Abstract
Background
The relationship between sleep disturbances and lung cancer is complex and bidirectional. This meta-epidemiological study aimed to explore the potential association between sleep disruption and the risk of pulmonary cancer.
Methods
We conducted a comprehensive literature search of the PubMed, Embase, Cochrane Library, and Web of Science databases to retrieve relevant studies. We employed the Newcastle–Ottawa Scale to assess the quality of the observational studies. Stata 17.0 was used to synthesize and conduct a meta-analysis of odds ratios (ORs) and corresponding 95% confidence intervals (CIs). We used funnel plot analysis and Egger’s regression test to evaluate potential publication bias.
Results
A total of 11 studies were included with 469,691 participants. The methodological quality of the included studies ranged from moderate to high. Compared with 7–8 h of sleep time, short sleep duration was associated with a 13% higher lung cancer risk [OR, 1.13; 95%CI: 1.02–1.25; I2 = 67.6%; P = 0.018] and long sleep duration with a 22% higher risk [OR, 1.22; 95%CI: 1.12–1.33; I2 = 6.9%; P < 0.001]. Insomnia symptoms [OR, 1.11; 95%CI: 1.07–1.16; I2 = 0%; P < 0.001] and evening chronotype [OR, 1.15; 95%CI: 1.05–1.26; P = 0.002] were all related to a higher risk of lung cancer. Egger’s test revealed no publication bias for sleep duration (P = 0.13).
Discussion
This systematic review is the first one which observes positive correction between sleep disturbances and the incidence of lung cancer. While the plausible mechanism is not clear, it is hypothesized that the association of short sleep duration and lung cancer mainly mediated by melatonin secretion and the immune-inflammatory balance. Further studies are needed to examine whether other risk factors, such as age, occupation, cumulative effect of sleep disturbances might mediate the relationship between sleep disturbances and lung cancer risk.
Conclusion
The present study revealed that insufficient and excessive sleep duration, insomnia symptoms, and evening chronotype were significantly predictive of an increased risk of lung cancer. This finding underscores the need to account for sleep disturbances as an independent risk factor for evaluating susceptibility to lung cancer.
Trial registration
CRD42023405351.
Similar content being viewed by others
Background
Lung cancer, the second most prevalent form of cancer, remains a significant health challenge and threat to life worldwide [1]. Studies indicate that the 5-year survival rate of patients with lung cancer ranges from 10 to 20% in most countries [2]. Moreover, lung cancer became the primary cause of cancer-related mortality in 2020, with an estimated 1.8 million deaths accounting for 18% of all fatalities globally [3]. Therefore, the etiology and pathogenesis of lung cancer have garnered considerable attention.
Sleep disturbance is a disorder that encompasses difficulties in sleep initiation and maintenance, or excessive sleepiness [4]. A study in Sweden revealed that approximately 20–30% of adults frequently experience sleep disturbances [5]. Recent research contends that sleep disturbance may serve as a risk factor for breast, colorectal, and gastric cancer [6,7,8]. Multiple studies have suggested that sleep disturbances could be associated with cancer, specifically through the disruption of circadian rhythms, reduction of melatonin secretion, and inflammatory responses, leading to unregulated cell proliferation [9,10,11].
Animal studies have shown that circadian disruption can promote lung cancer tumor growth and metastasis in male C57BL/6 mice by affecting the expression of tumor-related genes [12]. According to a previous cross-sectional study, the concentration of melatonin in patients with non-small cell lung cancer was significantly lower than that in healthy volunteers [13]. Chen et al. found that melatonin has a good anticancer effect by inhibiting lung cancer growth in a Lewis mouse model [14]. Accordingly, we propose an underlying association between lung cancer risk and sleep disturbances, which may be related to circadian rhythms and melatonin secretion.
However, few pooled analyses have explored the effects of multiple sleep parameters on the occurrence of lung cancer, resulting in inconsistent results. Detecting sleep features associated with the occurrence of lung cancer may enable the identification of individuals at risk for this disease and promote primary prevention efforts. Therefore, this meta-analysis aimed to quantitatively assess the relationship between sleep disturbances and lung cancer to provide valuable insights into future lung cancer prevention efforts.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [15]. The protocol for this meta-analysis was registered with the International Prospective Register of Systematic Reviews under the registration number CRD42023405351.
Data sources
To identify studies exploring the potential links between sleep disturbances and lung cancer risk, we conducted a comprehensive search of four electronic databases (PubMed, Embase, Cochrane Library, and Web of Science) covering studies published up to March 5, 2023. Additionally, we manually screened review article reference lists for relevant reports.
The search strategy encompassed two themes that delineated the diverse sleep characteristics during both physiological and pathological conditions as exposure variables, while considering lung cancer risk as the outcome variable. No restrictions were established concerning study types or geographic boundaries to enhance the comprehensiveness of the literature retrieval. The search string used for sleep disturbances contained: "Sleep [Mesh]","Sleep Initiation and Maintenance Disorders [Mesh] ","Sleep Wake Disorders [Mesh] ","Sleep* Habit*","Nap*","Daytime sleep", "Siesta", "Daytime sleepiness", "Daytime somnolence", "Insomnia*","Disorders of Initiating and Maintaining Sleep", "Early Awakening". The following terms are used for lung cancer: "Lung Neoplasm [Mesh]","Pulmonary Neoplasm*","Lung* Neoplasm*","Lung Cancer*", " Pulmonary Cancer*."
Eligibility criteria
The present study included original literature that met the following inclusion criteria:1) observational studies utilizing a cross-sectional, case–control, retrospective or prospective cohort design; 2) investigations that explored the implications of sleep disturbances, such as insufficient or excessive sleep duration, insomnia symptoms, and subjective breathing disorders, on the development of lung cancer; 3) sleep disruption was determined through self-reported questionnaires, face-to-face questioning, or clinical diagnosis in patients experiencing symptoms of sleep disorders; and 4) studies that provided risk estimates, including odds ratios (ORs), risk ratios (RRs), hazard ratios (HRs) with 95% confidence intervals (95% CIs), or adequate data for computing.
The exclusion criteria were as follows: 1)case studies, reports, reviews, conference abstracts, or genetic, animal and cell studies;2) assessing sleep disorders after lung cancer; 3) the cohort was examined for multiple cancer types, but no subgroup analyses were conducted specifically for lung cancer; 4) only survival or mortality was used as the outcome measure;5) outcome measures was compound unit about the length of population survival time; and 6) studies that reported repeated outcome measures within the overlapping sample, which had a small sample size.
Study selection
Two researchers (WZC and QCX) performed primary screening by reading the study titles and abstracts, and then downloaded and browsed the full text of potentially eligible studies to determine the final literature that met the eligibility criteria. When the two researchers disagreed on the selection, the decision was made through discussion and consultation with a third researcher (ZT).
Data extraction
Two researchers (WZC and QCX) independently collected information using a predesigned checklist. In case of disputes, the final decision was made by the researchers (ZT and WS) after a full-text review. The checklist aimed to obtain details such as publication information (first author, publication year, country), study features (type of study, follow-up year, mean age/age range, number of cases, and control group), exposure and outcome diagnostic criteria, sleep disturbance categorization, subgroup stratifications (including specific sleep duration, insomnia symptoms, morning/evening chronotype, and subjective sleep-disorder breathing features), adjustment for confounding factors, and effect sizes with corresponding 95% CIs (effect sizes adjusted for all the available covariates were selected). To ensure a consistent definition of sleep duration, sleep durations of less than 6 h, less than 6.5 h, or 7 h were categorized as short durations, while durations exceeding 8 h were categorized as long durations. Furthermore, to eliminate the possible influence of newly diagnosed lung cancer on physiological state and sleep duration, we prioritized the statistical results after eliminating cases diagnosed within 2 years of baseline.
Risk of bias
Two independent researchers (HSH and WXY) used the Newcastle–Ottawa scale [16] to assess the quality of the studies, which were classified into three levels: low (7 or more), moderate (4–6), and high risk (3 or less) of bias.
Statistical analysis
Pooled ORs and corresponding 95% CIs were calculated as effect measures for lung cancer risk irrespective of the study design. Owing to the low incidence of lung cancer within populations, ORs were considered equivalent to RRs and HRs in this meta-analysis. In the presence of significant heterogeneity (P < 0.1 or I2 > 50%), a random-effects model was used; otherwise, a fixed-effects model was used. Sensitivity analyses were performed to assess the robustness of our pooled results and to examine the influence of individual studies on the overall estimates. Publication bias was evaluated using funnel plots and Egger’s test. All statistical analyses were conducted using Stata software (version 17.0).
Results
Study selection
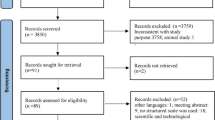
As presented in Fig. 1, 6907 records were retrieved from the electronic databases. After removing duplicates using an automatic tool and manual searches, 6028 articles were included in the initial screening. Of these, 5996 articles were excluded because of untargeted participants or outcome data. Finally, 10 studies [17,18,19,20,21,22,23,24,25,26] reached eligibility criteria after browsing the full‐text.
Study characteristics
The ten studies were published between 2014 and 2022, with sample sizes ranging from 950 to 469,691 participants (8,288 with lung cancer events). Eight studies [17, 19,20,21,22,23,24,25] were cohort studies, and two [18, 26] were case–control studies. Most studies were conducted in Europe [19, 22, 24, 26] and America [21, 23, 25], two in Canada [18, 20], and one in China [17]. Self-reported questionnaires were the most widely used method for assessing sleep parameters; only one study [17] used face-to-face questioning. The diagnosis of lung cancer was verified through cancer registries or medical records and classified according to the International Classification of Diseases (ICD 9 or 10). All studies showed adjusted HRs or ORs with corresponding 95% CIs; however, the adjusted confounders varied slightly between the studies. The characteristics of the included studies are summarized in Table 1.
Quality assessment
According to Newcastle–Ottawa Scale, the mean score of included studies was 7.00 ± 0.92, with a media of 7. Seven of the studies were judged as having a low risk of bias and three as having a moderate risk, indicating that the methodological quality of the included studies was medium or high. Eight studies [18, 20,21,22,23,24,25,26] used a self-reported questionnaire as the main assessment scale, which was considered to have a selection bias.
Short sleep duration and lung cancer risk
Nine eligible studies [17,18,19,20,21,22,23,24, 26] assessed the relationship between short sleep duration and the risk of lung cancer. The OR for short duration associated with the risk of lung cancer was 1.13 in a random-effects model (95%CI: 1.02–1.25, I2 = 67.6%, P = 0.018; Fig. 2). There was high heterogeneity in the short duration; therefore, we performed a sensitivity analysis. The sensitivity analysis suggested that removing Noah’s study [24] as an outlier significantly reduced heterogeneity (I2 = 15.9%, P = 0.002) but had no effect on the pooled OR (OR, 1.16; 95%CI: 1.11–1.23; Fig. 2). The specificity of this study is that it excluded participants who reported shift work at baseline and did not adjust for occupation. The discrepancy in the inclusion of participants may be the main reason why this is inconsistent with the conclusions regarding short sleep duration in other studies.
Long sleep duration and lung cancer risk
Eight eligible studies [18,19,20,21,22,23,24, 26] assessed the predictive association between long sleep disturbances and incidence of lung cancer. Individuals who reported long sleep duration had a 1.22-fold higher risk of developing lung cancer than subjects with normal sleep duration in a fixed-effects model (95%CI: 1.12–1.33, I2 = 6.9%, P < 0.001; Fig. 3).
Insomnia symptoms and lung cancer risk
The meta-analysis of five eligible studies [17, 19, 22, 24, 25] exploring the association between insomnia symptoms and lung cancer, including 903,731 insomnia patients, indicated that insomnia symptoms increased the risk of lung cancer (OR, 1.11; 95%CI: 1.07–1.16, P < 0.001; Fig. 4), which showed low heterogeneity (I2 = 0%).
Chronotype and lung cancer risk
Four eligible studies [19, 20, 24, 26] provided pooled estimates of morning and evening chronotypes and lung cancer. The incidence of lung cancer was associated with evening chronotype (OR, 1.15; 95%CI, 1.05–1.26, P = 0.002; Fig. 5), but not with morning chronotype (OR, 1.03; 95%CI, 0.95–1.12, P = 0.466; Fig. 5).
Sleep disordered breathing and lung cancer risk
Four studies [17, 19, 22, 25] described exposure variables about subjective sleep disordered breathing. The random-effects model revealed that overall subjective sleep disordered breathing did not increase the risk of lung cancer (OR, 0.99; 95%CI: 0.932–1.059; I2 = 46.1%, P = 0.836; Fig. 6).
Publication bias
The funnel plot for sleep duration and insomnia symptoms was relatively symmetrical, indicating no publication bias. Egger’s test (P = 0.13) revealed no publication bias for sleep duration (Fig. 7).
Discussion
Main finding
After conducting a meta-analysis of studies involving 469,691 individuals, we determined that common sleep disturbances, namely extreme sleep duration, symptoms of insomnia, and an evening chronotype, were associated with an increased risk of lung cancer. However, no analogous relationship was observed between morning chronotype or S-SDB and lung cancer risk. Shorter sleep duration was linked to a 13% higher risk of lung cancer than sleep for 7–8 h. Conversely, individuals who slept for longer periods had a surprising 22% increase in lung cancer risk. Meanwhile, those with insomnia symptoms or an evening chronotype exhibited a 1.11–1.15 times higher risk of lung cancer than individuals without sleep disturbances. Overall, our findings suggest that extreme sleep duration, insomnia symptoms, and evening chronotype are significant risk factors for lung cancer.
Interpretation of findings
Mounting evidence suggests plausible links between abnormal sleep characteristics and various cancers, including skin, ovarian, and colorectal cancer [6, 27, 28]. However, to the best of our knowledge, this systematic review is the first to observe positive associations between multiple sleep disturbances and the incidence of lung cancer, despite no significant increase in the risk of lung cancer reported in previous meta-analyses. As demonstrated by Chen [28], there were no significant association between extreme sleep duration and lung cancer (short: RR = 1.04, 95% CI = 0.88–1.22, I2 = 46.1%; long: RR = 1.01, 95% CI = 0.83–1.23, I2 = 41.6%). It is worth noting that Chen's investigation encompassed a limited scope, considering only four studies. To enhance the comprehensiveness and accuracy of our research findings, we expanded our analysis to include six studies conducted between 2018 and 2022. These studies exhibited significant improvements in various aspects, such as sample size, duration of follow-up, and acquisition of sleep-related characteristics. Consequently, our research outcomes were characterized by enhanced precision and a more comprehensive evaluation. Moreover, while Chen's study only focused on sleep duration as a singular characteristic, our research considered multiple dimensions, including insomnia symptom, chronotype and sleep-disordered breathing, thereby providing a multifaceted evaluation of the relationship between sleep and lung cancer. Remarkably, our investigation revealed a positive correlation, further substantiating our conclusions regarding this positive association. Various biologically plausible mechanisms have been proposed to account for the association between sleep disorders and cancer, including impaired antitumor immunity, initiation of inflammation, melatonin decline, and disruption of circadian rhythms.
Sleep quality has been shown to be directly correlated with impaired anti-tumor immunity. Previous animal studies have indicated that sleep deprivation detrimentally affects the number of cytotoxic cells, thereby decreasing the ability of the immune system to respond to tumor growth [29]. Sleep deprivation promotes inflammation, which can initiate the development of lung cancer [30]. This is due to the activation of proinflammatory cytokine secretion, which induces an inflammatory microenvironment in both animal models and humans [31, 32]. The relationship between lung cancer and sleep disturbances has its own particularity, which is mainly mediated by melatonin secretion and the immune-inflammatory balance, which influences the occurrence and development of lung cancer. First, environmental lighting conditions may influence the secretion of melatonin, which can inhibit lung cancer cell migration and proliferation by decreasing TOX3 expression through the direct activation of miR-135b-3p [33]. Melatonin is a promising molecule and is considered a super adjuvant therapy for lung cancer. Adjuvant melatonin following curative intent resection has been reported to increase disease-free survival in patients with late-stage non-small cell lung cancer [10]. Second, the immune-inflammatory balance plays a crucial role in lung cancer risk. Short sleep durations activate the sympathetic effector pathway, which converts the basal gene expression profile into an increased proinflammatory state.
These theories explain why individuals who experience short sleep durations and insomnia symptoms are at a higher risk of developing lung cancer. Interestingly, our findings suggest that long sleep duration is associated with an increased risk of lung cancer, despite prior studies demonstrating its adverse effects on cardiovascular function, neurocognition, and metabolite level [34,35,36]. However, further experimental exploration is required to uncover the possible pathophysiological mechanisms through which long sleep duration influences lung cancer risk.
Notably, our study showed that individuals with the evening chronotype were at a higher risk of developing lung cancer, whereas no significant association was found in those with the morning chronotype. One explanation for this association is that the extra burden of delayed circadian rhythms caused by the evening chronotype may further exacerbate tumor cell autonomic loss of circadian regulation, leading to the desynchronization of circadian control of cellular metabolism [37,38,39].
Implications and limitations
To our knowledge, this meta-analysis is the first to present a comprehensive synthesis of observational studies on multiple sleep characteristics and lung cancer risk. Notably, we excluded the pooled data of cases diagnosed within 2 years of baseline, which complemented the more precise estimates. Moreover, we performed a sensitivity analysis to ensure the stability of the results and enhance the validity of our findings. Our study demonstrated that sleep disturbances should be considered as an independent risk factor for the onset of lung cancer and that appropriate management of sleep disturbances may decrease the risk of lung cancer.
Some limitations of our study should be considered. First, most included studies relied on self-reported questionnaires to identify data on sleep characteristics, which may have introduced a potential bias. Additionally, the adjusted confounders varied among the included studies, which increased the risk of information bias and heterogeneity. Considering that the majority of individuals with lung cancer are often seniors and that a significant correlation exists between sleep parameters and certain occupations, such as nurses and flight attendants, performing subgroup analysis based on age and certain occupations holds significant potential for exploration. Unfortunately, due to the lack of stratified data in the original studies, we were unable to conduct a subgroup analysis of significant risk factors for lung cancer, such as age, occupation, cumulative effect of sleep disturbances, smoking status, and family history of cancer, highlighting the need for further research examining the relationship between sleep traits and other risk factors for lung cancer.
Conclusion
Our study showed that extreme sleep duration, insomnia symptoms, and evening chronotype were positively associated with the risk of lung cancer. This result suggests that early detection and management of sleep disturbances could be a promising means of mitigating the burden of lung cancer.
Availability of data and materials
All the research data and materials in this study were obtained from publicly available sources. The following sources were used: PubMed, EMBASE, Cochrane Library, and Web of Science.
Abbreviations
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Mata-Analyses
- OR:
-
Odds ratio
- RR:
-
Risk ratio
- IS:
-
Insomnia symptoms
- S-SDB:
-
Subjective sleep disordered breathing
- ICD:
-
International Classification of Diseases,10th revision
- BMI:
-
Body mass index
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries [J]. CA Cancer J Clin. 2021;71(3):209–49.
Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries [J]. The Lancet. 2018;391(10125):1023–75.
Alberg AJ, Gupta RD, Akonde M. Cancer: epidemiology of lung cancer [M]//CABALLERO B. Encyclopedia of human nutrition. 4th ed. Oxford: Academic Press; 2023. p. 154–61.
American Academy of Sleep Medicine. International Classification of Sleep Disorders, revised: Diagnostic and Coding Manual. Chicago: American Academy of Sleep Medicine; 2001.
Mallon L, Broman JE, Akerstedt T, et al. Insomnia in sweden: a population-based survey [J]. Sleep Disord. 2014;2014: 843126.
Li X, Huang D, Liu F, et al. Sleep characteristics and cancer-related outcomes: an umbrella review of systematic reviews and meta-analyses of observational studies [J]. J Clin Med. 2022;11(24):7289.
Sateia MJ, Lang BJ. Sleep and cancer: recent developments [J]. Curr Oncol Rep. 2008;10(4):309–18.
Gu F, Xiao Q, Chu LW, et al. Sleep duration and cancer in the NIH-AARP diet and health study cohort [J]. PLoS ONE. 2016;11(9): e161561.
Li Y, Li S, Zhou Y, et al. Melatonin for the prevention and treatment of cancer [J]. Oncotarget. 2017;8(24):39896–921.
Prather AA, Vogelzangs N, Penninx BW. Sleep duration, insomnia, and markers of systemic inflammation: results from the Netherlands Study of Depression and Anxiety (NESDA) [J]. J Psychiatr Res. 2015;60:95–102.
Truong KK, Lam MT, Grandner MA, et al. Timing matters: circadian rhythm in sepsis, obstructive lung disease, obstructive sleep apnea, and cancer [J]. Ann Am Thorac Soc. 2016;13(7):1144–54.
Wu M, Zeng J, Chen Y, et al. Experimental chronic jet lag promotes growth and lung metastasis of Lewis lung carcinoma in C57BL/6 mice [J]. Oncol Rep. 2012;27(5):1417–28.
Hu S, Shen G, Yin S, et al. Melatonin and tryptophan circadian profiles in patients with advanced non-small cell lung cancer [J]. Adv Ther. 2009;26(9):886–92.
Chen X, Hao B, Li D, et al. Melatonin inhibits lung cancer development by reversing the Warburg effect via stimulating the SIRT3/PDH axis [J]. J Pineal Res. 2021;71(2): e12755.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews [J]. BMJ. 2021;372: n71.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses [J]. Eur J Epidemiol. 2010;25(9):603–5.
Cao Q, Zhang Q, Li X, et al. Impact of sleep status on lung adenocarcinoma risk: a prospective cohort study [J]. Eur Rev Med Pharmacol Sci. 2022;26(20):7641.
Murphy RA, Darvishian M, Qi J, et al. Lifestyle factors and lung cancer risk among never smokers in the Canadian Partnership for Tomorrow’s Health (CanPath) [J]. Cancer Causes Control. 2022;33(6):913–8.
Xie J, Zhu M, Ji M, et al. Relationships between sleep traits and lung cancer risk: a prospective cohort study in UK Biobank [J]. Sleep. 2021;44(9):zsab089.
McNeil J, Barberio AM, Friedenreich CM, et al. Sleep and cancer incidence in Alberta’s Tomorrow Project cohort [J]. Sleep. 2019;42(3):zsy252.
Hurley S, Goldberg D, Bernstein L, et al. Sleep duration and cancer risk in women [J]. Cancer Causes Control. 2015;26(7):1037–45.
Luojus MK, Lehto SM, Tolmunen T, et al. Sleep duration and incidence of lung cancer in ageing men [J]. BMC Public Health. 2014;14(1):295.
Khawaja O, Petrone AB, Aleem S. Sleep duration and risk of lung cancer in the physicians’ health study [J]. Zhongguo Fei Ai Za Zhi. 2014;9(17):649–55.
Peeri NC, Tao M, Demissie S, et al. Sleep duration, chronotype, and insomnia and the risk of lung cancer: United Kingdom biobank cohort [J]. Cancer Epidemiol Biomark Prev. 2022;31(4):766–74.
Sillah A, Watson NF, Peters U, et al. Sleep problems and risk of cancer incidence and mortality in an older cohort: The Cardiovascular Health Study (CHS) [J]. Cancer Epidemiol. 2022;76: 102057.
Cordina-Duverger E, Uchai S, Tvardik N, et al. Sleep traits, night shift work and lung cancer risk among women: results from a population-based case-control study in France (The WELCA Study) [J]. Int J Environ Res Public Health. 2022;19(23):16246.
Erren TC, Morfeld P, Foster RG, Westermann IK, et al. Sleep and cancer: Synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol Int. 2016;33(4):325–50.
Chen Y, Tan F, Wei L, et al. Sleep duration and the risk of cancer: a systematic review and meta-analysis including dose–response relationship [J]. BMC Cancer. 2018;18(1):1149.
De Lorenzo BHP, Novaes EBRR, Paslar LT, et al. Chronic sleep restriction impairs the antitumor immune response in mice [J]. Neuroimmunomodulation. 2018;25(2):59–67.
Engels EA. Inflammation in the development of lung cancer: epidemiological evidence [J]. Expert Rev Anticancer Ther. 2008;8(4):605–15.
Yehuda S, Sredni B, Carasso RL, et al. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation [J]. J Interferon Cytokine Res. 2009;29(7):393–8.
Irwin MR, Witarama T, Caudill M, et al. Sleep loss activates cellular inflammation and signal transducer and activator of transcription (STAT) family proteins in humans [J]. Brain Behav Immun. 2015;47:86–92.
Wang Y, Wang Z, Shao C, et al. Melatonin may suppress lung adenocarcinoma progression via regulation of the circular noncoding RNA hsa_circ_0017109/miR-135b-3p/TOX3 axis [J]. J Pineal Res. 2022;73(2): e12813.
Beaman A, Bhide MC, McHill AW, et al. Biological pathways underlying the association between habitual long-sleep and elevated cardiovascular risk in adults [J]. Sleep Med. 2021;78:135–40.
Ostadrahimi A, Nikniaz Z, Faramarzi E, et al. Does long sleep duration increase risk of metabolic syndrome in Azar cohort study population? [J]. Health Promot Perspect. 2018;8(4):290–5.
Kaur SS, Tarraf W, Wu B, et al. Modifying pathways by age and sex for the association between combined sleep disordered breathing and long sleep duration with neurocognitive decline in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) [J]. Alzheimers Dement. 2021;17(12):1950–65.
Hittle BM, Gillespie GL. Identifying shift worker chronotype: implications for health [J]. Ind Health. 2018;56(6):512–23.
Papagiannakopoulos T, Bauer MR, Davidson SM, et al. Circadian rhythm disruption promotes lung tumorigenesis [J]. Cell Metab. 2016;24(2):324–31.
Peek CB, Levine DC, Cedernaes J, et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle [J]. Cell Metab. 2017;25(1):86–92.
Acknowledgements
Not applicable.
Funding
This project was funded by Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (No. CI2021A01801), National Natural Science Foundation of China (No. 82074239) and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202205). The sponsors are not involved in design, execution, or writing the study.
Author information
Authors and Affiliations
Contributions
Tong Zhou, Zichen Wang: collecting data, statistical analysis, writing the article, final approval of the article. Zichen Wang, Chenxi Qiao, Shuo Wang: primary screening, determining the final literature, extracting data. Shuaihang Hu, Xinyan Wang: quality evaluation, final approval of the article. Xiumei Ma, Dandan Wang: developing search strategy. Jinglei Li, Zheng Li: developing criteria, critical revision. Wei Hou: supervising the whole process, critical revision of the article, and final approval of the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Due to the publicly available nature of all data used in this study, no ethical approval was required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
PubMed (Mar 05, 2023). Table S2. Cochrane Library (Mar 05, 2023). Table S3. Embase(Mar 05, 2023). Table S4. Web of science(Mar 05, 2023).
Additional file 2: Table S5.
The quality assessment of cohort and case-control studies.
Additional file 3: Table S6.
Details of the Excluded Literature.
Additional file 4.
Supplementary Material Lists of excluded literature.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, T., Wang, Z., Qiao, C. et al. Sleep disturbances and the risk of lung cancer: a meta-epidemiological study. BMC Cancer 23, 884 (2023). https://doi.org/10.1186/s12885-023-11392-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11392-2