iPFS, intracranial progression-free survival. Fig S2 Sensitivity analysis. OS, overall survival; iPFS, intracranial progression-free survival.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, Y., Lin, S., Chen, J. et al. First-line treatment with TKI plus brain radiotherapy versus TKI alone in EGFR-mutated non-small cell Lung cancer with brain metastases: a systematic review and meta-analysis. BMC Cancer 23, 1043 (2023). https://doi.org/10.1186/s12885-023-11548-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11548-0