Abstract
Purpose
To assess survival of treatment patterns based on concurrent chemoradiotherapy (CCRT) in patients with stage IIB cervical squamous cell carcinoma (CSCC).
Materials and methods
Patients with stage IIB CSCC receiving CCRT were investigated from June 2012 to June 2019 in Guangxi Medical University Cancer Hospital. Baseline characteristics and treatment patterns were described. Survival between treatment patterns were compared using Kaplan-Meier methods.
Results
A total of 232 patients were included: 39.7% of patients received CCRT alone, 6.5% of patients received neoadjuvant chemotherapy (NACT) + CCRT, 45.6% of patients received CCRT + adjuvant chemotherapy (AC), and 8.2% of patients received NACT + CCRT + AC. CCRT + AC showed similar overall survival (OS; hazard ratio [HR] = 0.95, 95% confidence interval [CI]: 0.41–2.17; P = 0.894) and locoregional-free survival (LRFS; HR = 2.39, 95% CI: 0.45–12.63; P = 0.303) compared with CCRT. However, CCRT + AC had a worse distant metastasis-free survival (DMFS; HR = 5.39, 95% CI: 1.14–25.57; P = 0.034). After propensity score matching, CCRT + AC had comparable OS (HR = 0.89, 95% CI: 0.29–2.70; P = 0.833), LRFS (HR = 3.26, 95% CI: 0.30-35.38; P = 0.331), and DMFS (HR = 4.80, 95% CI: 0.55–42.26; P = 0.157) compared to CCRT.
Conclusion
AC did not improve survival in patients with stage IIB CSCC receiving CCRT.
Similar content being viewed by others
Introduction
Cervical squamous cell carcinoma (CSCC) is a major health threat of women worldwide [1]. In developing countries, patients usually present with locally advanced diseases [2]. Concurrent chemoradiotherapy (CCRT) is the standard treatment for these patients [3]. However, approximately 17% of patients experienced local recurrences and 18% of patients developed distant metastases [4,5,6].
Neoadjuvant chemotherapy (NACT) and adjuvant chemotherapy (AC) were expected to improve local control and reduce distant metastasis. However, studies investigating NACT and AC combined with CCRT have yielded inconsistent results [7,8,9,10,11,12,13,14,15,16,17,18,19]. The optimal treatment strategy remains uncertain, especially in the stage IIB subgroup. This study aims to evaluate treatment patterns and outcomes in patients with stage IIB CSCC.
Materials and methods
Patients
We identified CSCC patients who were treated at Guangxi Medical University Cancer Hospital from June 2012 to June 2019. Inclusion criteria were as follows: [1] pathologically confirmed cervical cancer, [2] stage IIB according to the FIGO staging system, and [3] squamous cell carcinoma. Exclusion criteria were as follows: [1] patients refused treatments, [2] patients had incomplete data, [3] patients did not finish treatments, and [4] patients received surgery.
Clinical characteristics (age, Eastern Cooperative Oncology Group [ECOG] performance status, tumor grade, hemoglobin, human papilloma virus [HPV] infection status, tumor diameter, and concurrent chemotherapy [CCT] cycles) and treatment patterns were extracted.
Treatments
Patients underwent pelvic external beam radiotherapy in combination with high-dose-rate intracavitary brachytherapy. The pelvic external beam radiotherapy involved a dose of 48–50 Gy delivered in 24–25 fractions using intensity-modulated radiotherapy. The high-dose-rate intracavitary brachytherapy was given at 28–35 Gy delivered in 4–5 fractions to the high-risk clinical target volume.
Platinum-based NACT was administered every 3 weeks before CCRT. The CCT consisted of either cisplatin at 30–40 mg/m2 on day 1 or nedaplatin at 50 mg/m2 on day 1 per week, during the course of radiotherapy. After CCRT, platinum-based AC was administered every 3 weeks.
Endpoints
Treatment failures were identified based on records, including pathology reports and/or imaging reports. Death events were determined from official statements.
The primary endpoint of the study was overall survival (OS). OS was defined as the duration from the date of diagnosis to the date of death due to any cause. The secondary endpoints were locoregional-free survival (LRFS), which was defined as the duration from the date of diagnosis to the date of locoregional recurrence, and distant metastasis-free survival (DMFS), which was defined as the duration from the date of diagnosis to the date of distant metastasis.
Statistical analysis
The continuous variable of tumor diameter was categorized based on a threshold of 4 cm [20]. Similarly, the continuous variables of age and hemoglobin were transformed into categorical variables using their respective median values. Categorical variables, including age, ECOG, tumor grade, hemoglobin, HPV infection status, tumor diameter, and CCT cycles were analyzed using the χ2 test or Fisher’s exact test.
For the analysis of OS, LRFS, and DMFS between treatment patterns, the Kaplan-Meier method with log-rank test statistics was employed. Pairwise comparisons were conducted among the different treatment patterns. The identification of independent prognostic factors was carried out using multivariable proportional hazards regressions, which adjusted for factors including age, ECOG, tumor grade, hemoglobin, HPV infection status, tumor diameter, and treatment patterns. The results were recorded as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs).
To mitigate selection bias between CCRT and CCRT + AC subgroups, a matched case-control analysis was performed using propensity score matching (PSM). Patients who received CCRT were considered the dependent variable in calculating the propensity scores. One-to-one matching without replacement was implemented in the logistic regression model, utilizing a caliper of 0.02 on the logit of the propensity score.
This study used SPSS Statistics Version 26.0 software (IBM Co., Armonk, NY, USA) and R software (version 4.2.1) to perform statistical analyses. Two-tailed P values < 0.05 were considered statistically significant.
Ethical approval for this study was obtained from the Guangxi Medical University Cancer Hospital Ethics Committee. The study was conducted in compliance with the principles outlined in the Declaration of Helsinki. However, informed consent was not obtained due to the retrospective nature of the study.
Results
Baseline characteristics
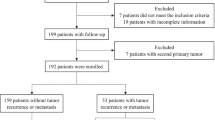
Figure 1 illustrates the patient selection process, wherein 232 patients were included after investigating a total of 721 patients. Table 1 provides a summary of the patient characteristics. The last follow-up time was October 2021. The median follow-up time was 54 months (interquartile range: 37–77 months). Thirty patients were lost to follow-up, resulting in a follow-up rate of 87.1%.
Treatment patterns
The treatment patterns investigated in this study included CCRT, NACT + CCRT, CCRT + AC, and NACT + CCRT + AC. The most commonly used treatment modalities were CCRT, accounting for 39.7% of the patients, and CCRT + AC, accounting for 45.7% of the patients. However, the sample sizes for the NACT + CCRT (6.5%) and NACT + CCRT + AC (8.1%) subgroups were relatively limited.
The CCRT subgroup had a higher proportion of patients with age > 54 years, while the AC subgroup had a higher proportion of patients with tumor diameter > 4 cm. However, factors including ECOG, tumor grade, hemoglobin level, and HPV infection status did not exhibit significant differences among the various treatment patterns.
Overall survival
The 5-year OS was 85.6%, 60.0%, 85.8%, and 73.3% for CCRT, NACT + CCRT, CCRT + AC, and NACT + CCRT + AC groups, respectively (Fig. 2A). NACT + CCRT subgroup had a worse 5-year OS compared to both the CCRT (P = 0.003) and CCRT + AC (P = 0.003) subgroups. However, there was no difference in the 5-year OS between the CCRT and CCRT + AC subgroups (P = 0.811). Multivariate regression analysis revealed that NACT + CCRT was identified as an independent prognostic factor for OS (HR = 3.54, 95% CI: 1.22–10.30; P = 0.020; Fig. 2B).
Overall survival of treatment patterns. (A) Overall survival between the CCRT, NACT + CCRT, CCRT + AC, and NACT + CCRT + AC subgroups. (B) Multivariable proportional hazards regressions of overall survival. CCRT: concurrent chemoradiotherapy. AC: adjuvant chemotherapy. NACT: neoadjuvant chemotherapy
Locoregional-free survival
The 5-year LRFS was 96.9%, 92.9%, 94.2%, and 82.5% for CCRT, NACT + CCRT, CCRT + AC, and NACT + CCRT + AC subgroups, respectively (Fig. 3A). The NACT + CCRT + AC subgroup exhibited a worse 5-year LRFS compared to the CCRT subgroup (P = 0.013). However, there were no differences in the 5-year LRFS between the CCRT, NACT + CCRT, and CCRT + AC subgroups. Multivariate regression analysis revealed that NACT + CCRT + AC was not identified as an independent prognostic factor for LRFS (HR = 5.68, 95% CI: 0.85–37.74; P = 0.073; Fig. 3B).
Locoregional-free survival of treatment patterns. (A) Locoregional-free survival between the CCRT, NACT + CCRT, CCRT + AC, and NACT + CCRT + AC subgroups. (B) Multivariable proportional hazards regressions of locoregional-free survival. CCRT: concurrent chemoradiotherapy. AC: adjuvant chemotherapy. NACT: neoadjuvant chemotherapy
Distant metastasis-free survival
The 5-year DMFS was 97.4%, 77.8%, 87.9%, and 84.2% for CCRT, NACT + CCRT, CCRT + AC, and NACT + CCRT + AC subgroups, respectively (Fig. 4A). The CCRT subgroup had better 5-year DMFS rates compared to the NACT + CCRT (P = 0.015), CCRT + AC (P = 0.016), and NACT + CCRT + AC (P = 0.008) subgroups. Multivariate regression analysis revealed that both CCRT + AC (HR = 5.39, 95% CI: 1.14–25.57; P = 0.034) and NACT + CCRT + AC (HR = 8.32, 95% CI: 1.28–53.95; P = 0.026) were identified as independent prognostic factors for DMFS (Fig. 4B).
Distant metastasis-free survival of treatment patterns. (A) Distant metastasis-free survival between the CCRT, NACT + CCRT, CCRT + AC, and NACT + CCRT + AC subgroups. (B) Multivariable proportional hazards regressions of distant metastasis-free survival. CCRT: concurrent chemoradiotherapy. AC: adjuvant chemotherapy. NACT: neoadjuvant chemotherapy
Survivals between CCRT and CCRT + AC subgroups after PSM
In the multivariate logistic regression analysis, it was observed that patients with age > 54 years were less likely to receive CCRT + AC (odds ratio = 0.27, 95% CI: 0.14–0.52; P < 0.001; Fig. 5). After PSM, 55 patients who received CCRT and 55 patients who received CCRT + AC were matched. Table 2 summarizes the patient characteristics after PSM. The patient characteristics were found to be well-balanced across all covariates after PSM (P > 0.05).
CCRT + AC did not improve the 5-year OS (85.9% vs. 86.0%; P = 0.920, Fig. 6A), LRFS (96.8% vs. 94.5%; P = 0.328, Fig. 6B), or DMFS (98.1% vs. 89.4%; P = 0.104, Fig. 6C) compared to CCRT. Multivariate regression analysis revealed that CCRT + AC was not identified as an independent prognostic factor for OS (HR = 0.89, 95% CI: 0.29–2.70; P = 0.833), LRFS (HR = 3.26, 95% CI: 0.30-35.38; P = 0.331), or DMFS (HR = 4.80, 95% CI: 0.55–42.26; P = 0.157) (Table 3).
Discussion
This study revealed two main findings. First, the most common treatment modalities for stage IIB CSCC were CCRT and CCRT + AC. Second, the addition of chemotherapy before or after CCRT did not improve survival for patients with stage IIB CSCC. Consequently, well-designed prospective, randomized controlled trials are needed to explore alternative treatments that may enhance survival rates in this patient population.
NACT can inhibit cancer cells implantation and eliminate cancer cells in the circulation, thus reducing subclinical metastasis. Additionally, NACT can effectively decrease the tumor load in the local and regional areas, ultimately leading to an increase in the rate of locoregional tumor control. However, despite its potential benefits, previous studies have reported that NACT may result in decreased disease-free survival and OS in patients with locally advanced diseases, [11, 18] particularly in stage IIB diseases [21, 22]. Our study also yielded similar findings, where patients receiving NACT exhibited worse OS (P = 0.003), LRFS (P = 0.013), and DMFS (P = 0.015) in comparison to those who underwent CCRT.
The reasons behind the detrimental effect of NACT in some cases remain unclear. Several possible explanations have been proposed: First, the delay caused by administering NACT before CCRT could potentially decrease survival rates. The time lapse between the two treatments may allow the cancer to progress or become more aggressive, affecting patient outcomes [23]. Second, cancer cells may acquire resistance to the treatment during the course of NACT. This resistance could make the cancer more difficult to control or eliminate during subsequent CCRT [24]. Third, NACT may lead to significant toxicity in some patients, which could affect their ability to tolerate and complete subsequent CCRT. The adverse events associated with NACT might interfere with the optimal delivery of subsequent CCRT, impacting treatment efficacy [18].
Due to the current limited data and conflicting findings from previous studies, further investigation is warranted. The ongoing head-to-head phase III INTERLACE trial (ClinicalTrials.gov identifier: NCT01566240) is specifically designed to evaluate the efficacy of NACT in patients with locally advanced diseases. It will provide a more comprehensive understanding of the role of NACT in the management of this patient population.
AC aims to eliminate potential residual tumor, both within the pelvis and beyond. A meta-analysis reported that CCRT + AC was associated with improved OS (HR = 0.78, 95% CI: 0.69–0.88; P < 0.0001) and progression-free survival (HR = 0.80, 95% CI: 0.73–0.87; P < 0.0001) compared to CCRT [7]. However, outcomes of AC were not consistent across studies. Two phase III trials (ACTLACC and OUTBACK trials) demonstrated that adjuvant carboplatin and paclitaxel chemotherapy did not improve OS but led to increased toxicity when compared to CCRT [14, 15].
Several possible explanations for the inconsistent results of AC are as follows: First, studies included different pathological subtypes [7, 8]. The efficacy of AC was different among different histological subtypes (squamous cell carcinoma and adenocarcinoma) [25]. Second, the paclitaxel plus carboplatin chemotherapy regimen may not be effective [26,27,28]. Third, studies included different FIGO stages [7, 8]. The benefits of AC might differ among different FIGO stages [26]. Due to these variations and potential confounding factors, efficacy of AC in patients with locally advanced diseases needs further assessment.
Our study revealed that AC did not improve survivals in patients with stage IIB CSCC. The result was consistent with the results from the OUTBACK trial [14]. One possible explanation for this lack of benefit was that stage IIB disease has a relatively lower tumor burden compared to other locally advanced diseases. As a result, CCRT may already provide satisfactory treatment outcomes in this subgroup of patients. In contrast, AC leads to an increase in treatment-related toxicities. These adverse events could potentially impact patient survival adversely [15]. Furthermore, patients should be divided into different risk subgroups based on various prognostic factors. AC may be more beneficial for high-risk patients, while it may not provide significant advantages for low-risk patients [29].
A major limitation of this study should be considered. The sample sizes of NACT + CCRT and NACT + CCRT + AC subgroups were quite small. Small sample sizes can limit the statistical power to detect significant differences in survival outcomes between treatment patterns. Although efforts were made to adjust for all the factors, including age, ECOG, tumor grade, hemoglobin, HPV infection status, tumor diameter, and treatment patterns through multivariable proportional hazards regressions, potential unmeasured statistical biases might still exist. These biases could influence the conclusions and interpretations of this study. To address this limitation and further validate the findings, large sample size randomized controlled trials are needed.
In conclusion, our study suggested that AC did not improve treatment outcomes in patients with stage IIB CSCC receiving CCRT.
Data Availability
The datasets used and/or analyzed during the current study are available from the Supplemental file.
Abbreviations
- CCRT:
-
concurrent chemoradiotherapy
- CSCC:
-
cervical squamous cell carcinoma
- NACT:
-
neoadjuvant chemotherapy
- AC:
-
adjuvant chemotherapy
- OS:
-
overall survival
- LRFS:
-
locoregional-free survival
- DMFS:
-
distant metastasis-free survival
- HR:
-
hazard ratio
- CI:
-
confidence interval
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Zhang M, Zhong Y, Zhao Z, Huang Z, Zhang X, Li C, et al. Cervical Cancer Screening Rates among Chinese Women - China, 2015. China CDC Wkly. 2020;2(26):481–6.
Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–53.
Chemoradiotherapy for Cervical Cancer Meta-Analysis C. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncology: Official J Am Soc Clin Oncol. 2008;26(35):5802–12.
Cibula D, Potter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie-Meder C, et al. The european Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the management of patients with cervical Cancer. Virchows Arch. 2018;472(6):919–36.
Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl4):iv72–iv83.
Zhong L, Li K, Song L, Yin R. The effect of consolidation chemotherapy after concurrent chemoradiation on the prognosis of locally advanced cervical cancer: a systematic review and meta-analysis. J Obstet Gynaecology: J Inst Obstet Gynecol. 2022;42(5):830–7.
Liu H, Ma X, Sun C, Wu M, Xu Z, Zhou S, et al. Concurrent chemoradiotherapy followed by adjuvant chemotherapy versus concurrent chemoradiotherapy alone in locally advanced cervical cancer: a systematic review and meta-analysis. Front Oncol. 2022;12:997030.
Ma X, Fang J, Zhang L, Huang Y, Shen H, Ma X, et al. Efficacy and safety of adjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis. Crit Rev Oncol/Hematol. 2023;184:103953.
Tangjitgamol S, Katanyoo K, Laopaiboon M, Lumbiganon P, Manusirivithaya S, Supawattanabodee B. Adjuvant chemotherapy after concurrent chemoradiation for locally advanced cervical cancer. Cochrane Database Syst Rev. 2014;2014(12):CD010401.
Qiao Y, Li H, Peng B. Neoadjuvant and adjuvant treatments compared to concurrent chemoradiotherapy for patients with locally Advanced Cervical Cancer: a bayesian network Meta-analysis. Front Oncol. 2022;12:745522.
Mabuchi S, Isohashi F, Okazawa M, Kitada F, Maruoka S, Ogawa K, et al. Chemoradiotherapy followed by consolidation chemotherapy involving paclitaxel and carboplatin and in FIGO stage IIIB/IVA cervical cancer patients. J Gynecologic Oncol. 2017;28(1):e15.
Kou L, Zhang T, Yang X, Peng S, Wang Y, Yuan M, et al. Role of adjuvant chemotherapy after concurrent chemoradiotherapy in patients with locally advanced cervical cancer. Future Oncol. 2022;18(16):1917–5.
Mileshkin LR, Moore KN, Barnes EH, Gebski V, Narayan K, King MT, et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(5):468–82.
Tangjitgamol S, Tharavichitkul E, Tovanabutra C, Rongsriyam K, Asakij T, Paengchit K, et al. A randomized controlled trial comparing concurrent chemoradiation versus concurrent chemoradiation followed by adjuvant chemotherapy in locally advanced cervical cancer patients: ACTLACC trial. J Gynecologic Oncol. 2019;30(4):e82.
Ali N, Valimohammad AT, Abbasi AN, Mansha MA, Hafiz A, Qureshi BM. Chemoradiation and the role of Adjuvant Chemotherapy in Lymph nodal-metastatic cervical Cancer. J Global Oncol. 2018;4:1–4.
de Azevedo C, Thuler LCS, de Mello MJG, de Oliveira Lima JT, da Fonte ALF, Fontao DFS, et al. Phase II trial of neoadjuvant chemotherapy followed by chemoradiation in locally advanced cervical cancer. Gynecol Oncol. 2017;146(3):560–5.
da Costa SCS, Bonadio RC, Gabrielli FCG, Aranha AS, Dias Genta MLN, Miranda VC, et al. Neoadjuvant Chemotherapy with Cisplatin and Gemcitabine followed by Chemoradiation Versus Chemoradiation for locally Advanced Cervical Cancer: a randomized phase II trial. J Clin Oncology: Official J Am Soc Clin Oncol. 2019;37(33):3124–31.
McCormack M, Kadalayil L, Hackshaw A, Hall-Craggs MA, Symonds RP, Warwick V, et al. A phase II study of weekly neoadjuvant chemotherapy followed by radical chemoradiation for locally advanced cervical cancer. Br J Cancer. 2013;108(12):2464–9.
Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145(1):129–35.
Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvant Chemotherapy followed by radical surgery Versus Concomitant Chemotherapy and Radiotherapy in patients with Stage IB2, IIA, or IIB squamous cervical Cancer: a Randomized Controlled Trial. J Clin Oncology: Official J Am Soc Clin Oncol. 2018;36(16):1548–55.
Gadducci A, Cosio S. Neoadjuvant chemotherapy in locally Advanced Cervical Cancer: review of the literature and perspectives of Clinical Research. Anticancer Res. 2020;40(9):4819–28.
Chidambaram S, Owen R, Sgromo B, Chmura M, Kisiel A, Evans R et al. Delayed Surgical Intervention After Chemoradiotherapy in Esophageal Cancer: (DICE) Study. Annals of surgery. 2023.
Glynne-Jones R, Hoskin P. Neoadjuvant cisplatin chemotherapy before chemoradiation: a flawed paradigm? J Clin Oncology: Official J Am Soc Clin Oncol. 2007;25(33):5281–6.
Hu K, Wang W, Liu X, Meng Q, Zhang F. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma of cervix after definitive radiotherapy or concurrent chemoradiotherapy. Radiat Oncol. 2018;13(1):249.
Duenas-Gonzalez A, Zarba JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncology: Official J Am Soc Clin Oncol. 2011;29(13):1678–85.
Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv262.
Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169–82.
Yuan Y, You J, Li X, Wang W. Adjuvant chemotherapy after radiotherapy or concurrent chemoradiotherapy for pelvic lymph node-positive patients with locally advanced cervical cancer: a propensity score matching analysis. Int J Gynecol cancer: Official J Int Gynecol Cancer Soc. 2022;32(1):21–7.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: Xin-Bin Pan Methodology: Yan Lu Formal Analysis: You-Sheng Wei Investigation: Xin-Bin Pan Resources: Yan Lu Validation: Yan Lu and You-Sheng Wei Writing-Original Draft Preparation: Xin-Bin Pan Writing-Review & Editing: De-Sheng Yao.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
1. The requirement for ethics approval was approved by the ethics committee/Institutional Review Board of Guangxi Medical University Cancer Hospital. 2. The requirement for informed consent was waived by the ethics committee/Institutional Review Board of Guangxi Medical University Cancer Hospital. 3. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pan, XB., Lu, Y., Wei, YS. et al. Efficacy of treatment patterns based on concurrent chemoradiotherapy in patients with stage IIB cervical squamous cell carcinoma. BMC Cancer 24, 106 (2024). https://doi.org/10.1186/s12885-023-11372-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11372-6