Abstract
Aim
The aim of this study was to investigate genetic alterations within breast cancer in the setting of recurrent or de novo stage IV disease.
Patients and methods
: This study included 22 patients with recurrent breast cancer (n = 19) and inoperable de novo stage IV breast cancer (n = 3). For next generation sequencing, FoundationOneCDx (F1CDx) (Foundation Medicine Inc., Cambridge, MA, USA) was performed in 21 patients and FoundationOneLiquid CDx was performed in 1 patient.
Results
Median age was 62.9 years (range, 33.4–82.1). Pathological diagnoses of specimens included invasive ductal carcinoma (n = 19), invasive lobular carcinoma (n = 2), and invasive micropapillary carcinoma (n = 1). F1CDx detected a median of 4.5 variants (range, 1–11). The most commonly altered gene were PIK3CA (n = 9), followed by TP53 (n = 7), MYC (n = 4), PTEN (n = 3), and CDH1 (n = 3). For hormone receptor-positive patients with PIK3CA mutations, hormonal treatment plus a phosphoinositide 3-kinase inhibitor was recommended as the treatment of choice. Patients in the hormone receptor-negative and no human epidermal growth factor receptor 2 expression group had significantly higher tumor mutational burden than patients in the hormone receptor-positive group. A BRCA2 reversion mutation was revealed by F1CDx in a patient with a deleterious germline BRCA2 mutation during poly ADP ribose polymerase inhibitor treatment.
Conclusion
Guidance on tailored precision therapy with consideration of genomic mutations was possible for some patients with information provided by F1CDx. Clinicians should consider using F1CDx at turning points in the course of the disease.
Similar content being viewed by others
Introduction
Modern advanced diagnostic technology that includes radiological and genomic approaches enables patients to receive personalized precision care. For breast cancer, tailored treatment strategies consider not only clinicopathological findings but also genomic profiling results. It is estimated that 5 to 10% of women breast cancer cases are linked to germline mutations [1]. The well-known germline mutation associated with high breast cancer risk includes BRCA1/2 mutation. For the recurrent breast cancer patients harboring those germline mutations, the treatment of olaparib, a poly (ADP-ribose) polymerase inhibitor, is considered as an effective targeted therapy [2]. And alpelisib, phosphoinositide 3-kinase inhibitor, shows efficacy in PIK3CA-mutated recurrent breast cancer patients [3]. Unfortunately, for breast cancer patients who carry other mutations such as PTEN, TP53, CDH1 and MYC, no effective medication is introduced. Under the circumstances, 10 types of genome screening systems are currently available [4]. And only 3 of them are approved by the national health insurance system: OncoGuide™ NCC Oncopanel System (Sysmex Corporation, Kobe, Japan) [5], FoundationOne®CDx (F1CDx), and FoundationOne® Liquid CDx (F1CDx-Liquid) (Foundation Medicine Inc., Cambridge, MA, USA) [6,7,8]. Although the OncoGuide™ NCC Oncopanel System can evaluate germline mutations in blood samples, it can evaluate fewer variants that F1CDx (114 versus 324 variants, respectively). F1CDx is approved as a companion diagnosis method.
In this study, we performed comprehensive genomic profiling using F1CDx and F1CDx-Liquid for 22 patients with breast cancer, which included 19 patients with recurrent disease and 3 patients with de novo stage IV disease. This study was conducted using real-world clinical data. There are few publications about this type of investigation in the field of breast cancer treatment. The clinically applicable data presented in this study might contribute to improving treatment strategies for advanced and metastatic breast cancer.
Patients and methods
This study was conducted to compile the comprehensive genomic profiling data using F1CDx and F1CDx-Liquid retrospectively and to elucidate the specific genomic alterations of the breast cancer patients. And we weighted those genomic date against the conventional intrinsic subtype of breast cancer.
Patients
A total of 22 patients with breast cancer who had a F1CDx examination date between January 2020 and May 2022 were enrolled in this study. Among these 22 patients, 17 patients underwent surgery at Aichi Medical University Hospital (Nagakute, Aichi, Japan), 1 patient at Marumo Hospital (Nagoya, Aichi, Japan), and 1 patient at Aichi Cancer Center Hospital (Nagoya, Aichi, Japan). They were referred to our institution when recurrence was diagnosed. The remaining 3 patients were diagnosed with de novo stage IV cancer on the first visit to the outpatient clinic of our hospital. Patients’ past medical history and family history of cancer were summarized in Table 1. Information on TNM stage [9], operative procedure, and lines of previous chemotherapy were collected from medical records.
Tumor pathology
Pathological assessment of specimens from all patients was performed by the Department of Pathology at Aichi Medical University Hospital. Histologic type was determined according to World Health Organization criteria [10]. Estrogen receptor (ER) or progesterone receptor (PgR) positivity was defined as moderate-to-intense nuclear staining of ≥ 10%. Human epidermal growth factor 2 (HER2) positivity was defined based on fluorescence in situ hybridization (FISH) with the PathVysion®HER-2 DNA Probe kit (Abbott Pharmaceutical Co. Ltd., Lake Bluff, IL, USA); results were assessed according to the manufacturer’s instructions. For FISH score assessment, a HER2:centromeric probe 17 (CEP 17) ratio of ≥ 2 was defined as positive for HER2 amplification. A programmed death-ligand 1 (PD-L1) immunohistochemistry (IHC) assay was performed for patients whose hormonal and HER2 status was ER(-), PgR(-), and HER2(-). In this subgroup, patients who are PD-L1–positive might benefit from treatment with a immune checkpoint inhibitor such as pembrolizumab or atezolizumub [11]. For the SP142 IHC assay for PD-L1 (VENTANA OptiView PD-L1 (SP142), F. Hoffmann-La Roche Ltd., Basel, Switzerland), the percentage of immune cells was recorded as the percentage of tumor area (consisting of tumor cells and associated intratumoral and contiguous peritumoral stroma) occupied by immune cells with discernible PD-L1 staining of any intensity. Positivity was defined as ≥ 1% immunoreactive cells [12]. For the 22C3 IHC assay for PD-L1 (Dako, Carpinteria, CA, USA), the combined positive score (CPS) was defined as the number of PD-L1–stained cells (which includes tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells and multiplied by 100. Positivity was defined as CPS ≥ 1 [13].
BRCA1/2 germline mutations
For the assessment of germline mutations BRCA1/2, BRACAnalysis CDx® (Myriad Genetics, Inc., Salt Lake City, UT, USA) was used in all patients. This commercialized test was first established for ovarian cancer [14]. This examination was conducted at the time of diagnosis of recurrence for 19 patients and at time of diagnosis of de novo stage IV cancer for 3 patients. The results of this examination were confirmed by attending physicians.
FoundationOne®CDx
Comprehensive genomic analyses were performed using F1CDx in all but 1 patient for whom F1CDx-Liquid was used [6,7,8]. Each attending physician decided on whether to use F1CDx or F1CDx-Liquid. The specimens in the present series subjected to F1CDx analysis had (mean ± standard deviation) 38.6 ± 11.2% tumor nuclei. The F1CDx-targeted next-generation sequencing (NGS) platform has been previously described and validated. The methodology was demonstrated by Frampton et al. [7]. After formalin-fixed, paraffin-embedded samples were retrieved from biopsy or surgical specimens, 10 unstained sections and 1 hematoxylin and eosin stained section with thickness of 5 μm and tumor area > 25 mm2 were delivered to Foundation Medicine Inc. All samples were confirmed as adenocarcinoma and contained a minimum of 20% tumor cells. They were graded as Pass or Qualified. F1CDx applies NGS across the entire coding DNA of 324 genes proven to be solid tumor drivers. In addition, both tumor mutational burden (TMB) and microsatellite instability (MSI) was evaluated. TMB was presented as the number of mutations per megabase (Mut/Mb) of sequenced DNA. MSI was classified as stable, intermediate, or high. If the DNA sequence could not be determined with confidence, the result for TMB and MSI was reported as “cannot be determined.”
FoundationOne® Liquid CDx
F1CDx-Liquid is an NGS-based in vitro diagnostic method targeting 324 genes that is approved by the U.S. Food and Drug Administration. It uses circulating cell-free DNA (cfDNA) isolated from plasma derived from anti-coagulated peripheral whole blood of patients with cancer. All coding exons of 309 genes are targeted; select intronic or non-coding regions are targeted in 21 of these genes. Additionally, select intronic or non-coding regions are targeted in 15 genes, resulting in 324 total targeted genes. The assay detects substitutions, indels, genomic rearrangements, copy number alterations (CNAs) including amplifications and losses, and genomic signatures including blood TMB and MSI [8].
Statistical analysis
BellCurve for Excel (Social Survey Research Information, Tokyo, Japan) was used for statistical analyses. The Mann-Whitney U test was performed for line of chemotherapy, number of pathogenic variants, variants of unknown significance, and tumor mutational burden score. The significance level was defined as 0.05. To assess the correlation between line of chemotherapy and number of variants, linear regression was used. Correlations were expressed with Pearson correlation coefficients. The correlation coefficient was scaled with range from − 1 to + 1, where 0 indicates no linear association [15].
Results
Table 1 demonstrates the characteristics of the patients enrolled in this study. Median age at the date of F1CDx and F1CDx-Liquid examination was 62.9 years (range, 33.4–82.1 years). All patients were female. One patient had a history of adult T-cell leukemia, which had been completely cured before breast cancer treatment. Three patients had a family history of breast cancer and 1 patient each had a family history of lung cancer, cervical cancer, and endometrial cancer. Three patients were diagnosed with de novo stage IV breast cancer; they received hormonal or chemotherapeutic treatment without surgical intervention.
Among 22 patients enrolled, 2 patients experienced receptor conversion at sites of metastases (Table 2).
For those 2 patients, the F1CDx assay was carried out using the specimen biopsied at the sites of metastases, metastatic lymph node, and lung. Tumor cells at those sites had demonstrated receptor conversion. In the patient who had lung metastasis, HER2 overexpression in the primary breast cancer shifted to HER2-negative status. The fluorescence in situ hybridization (FISH) score, which indicates the HER2 status of this patient, changed from 2.64 (primary lesion) to 1.1 (metastatic lesion).
For all patients, the BRACAnalysis CDx® genetic test was used to detect germline BRCA1/2 mutations [14]. A germline BRCA2 mutation was found in only 1 patient who presented with a BRCA2 reversion mutation during treatment with olaparib, a poly(ADP-ribose) polymerase inhibitor.
Three patients (13.6%) had a high TMB score (> 10 mut/Mb). None of them received immunotherapy although patients with a high TMB would benefit from T-cell checkpoint inhibitors (Table 3). It is reported that hypermutation occurs in about 5% of all breast cancers [16].
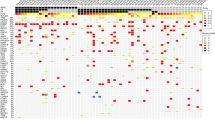
Variant alterations are summarized in Table 4. All patients had at least 1 identified variant that was either verified or likely pathogenic, with a median of 4.5 variants identified per patient (range, 1–11). The right side of Table 4 presents the probability of being loss-of-function intolerant (pLI) and combined annotation dependent depletion (CADD) scores. The pLI score reflects the tolerance of a target gene to the loss of its function on the basis of the number of protein truncating variants. A pLI score > 0.9 indicates that the mutation would cause disease with autosomal inheritance [17]. The CADD score is used to measure the deleteriousness of variants; the score predicts the pathogenicity of the variant [18]. Among the types of genomic functional effects, copy number alterations (CNAs) such as amplification and loss were recognized in 11 and 4 genes, respectively (Table 4).
Discussion
Breast cancer is the second leading cause of cancer deaths among women recently. In Japan, 94,519 people were diagnosed with breast cancer in 2018. Regrettably, 14,779 (15.6%) patients died due to disease progression in 2020 [19]. If patients are affected by distant metastases, the chance of survival is decreased, as indicated in the 5-year relative survival rate with distant metastases of 39.3% [19]. It is a crucial obligation for clinicians specializing in breast cancer treatment to improve patient survival as much as possible. We have to devise a new therapeutic strategy, forsaking outdated considerations based on temporal changes in levels of tumor markers such as carcinoembryonic antigen and carbohydrate antigen 15 − 3. Since the phenotype of breast cancer is frequently altered at the time of metastasis, re-biopsy at metastatic sites and subsequent decision-making about appropriate therapy with consideration of subtype are recommended [20,21,22]. Modern advanced analytic techniques have opened up a new era for in-depth assessment of tumors using genomic assays. Currently, germline mutations in BRCA1/2 and PD-L1 expression can be assessed easily with commercially available products [14, 23]. Obtaining this information has not been possible with classical IHC analyses. We clinicians have to take into consideration that genomic evaluation of cancer recurrence for personalized precision therapy is inevitable. As the latest frontier of comprehensive genomic profiling, we employed F1CDx as a NGS technology for the examination of entire exon regions of cancer-relevant genes. According to the literature research, Burstein MD et al. identified four distinct triple-negative breast cancer subtypes: (i) luminal androgen receptor, (ii) mesenchymal, (iii) basal-like immunosuppressed, and (iv) basal-like immune-activated by genomic profiling [24]. And the prevalence and distribution of immunotherapy responsiveness-associated gene mutations were identified by F1CDx [25].
The most prevalent altered gene was PIK3CA, with alterations detected in 9 (40.9%) patients. The median age of them was 63.1 years old (range, 43.7–78.2). PIK3CA alterations have been reported in 24–40% of patients with breast cancer [16, 26,27,28,29,30]. PIK3CA-activated alterations, like those in the 9 patients in the present series, might predict sensitivity to agents that target phosphoinositide 3-kinase, such as alpelisib. In the SOLAR-1 trial, median overall survival (OS) of the cohort with PIK3CA mutations (n = 341) was 39.3 months (95% CI, 34.1–44.9) for alpelisib in combination with fulvestrant and 31.4 months (95% CI, 26.8–41.3) for placebo-fulvestrant with a hazard ratio (HR) of 0.86 (95% CI, 0.64–1.15; P = 0.15). Alpelisib-fulvestrant failed to prolong OS, but median time to chemotherapy was significantly extended [3]. In the F1CDx report of each patient, this treatment was described as a clinically beneficial.
TP53 alteration was the second most commonly altered gene in our study. The 7 patients (31.8%) with TP53 mutations also had negative hormonal and HER2 status, i.e., triple-negative breast cancer (TNBC). And their median age was 46.4 years old (range, 33.4–81.9). TP53 is one of the most frequently mutated genes in breast cancer, with mutations detected in 27–37% of breast carcinoma samples [31,32,33,34,35,36]. TP53 mutations are also implicated in breast cancer susceptibility because TP53 mutation carriers have an 18–60–fold increased risk for early-onset breast cancer [37,38,39]. And 3 patients with TP53 alterations had high CADD scores and high allele fractions, above 0.5. However, no related pathogenic disorders were recognized in those 3 patients. Functional loss of the tumor suppressor p53, which is encoded by TP53, is commonly identified in aggressive advanced cancers [40]. Germline mutations in TP53 are associated with autosomal dominant disorder Li-Fraumeni syndrome and early onset of many caners [41,42,43]. Unfortunately, there are no approved treatments to address TP53 mutation or loss.
MYC amplification occurred in 4 patients: 3 patients with TNBC and 1 with luminal breast cancer. The median age of them was 47.4 years old (range, 38.0–66.8). The median amplification ratio was 2.165 (range, 1.9–2.52). MYC overexpression is reported to be higher in TNBC than in luminal breast cancer; it is associated with particularly poor outcomes and the loss of tumor suppressor pathways such as p53 [40, 44, 45]. In our series, all 3 TNBC-patients with MYC amplification harbored TP53 mutations with high allele fractions, ranging from 0.5337 to 0.6729.
PTEN alterations were detected in 3 patients (13.6%), 1 with loss and 2 with frameshift and nonsense mutations. The median age of them was 47.7 years old (range, 43.8–51.8). PTEN alterations are more frequently associated with triple-negative breast cancer than HER2 or hormone-positive breast cancer [46, 47]. Loss or reduction of PTEN expression is observed in 28% of invasive ductal carcinomas. It is associated with poor prognosis, including a shorter disease-free survival of approximately 2 years [48, 49]. In our series, the pathological diagnosis of the primary lesion in all 3 patients was triple-negative type breast cancer. Their disease-free interval was short: 22 months, 11 months, and 6 months. Their clinical courses seemed very aggressive. On the other hand, PTEN mutations cause inherited disorders such as Cowden syndrome. The incidence of Cowden syndrome is approximately 1 in 200,000 but is generally underestimated due to the high variability of this disorder [50]. In our series, the allele fraction of the 2 patients with frameshift and nonsense mutations was high at 0.8385 and 0.5590, respectively. Germline testing should be taken into consideration even when no family history of malignant cancer has been recognized. We could not perform germline testing because the patients did not consent to genetic testing.
CDH1 mutations were identified in 3 patients. The median age of them was 68.2 years old (range, 48.7–82.1). CDH1 encodes the transmembrane protein E-cadherin, which plays an important role in epithelial cell-cell adhesion [51]. Inactivation of CDH1 is considered to be a genetic hallmark of invasive lobular breast carcinoma, with CDH1 mutations in 46–65% of cases [50,51,52,53]. In our series, 2 patients with CDH1 mutations were classified as having invasive lobular carcinoma and 1 was classified as having invasive ductal carcinoma (n = 1).
Another noteworthy mutation was detected in the SDHA gene. Mutations in this gene are associated with hereditary paraganglioma-pheochromocytoma syndrome and mitochondrial complex II deficiency (Clin Var, https://www.ncbi.nlm.nih.gov/clinvar/) [54]. In our series, this mutation was detected in 2 patients (54.9 and 65.4 years) with an allele frequency of 0.5006 and 0.4739, respectively. As their allele frequencies were relatively high, germline testing of SDHA should have been performed. However, we did not propose such testing because there was no confirmed family history.
In Table 5, we reevaluated the comprehensive genomic profile from the viewpoint of intrinsic molecular subtypes of breast cancer. In this series, there were 8 patients with TNBC (HR(-), HER2(-)) and 14 patients with luminal breast cancer (HR(+), HER2(-)). No significant differences were identified in the number of variants, including pathogenic variants and variants of unknown significance. However, TNBC had a significantly higher TMB score than luminal breast cancer. The elevation pf PD-L1 status was recognized in 4 (50%) patients with TNBC. It is reported that BRAF and PBRM1 mutations would benefit by immune check point inhibitor, however, such genomic alterations could not be identified in those 4 patients with elevated PD-L1 status [25]. On the other hand, somatic mutations were expected to be induced with cancer chemotherapy. The lines of chemotherapy before F1CDx examination were considered to be low. Linear regression did not identify any correlations between the line of chemotherapy and the number of variants. The multiple correlation coefficient of those two factors was almost zero (P < 0.001).
Herein we describe the case of a 67-year-old female who presented with a BRCA2 reversion mutation. The patient had metastatic recurrence in a left subclavian lymph node. Because BRACAnalysis CDx® revealed a germline BRCA2 mutation (c.6446_6450delTTAAA), olaparib was administered. This treatment remained efficacious for approximately 13 months. Subsequently, the lymph node showed re-growth, overcoming olaparib treatment. F1CDx performed using the re-biopsy specimen of the swollen left subclavian lymph node showed putative somatic BRCA2 reversion mutations c.6419_6457del129 and c.6466_6469delTCTC with allele fractions of 0.0541 and 0.2772, respectively. The allele fraction of the germline BRCA2 mutation diagnosed with F1CDx was 0.8739 in a re-biopsied lymph node. The secondary BRCA2 mutations presented here removed an initial deleterious mutation and resulted in partial restoration of BRCA function [55, 56]. Therefore, abemaciclib, a cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitor, and letrozole were chosen as the next therapeutic agents. The curative effect of this treatment was acceptable. The lymph node remained stable in size without re-growth for a year and half. The patient was classified as having stable disease overall.
One useful feature of the F1CDx assay is prediction of response to gene-targeted therapies by profiling the total number of synonymous and non-synonymous mutations across the coding regions of 324 cancer-associated genes. In our series of F1CDx assays, we could not propose an approved therapy available in Japan that is tailored to the genomic alterations for all patients. PIK3CA alteration, which is most prevalent mutation in our study, is eligible for alpelisib treatment, nevertheless the agent has not been approved in Japan. However, we identified several other alterations including TP53 mutations and MYC amplifications, both of which are associated with poor prognosis in breast cancer. Those 2 variants were recognized in TNBC, which were considered to have aggressive features. We identified a SDHA mutation that was potentially a pathogenic germline mutation. TMB score, which was significantly higher in TNBC, was also evaluated with the F1CDx assay. TMB is reported to be associated with sensitivity to programmed death-1 and immune checkpoint inhibitors that target PD-L1 [6]. Throughout each patient’s long cancer journey, genomic alterations assessed with F1CDx might reflect transitory genetic variations affected by invasive treatment. To promote individualized care, clinicians should share personalized genetic information with patients and make evidence-based decisions in consideration of genetic heterogeneity.
For F1CDx, re-biopsy of the recurrent lesion is recommended. We experienced a case of a BRCA2 reversion mutation and a case of HER2 receptor conversion. The F1CDx assay and FISH analysis of the resected lung metastasis revealed the loss of HER2 expression in a 62-year-old female patient. We should confirm whether biological features of tumors continue to change despite medical interventions.
This study had some limitations. First, although the gene assays of 2 patients, which consisted of assays performed after neoadjuvant chemotherapy and skin metastasis, respectively, were performed completely, the quality of the process was not optimal due to the low tumor nuclei content. Second, because the number of patients enrolled in this study was small, we evaluated only a limited range of genetic alterations. Third, the F1CDx assay can detect many CNAs, but CNA frequency in the study patients cannot be demonstrated with this assay.
In conclusion, the use of F1CDx in clinical settings will contribute to encouraging tailored precision treatment for patients with breast cancer. Clinicians should consider using comprehensive genomic profiling at turning points in the course of the disease.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Abbreviations
- F1CDx:
-
FoundationOneCDx
- F1CDx-Liquid:
-
FoundationOne® Liquid CDx
- ER:
-
estrogen receptor
- PgR:
-
progesterone receptor
- HER2:
-
human epidermal growth factor 2
- CEP 17:
-
centromeric probe 17
- PD-L:
-
programmed death-ligand 1
- IHC:
-
immunohistochemistry
- CPS:
-
combined positive score
- NGS:
-
next-generation sequencing
- TMB:
-
tumor mutational burden
- MSI:
-
microsatellite instability
- Mut/Mb:
-
mutations per megabase
- cfDNA:
-
cell-free DNA
- CTA:
-
clinical trial assay
- CI:
-
confidence interval
- FISH:
-
fluorescence in situ hybridization
- HR:
-
hazard ratio
- TNBC:
-
triple-negative breast cancer
- pLI:
-
probability of being loss-of-function intolerant
- CADD:
-
combined annotation dependent depletion
- CAN:
-
copy number alteration
- HR:
-
hormonal receptor
- CDK4/6:
-
cyclin-dependent kinases 4 and 6
References
Loman N, Johannsson O, Bendahl PO, Borg A, Fernö M, et al. Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer. 1998;83:310–9.
Robson ME, Tung N, Conte P, Im SA, Senkus E, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–66.
André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, et al. Alpelisib for PIK3CA-Mutated, hormone receptor-positive advanced breast Cancer. N Engl J Med. 2019;380:1929–40.
Ebi H, Bando H. Precision oncology and universal health coverage system in Japan. JCO Precis Oncol. 2019;3:PO1900291.
Sunami K, Ichikawa H, Kudo T, Kato M, Fujiwara Y, et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: a hospital-based study. Cancer Sci. 2019;110:1480–90.
Kawaji H, Kubo M, Yamashita N, Yamamoto H, Kai M, et al. Comprehensive molecular profiling broadens treatment options for breast cancer patients. Cancer Med. 2021;10:529–39.
Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;11:1023–31.
Woodhouse R, Li M, Hughes J, Delfosse D, Skoletsky J, et al. Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PLoS ONE. 2020;15:e0237802.
Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 8th ed. Chichester, JohnWiley and Sons; 2017.
Tan PH, Ellis I, Allison K, Brogi E, Fox SB, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77:181–5.
Kwapisz D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol Immunother. 2021;70:607–17.
VENTANA. PD-L1 (SP142) assay (CE-IVD) [package insert]. Tucson, AZ: Ventana Medical Systems, Inc; 2019.
DAKO. PD-L1 IHC 22C3 pharmDx assay [instructions for use]. Carpinteria, CA: Dako North America, Inc; 2018.
Gunderson CC, Moore KN. BRACAnalysis CDx as companion diagnostic tool for Lynparza. Expert Rev Mol Diagn. 2015;15:1111–6.
Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–8.
Barroso-Sousa R, Jain E, Cohan O, Kim D, Buendia-Buendia J, et al. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol. 2020;31:387–94.
Ziegler A, Colin E, Goudenège D, Bonneau D. A snapshot of some pLI score pitfalls. Hum Mutat. 2019;40:839–41.
van der Velde KJ, Kuiper J, Thompson BA, Plazzer JP, van Valkenhoef G, et al. InSiGHT Group. Evaluation of CADD Scores in Curated Mismatch Repair Gene Variants yields a model for clinical validation and prioritization. Hum Mutat. 2015;36:712–9.
Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare).
Fujii K, Watanabe R, Ando T, Kousaka J, Mouri Y, et al. Alterations in three biomarkers (estrogen receptor, progesterone receptor and human epidermal growth factor 2) and the Ki67 index between primary and metastatic breast cancer lesions. Biomed Rep. 2017;7:535–42.
Schrijver WAME, Suijkerbuijk KPM, van Gils CH, van der Wall E, Moelans CB, et al. Receptor Conversion in distant breast Cancer Metastases: a systematic review and Meta-analysis. J Natl Cancer Inst. 2018;110:568–80.
Hoefnagel LDC, van der Groep P, van der Vijver M, Boers JE, Wesseling P, et al. Discordance in ERα, PR and HER2 receptor status across different distant breast cancer metastases within the same patient. Ann Oncol. 2013;24:3017–23.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomized, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–28.
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–98.
Sivapiragasam A, Ashok Kumar P, Sokol ES, Albacker LA, Killian JK, et al. Predictive biomarkers for Immune checkpoint inhibitors in metastatic breast Cancer. Cancer Med. 2021;10:53–61.
Loi S, Michiels S, Baselga J, Bartlett JMS, Singhai SK, et al. PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer. PLoS ONE. 2013;8:e53292.
Christgen M, Noskowicz M, Schipper E, Christgen H, Heil C, et al. Oncogenic PIK3CA mutations in lobular breast cancer progression. Genes Chromosomes Cancer. 2013;52:69–80.
Ramirez-Ardila DE, Helmijr JC, Look MP, Lurkin I, Ruigrok-Ritstier K, et al. Hotspot mutations in PIK3CA associate with first-line treatment outcome for aromatase inhibitors but not for tamoxifen. Breast Cancer Res Treat. 2013;139:39–49.
Kalinsky K, Jacks LM, Heguy A, Patil S, Drobnjak M, et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15:5049–59.
Mosele F, Stefanovska B, Lusque A, Dien AT, Garberis I, et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol. 2020;31:377–86.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70.
Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter S, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–9.
Stephens PJ, Tarpey PS, Davis H, Loo PV, Greenman C, et al. The landscape of cancer genes and mutational process in breast cancer. Nature. 2012;486:400–4.
Alsner J, Jensen V, Kyndi M, Offersen BV, Vu P, et al. A comparison between p53 accumulation determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol. 2008;47:600–7.
Alkam Y, Mitomi H, Nakai K, Himuro T, Saito T, et al. Protein expression and methylation of DNA repair genes hMLH1, hMSH2, MGMT and BRCA1 and their correlation with clinicopathological parameters and prognosis in basal-like breast cancer. Histopathology. 2013;63:713–25.
Uji K, Naoi Y, Kagara N, Shimoda M, Shimomura A, et al. Significance of TP53 mutations determined by next-generation deep sequencing in prognosis of estrogen receptor-positive breast cancer. Cancer Lett. 2014;342:19–26.
Walsh T, Casadei S, Coats KH, Swisher E, Stray SM et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer.
Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23:276–92.
Apostolou P, Fostira F. Hereditary breast cancer: the era of new susceptibility genes. Biomed Res Int. 2013;2013:747318.
Brown CJ, Lain S, Verma CS, Fersht AR, Kane DP, et al. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–73.
Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol. 2015;33:2345–52.
Sorrell AD, Espenschied CR, Culver JO, Weitzel JN. Tumor protein p53 (TP53) testing and Li-Fraumeni syndrome: current status of clinical applications and future directions. Mol Diagn Ther. 2013;17:31–47.
Nichols KE, Malkin D, Garber JE, Fraumeni JF Jr, Li FP. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol Biomarkers Prev. 2001;10:83–7.
Pereira CBL, Leal MF, de Souza CRT, Montenegro RC, Rey JA, et al. Prognostic and predictive significance of MYC and KRAS alterations in breast cancer from women treated with neoadjuvant chemotherapy. PLoS ONE. 2013;8:e60576.
Chen Y, Olopade OI. MTC in breast tumor progression. Expert Rev Anticancer Ther. 2008;8:1689–98.
Hohensee I, Lamszus K, Riethdorf S, Meyer-Staeckling S, Glatzel M, et al. Frequent genetic alterations in EGFR- and HER2-driven pathways in breast cancer brain metastases. Am J Pathol. 2013;183:83–95.
Perez EA, Dueck AC, McCullough AE, Chen B, Geiger XJ, et al. Impact of PTEN protein expression on benefit from adjuvant trastuzumab in early-stage human epidermal growth factor receptor 2-positive breast cancer in the North Central Cancer Treatment Group N9831 trial. J Clin Oncol. 2013;31:2115–22.
Tsutsui S, Inoue H, Yasuda K, Suzuki K, Higashi H, et al. Reduced expression of PTEN protein and its prognostic implications in invasive ductal carcinoma of the breast. Oncology. 2005;68:398–404.
Capodanno A, Camerini A, Orlandini C, Baldini E, Resta ML, et al. Dysregulated PI3K/Akt/PTEN pathway is a marker of a short disease-free survival in node-negative breast carcinoma. Hum Pathol. 2009;40:1408–17.
Blumenthal GM, Dennis PA. PTEN hamartoma tumor syndromes. Eur J Hum Genet. 2008;16:1289–300.
Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34.
Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–19.
Pereira B, Chin SF, Rueda OM, Vollan HKM, Provenzano E, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479.
Landrum MJ, Lee LM, Benson M, Brown GR, Chao C, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46(D1):D1062–7.
Dhillon KK, Bajrami I, Taniguchi T, Lord CJ. Synthetic lethality: the road to novel therapies for breast cancer. Endocr Relat Cancer. 2016;23:T39–55.
Weigelt B, Comino-Méndez I, de Bruijn I, Tian L, Meisel JL, et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian Cancer. Clin Cancer Res. 2017;23:6708–20.
Acknowledgements
We thank all the members of Expert Panel, which is based at Nagoya University Hospital as a core institution for caner genomic medicine, for reviewing the F1CDx results.
Funding
This research study did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design: K. Fujii and M. Ido. Provision of study material or patients: All authors. Collection and/or assembly of data: K. Fujii, H. Mishima, and A. Kubo. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Ethical approval and Consent to participate
The study received approval from the institutional review board of Aichi University Hospital. The committee’s reference number is 2022 − 186. And the data collected form the patients’ medical record was managed in accordance with the Declaration of Helsinki. As this study is considered to be a retrospective observational study not an intervention one, the institutional review board of Aichi University Hospital declared that it was not necessary to obtain the informed consent from the patients enrolled in this study. However, the information of this study is disclosed and the means to opt out of this study is provided in the internet home page of Aichi Medical University Hospital.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ido, M., Fujii, K., Mishima, H. et al. Comprehensive genomic evaluation of advanced and recurrent breast cancer patients for tailored precision treatments. BMC Cancer 24, 85 (2024). https://doi.org/10.1186/s12885-023-11442-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11442-9