Abstract
Background
Currently, there is no standard treatment for managing relapse in patients with acute myeloid leukemia and myelodysplastic syndrome (AML/MDS) after allogeneic hematopoietic cell transplantation. Venetoclax-based therapies have been increasingly used for treating post-transplantation relapse of AML. The aim of this systematic review and meta-analysis was to evaluate the efficacy and adverse events of Venetoclax combined with hypomethylating agents (HMAs) for AML/MDS relapse post-transplantation.
Methods
We searched PubMed, Web of Science, Excerpta Medica Database, Cochrane Library, and Clinical. gov for eligible studies from the inception to February 2022. The Methodological Index for Non-Randomized Studies was used to evaluate the quality of the included literatures. The inverse variance method calculated the pooled proportion and 95% confidence interval (CI).
Results
This meta-analysis included 10 studies involving a total of 243 patients. The pooled complete response and complete response with incomplete blood count recovery rate of Venetoclax combined with HMAs for post-transplantation relapse in AML/MDS was 32% (95% CI, 26-39%, I2 = 0%), with an overall response rate of 48% (95% CI, 39-56%, I2 = 37%). The 6-month survival rate was 42% (95% CI, 29-55%, I2 = 62%) and the 1-year survival rate was 23% (95% CI, 11-38%, I2 = 78%).
Conclusion
This study demonstrated a moderate benefit of Venetoclax in combination with HMAs for patients with relapsed AML/MDS post-transplantation (including those who have received prior HMAs therapy), and may become one of treatment options in the future. Large-scale prospective studies are needed to confirm the potential benefit from venetoclax combined with HMAs.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) as a group of complex hematopoietic malignancies is often characterized by adverse molecular biological features and an unfavorable clinical prognosis [1, 2]. Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal hematopoietic stem cell neoplasms that are characterized by ineffective hematopoiesis and a high risk of transformation into AML. The overall prognosis of MDS is poor, especially for high-risk MDS, with a survival time shorter than 1 year [3, 4]. Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is one of the most promising options for treating AML/MDS. However, relapse remains one of the most critical factors leading to the failure of allo-HSCT [5, 6]. The 2-year survival rate is fewer than 20% for about 30 − 40% of patients with myeloid malignancies relapse after transplantation, especially in these patients with a poor prognosis and an early relapse [7,8,9].
There is no standard regimen for the treatment of post-transplantation relapse due to the disease heterogeneity, fitness of a patient, ways of transplantation, time to post-transplantation relapse, etc. At present, post-transplantation relapse treatment options primarily include supportive care, withdrawal of immunosuppression, donor lymphocyte infusion (DLI), intensive chemotherapy (IC) ± DLI, hypomethylating agents (HMAs) therapy, and secondary allo-HSCT [10,11,12,13]. Among the treatment regimens mentioned above, patients either have unfitness for receiving intensive chemotherapy or secondaryallo-HSCT, or patients with hematological relapse have poor efficacy for DLI and HMAs treatment. Hence, novel therapeutic targets is urgent and necessary to be explored for the relapse of AML/MDS post-transplantation. Venetoclax, a selective BCL2 inhibitor which was approved in 2018 by Food and Drug Administration (FDA), is used in elderly or ineligible patients with newly diagnosed AML,. Venetoclax combined with HMAs (Ven-HMAs) has been gradually adopted to show favorable results in relapsed/refractory AML (R/R AML) and MDS [14,15,16,17]. Notably, a minority of the enrolled patients were relapsed AML/MDS post-transplantation who presented effectiveness of Ven-HMAs initially demonstrated by these clinical studies Therefore, Ven-HMAs therapy for treating relapse in AML/MDS post-transplantation has been gradually emerged.
Due to the effect of Ven-HMAs in relapse of patients with AML/MDS post-transplantation has been reported but only on a small scale, leading to an undetermined efficacy and safety, therefore, we aimed to conduct a systematic review and meta-analysis to assess the efficacy and adverse events (AEs) of Ven-HMAs in relapse of patients with AML/MDS post-transplantation.
Materials and methods
Data sources and literature search strategy
This meta-analysis has been conducted in the International Prospective System of Register of systematic reviews (PROSPERO) (Registration No. CRD42023398349). The present work was conducted by six researchers. PubMed, Web of Science, Embase, Cochrane Library, and ClinicalTrials.gov were systematically searched from inception to February 2023, and the language was limited to English. The subject terms of this study were “acute, myeloid, leukemia”, “myelodysplastic syndrome”, “venetoclax”, “hematopoietic stem cell transplantation”, “azacitidine”, “decitabine”, and “recurrence”. The complete search strategy is shown in Table S1.
Literature inclusion and exclusion criteria
Inclusion criteria: (1) Study population: Adult AML/MDS patients who relapsed after transplantation; (2) Intervention: Ven-HMAs regimens; (3) Comparison: IC ± DLI group, DLI group, supportive care group or single arm study; (4) Outcome: The primary outcome indicators were complete response (CR)/complete response with incomplete blood count recovery (CRi) rate, overall response rate (ORR), and survival rate; the secondary indicators were grade 3–4 hematological AEs and incidence of infection; (5) Study design: randomized controlled trial (RCT), retrospective study, prospective study; (6) The number of participants in each study was more than 10 patients.
Exclusion criteria: (1) Case reports, reviews, meta-analysis, commentaries, and non-human studies; (2) Treatment regimens including agents other than Venetoclax in combination with HMAs. (3) Study results without primary outcome indicators; (4) Duplicate publication of literature.
Three investigators (YFD, CHL, and ZJZ), independently read the title, abstract, and full text to decide whether to include a study or not. Any discrepancy in included literatures was resolved by consensus or by consulting another senior investigator.
Definition of treatment response
We selected included CR/CRi rate, ORR, and survival rate as primary outcome indicators. ORR included the rate of CR/CRi, morphologic leukemia-free status (MLFS), partial remission (PR), and stable disease (SD). Survival rate was defined as the percentage of treated patients who survived after a period of follow-up. The response criteria referred to the 2003 International Working Group, 2017 European Leukemia Network (ELN-2017), and the International Response Unified Criteria revised by the MDS International Working Group in 2006 [18,19,20].
Grade 3–4 hemocytopenia and incidence of infection were secondary outcome indicators in this research. The different degrees of AEs were rated according to the National Cancer Institute Common Terminology Standard for Adverse Events VERSION 4.03 [21].
Article quality evaluation
This study assessed the risk of bias using the Methodological Index of Non-Randomized Studies (MINORS) guidelines [22]. Among a total of 12 items in MINORS, the first 8 items are related to non-comparative studies, and the rest 12 items are related to comparative studies. These items were scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported but adequate). Three investigators (YFD, CHL, and ZJZ) independently evaluated the risk bias of the included studies, and any discrepancy was resolved by consensus.
Data extraction and statistical analysis
Data were independently collected by three investigators (YFD, CHL, and ZJZ) according to a pre-designed Excel sheet. The discrepancy was resolved by consensus or consultation with senior collaborators. R version 4.3.1 software was utilized in the meta-analysis. Analysis of dichotomous variables was performed for dichotomous information CR/CRi, ORR, and AEs using generalized inverse variance without a control group. A 95% confidence interval (CI) was reported for each measure. Q-test and I2 test (I2 ≤ 25%: no heterogeneity; 26–50%: low heterogeneity; 51–75%: moderate heterogeneity, and > 75%: high heterogeneity) were used for heterogeneity analysis. p < 0.1 or I2 > 50% indicated a statistical heterogeneity among references, and a random-effects model was used. When p > 0.1 or I² < 50%, a fixed-effects model was employed for analysis [23]. Subgroup analysis or sensitivity analysis was carried out for results with high heterogeneity to determine the cause of heterogeneity. The sensitivity analysis was performed to evaluate the effect of each study on the statistical results by excluding individual studies one by one.
Results
Literature search results
A total of 549 articles were retrieved. Specifically, 173 duplicates and 317 unrelated publications were excluded. Upon reviewing the remained 31 studies in full text, 21 were further eliminated for data unavailability or a lack of primary outcome. The final 10 studies met for the inclusion criteria [24,25,26,27,28,29,30,31,32,33]. The literature screening flow chart was prepared using Revman software (shown in Fig. 1).
Characteristics of the included studies
In total, 10 studies were included in this meta-analysis, all of which were retrospective studies. All the studies included were published from 2019 to 2022. Five studies were based in America, and the other 4 was based in Germany, Italy, Turkey, and China, respectively. Eight studies reported CR/CRi and ORR, respectively. Six studies reported 6-month and 1-year survival rates. AEs were reported in four studies. The included studies are presented in Table 1.
Clinical characteristics of included patients
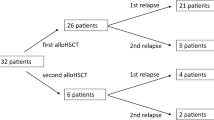
A total of 243 patients were included in the analysis (Table 1). The patients included 230 patients (94.7%) with AML in this meta-analysis. With a sample size of each study ranging from 11 to 41 patients (median size: 26) and an age range of 20-73.7 years old, the proportion of countable males in the trials was 51.7%. This study used ELN-2017 criteria for risk stratification on the included population and suggested that 92.1% of the patients had an intermediate-high risk (intermediate risk: 41.3%, high risk: 50.8%). The morphological/ hematological relapse was observed in 97.5% of patients. In this study, 25.0% of the patients were treated with a combination of DLI. An estimated 52.7% of patients were previously exposed to HMAs.
Quality assessment of the included studies
The 10 included studies were assessed using the MINORS tool sheet. As all the studies were single-group rate studies, we used the first 8 items for assessment. The quality of evaluation score for each included literature is 12, as shown in Table S2.
Efficacy of the study
The primary outcomes observed in this meta-analysis were CR/CRi, ORR, and Survival rate, which were analyzed using a random-effects model for the included studies. We estimated that the CR/CRi of Ven-HMAs for treating relapse in patients with AML/MDS post-transplantation was 32% (95% CI, 26-39%, I2 = 0%; Fig. 2) and the ORR was 48% (95% CI, 39-56%, I2 = 37%; Fig. 3), indicating a low heterogeneity. In this study, 6-month survival rate was 42% (95% CI, 29-55%, I2 = 62%; Figs. 4) and 1-year survival rate 23% (95% CI, 11-38%, I2 = 78%; Fig. 5), respectively. The analysis results in terms of race were as follows: Pooled CR/CRi and ORR in North America were 31% (95%CI, 20-43%, I2 = 0%) and 51% (95%CI, 32-70%, I2 = 63%), in Europe were 30% (95% CI, 17-45%. I2 = 0%) and 42% (95%CI, 27-57%, I2 = 0%), and in Asia were 34% (95%CI, 25-45%, I2 = 0%) and 51% (95% CI, 37-66%, I2 = 45%) (Figure S1-S2).
AEs of the study
This study analyzed the incidence of three AEs (grade 3/4) including neutropenia, thrombocytopenia, and infection. Each of these 3 AEs derived from 5 studies was included for further analysis. Specifically, thrombocytopenia was 81% (95% CI, 64-94%, I2 = 81%), neutropenia 88% (95% CI, 74-98%, I2 = 79%), and infection 54% (95% CI, 42-67%, I2 = 47%) (Fig. 6). The three outcomes had moderate to high heterogeneity. Gao et al. reported an an early death in induction related to mortality rates of 11.4% and 22.7% within 30 and 60 days, respectively [30].
Sensitivity analysis
In this study, sensitivity analyses on the main observed outcomes were performed using the “leave one out” method to further evaluate whether the exclusion of a study would significantly alter the results based on the remaining studies. For a high heterogeneity, potential origins were explored. Sensitivity analysis showed that the results of the meta-analysis of this study were robust (Figure S3-S6).
Discussion
This meta-analysis included 10 studies of 243 patients who relapsed after receiving AML/MDS transplantation to evaluate the efficacy and safety of the Ven-HMAs. Combined data for the included studies showed that the CR/CRi and ORR of this regimen for managing post-transplantation relapse in AML/MDS were 32% and 48%, respectively, with low heterogeneity. Survival analysis showed a 42% and 23% survival rate at 6 and 12 months, respectively, with a moderate and high heterogeneity. We hypothesized that the heterogeneity might be caused by the heterogeneity of clinical characteristics of patients who relapsed after transplantation (e.g., method of transplantation) on the one hand, on the other hand, the heterogeneity of the number of cycles and duration of treatment after transplantation. For this study, adverse reactions were reported in five articles, and common AEs were thrombocytopenia, neutropenia, and infection. Causes of death during treatment with the Ven-HMAs was not reported in most articles.
B-cell lymphoma-2 (BCL-2) gene was initially regarded as a growth-driven oncogene and was later identified to promote tumor cell survival by inhibiting apoptosis [34, 35]. BCL-2 family proteins are important regulators of the apoptosis signaling pathway. According to protein function, they are divided into anti-apoptotic proteins (BCL-2, BCL-XL, BCL-W, BCL2-A1, MCL-1) and pro-apoptotic proteins (effector proteins: BAX/BAK; BH3-Only proteins: BID, BIK, BIM, BAD, PUMA, NOXA) [36, 37]. The relative levels of anti-apoptotic and pro-apoptotic proteins determine a cell survival or death [38]. High expression of BCL-2 in CD34 + AML cells promotes AML cell survival and is associated with chemotherapy resistance and poor prognosis [39]. Venetoclax has a high affinity for the BH3 binding domain of BCL-2 and promotes apoptosis as well as inhibits cell proliferation by suppressing high BCL-2 expression in AML cells [40, 41]. In 2018, the FDA approved Venetoclax-based therapy for AML, which has witnessed a gradual expansion of its application to the management of post-transplant relapse in AML/MDS in the last three years. This study found that the CR/CRi rate and 1-year survival rate after relapse from AML/MDS transplantation based on Ven-HMAs were 32% and 23%, respectively, which did not seem to show a significant advantage in comparison to the previous HMAs ± DLI regimen [42,43,44,45,46]. The possible reason may be related to different baseline characteristics of the populations included in each study, and the comparative results may be highly inaccurate. On the other hand, the majority (97.5%) of patients included in this study were hematological relapsed and experienced a higher tumor burden than in the previous HMAs ± DLI group, which also influenced the response rate in this study. It should be noticed that more than half of the patients (52.7%) included in this study were previously treated with HMAs and may be insensitive to HMAs alone, however, Ven + HMAs enable some of these patients to achieve CR/CRi again. This also suggested that Ven-HMAs enhanced the anti-leukemic effect, showing a synergistical effect. It is currently believed that the synergistic effect of Venetoclax and HMAs may be through several mechanisms: (1) HMAs induce the expression of the pro-apoptotic protein NOXA through the integrated stress response pathway, which enhances the activation of Venetoclax-induced apoptosis in AML cells [47]; (2) Venetoclax can enhance the sensitivity of AML cells to HMAs by inhibiting the antioxidant response pathway induced by HMAs [48]; (3) Ven-HMAs together impair the tricarboxylic acid cycle of leukemic stem cells (LSCs) and deplete ATP of LSCs leading to AML cell death [49]. Similarly, the CR/CRi rate of relapse after AML/MDS transplantation was low for Ven-HMAs when compared to the previous IC + DLI regimen [11, 50, 51]. In addition to the heterogeneity of the study population, this difference may also be due to inadequate Ven-HMAs combined with cell therapy (only 25% of patients were treated with DLI at the base of Ven-HMAs). Although IC + DLI has a high remission rate, the 2-year survival rate of the patients is not high, which may be associated with a higher rate of treatment-related death (TRM) caused by IC and an inability to maintain a long-term remission [12, 52]. Though TRM was not mentioned explicitly in the studies included in this meta-analysis, previous findings showed that Ven-HMAs did not cause a high rate of TRM in R/R-AML [53, 54]. Therefore, we speculated that Ven-HMAs regimens could be considered as a treatment option for AML/MDS relapsed after transplantation and intolerance to IC (including patients previously treated with HMAs). Relapse < 6 months from transplantation, high tumor burden, poorer karyotype/molecular biology mutations, and aGVHD at the time of relapse have been considered as a poor prognosis of relapse after transplantation in previous studies [8, 45, 55]. However, the study of Bewersdorf et al. suggested that transplantation < 12 months from relapse and SF3B1 mutation were poor prognostic factors, while mutations in TP53 were not relevant to response and survival rate [27]. Joshi et al. and Schuler et al. similarly observed that TP53 mutations were not associated with a poor prognosis in Ven-HMAs treatment [28, 29]. However, the study by Gao et al. found that TP53 mutations and relapse within 1 year of transplantation were independent risk factors for the outcome, while DNMT3A, NPM1, and IDH1/2 mutations may be associated with favorable CR/CRI rate [30]. It should be noted that the four studies were on a small scale and the prognostic impact of TP53 mutations still required observational studies based on large samples. A further finding in the study by Schuler et al. showed that only patients with molecular relapse achieved long-term survival from receiving Ven-HMAs therapy. These studies indicated that on one hand, Ven-HMAs regimens may overcome the effects of certain adverse molecular mutations and allow this group of patients to regain remission. On the other hand, it suggested that post-transplantation monitoring was particularly important, especially NGS-based molecular and flow cytometry-based MRD monitoring, and that preemptive treatment at the molecular relapse stage may stimulate a higher response rate to achieve a long-term survival [56,57,58].
The toxicity of Ven-HMAs regimens is substantial. This study found an incidence of grade 3/4 thrombocytopenia and neutropenia of 81% and 88%, respectively, with a higher incidence than previously reported in untreated AML patients [59, 60]. We hypothesized that the occurrence was related to cytopenia due to disease progression and decreased bone marrow hematopoiesis resulting from the disruption of the hematopoietic microenvironment by previous chemotherapeutic agents [61]. A long-term cytopenia may lead to treatment disruptions and shortened treatment cycles, and whether this would reduce treatment efficacy is unknown. The use of granulocyte-stimulating factors and thrombopoietin to a lower death risk due to cytopenia may also be rational. Granulocyte deficiency could cause a high incidence of infection, and the grade 3/4 infection rate in this study was 54%, which was similar to the results previously reported [53]. However, we speculated that the 54% infection rate was underestimated because some patients used antibiotics (e.g., posaconazole) prophylactically during the treatment period. In this study, one article mentioned that the common cause of death during follow-up was infection and three articles observed that the common cause of death was disease progression [25, 26, 30, 32]. Therefore, monitoring infections during treatment with this regimen was also crucial. Only the study by Gao et al. mentioned early death in induction, with mortality rates of 11.4% and 22.7% within 30 and 60 days, slightly higher than that reported by DiNardo et al. for de novo AML [30, 60]. The reason for this difference may be related to the poor performance status of patients who relapsed after transplantation.
There are several advantages of this meta-analysis. Firstly, a comprehensive search of the available studies revealed that there was no published meta-analysis of Ven-HMAs for AML/MDS relapsed after transplantation. Secondly, we developed a comprehensive search strategy with clear inclusion criteria and strict quality evaluation to ensure the reliability of the current results, and we reported strictly according to PRISMA standards. Finally, the current analysis also evaluated the results of studies from different geographical regions in order to provide a comprehensive reference for future studies.
However, this study inevitably has some limitations. First, the sample size of each study included was small, though we have adequately searched each database. This may be related to the fact that clinical studies of Ven-HMAs in AML/MDS relapse after transplantation were at a preliminary stage. The second, the included clinical studies were retrospective ones and the quality of clinical evidence as prospective studies was not high enough, therefore we hope that prospective studies will emerge to certify the analysis findings in our study. The third, more than half of the studies came from the United States and Asia, and it was unclear whether the current findings can be generalized to other regional populations. The fourthly, a moderate to a high heterogeneity in the data on survival and side effects was present, and we used sensitivity analysis to obtain some factors contributing to the heterogeneity, but a comprehensive analysis on the specific causes to the heterogeneity was difficult. In final, most of the patients included in this study were AML, however, the number of MDS patients included was muchsmall, therefore, more MDS data need to be accumulated to ensure the robustness of the current conclusions.
Conclusion
In general, this meta-analysis demonstrated a moderate benefit to AML/MDS patients with post-transplant relapses treated with Ven-HMAs regimens, including those who have received prior HMAs therapy. The most common AEs of the regimen were grade 3–4 hemocytopenia and infection. In the future, large prospective clinical studies are needed to validate our research.
Data Availability
This article and supplemental material included all the data generated during this study. For further inquiries, please contact the corresponding author.
References
Palmieri R, Paterno G, De Bellis E, Mercante L, Buzzatti E, Esposito F, et al. Therapeutic choice in older patients with Acute myeloid leukemia: a matter of fitness. Cancers (Basel). 2020;12(1):120. https://doi.org/10.3390/cancers12010120
Nilius-Eliliwi V, Gerding WM, Schroers R, Nguyen HP, Vangala DB. Optical genome mapping for Cytogenetic Diagnostics in AML. Cancers (Basel). 2023;15(6):1684. https://doi.org/10.3390/cancers15061684
Zavras PD, Sinanidis I, Tsakiroglou P, Karantanos T. Understanding the Continuum between High-Risk Myelodysplastic Syndrome and Acute Myeloid Leukemia. Int J Mol Sci. 2023;24(5):5018. https://doi.org/10.3390/ijms24055018
Bewersdorf JP, Carraway H, Prebet T. Emerging treatment options for patients with high-risk myelodysplastic syndrome. Ther Adv Hematol. 2020;11:2040620720955006. https://doi.org/10.1177/2040620720955006
Craddock C, Hoelzer D, Komanduri KV. Current status and future clinical directions in the prevention and treatment of relapse following hematopoietic transplantation for acute myeloid and lymphoblastic leukemia. Bone Marrow Transplant. 2019;54(1):6–16. https://doi.org/10.1038/s41409-018-0203-8
Piemontese S, Boumendil A, Labopin M, Schmid C, Ciceri F, Arcese W, et al. Acute Leukemia Working Party of the European Society for blood and marrow transplantation (EBMT). Leukemia relapse following unmanipulated haploidentical transplantation: a risk factor analysis on behalf of the ALWP of the EBMT. J Hematol Oncol. 2019;12(1):68. https://doi.org/10.1186/s13045-019-0751-4
Horowitz M, Schreiber H, Elder A, Heidenreich O, Vormoor J, Toffalori C, et al. Epidemiology and biology of relapse after stem cell transplantation. Bone Marrow Transplant. 2018;53(11):1379–89. https://doi.org/10.1038/s41409-018-0171-z
Schmid C, de Wreede LC, van Biezen A, Finke J, Ehninger G, Ganser A, et al. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the chronic Malignancies Working Party of the european society of blood and marrow transplantation. Haematologica. 2018;103(2):237–45. https://doi.org/10.3324/haematol.2017.168716
Bazarbachi A, Schmid C, Labopin M, Beelen D, Wolfgang Blau I, Potter V, et al. Evaluation of Trends and Prognosis over Time in patients with AML Relapsing after allogeneic hematopoietic cell transplant reveals Improved Survival for Young patients in recent years. Clin Cancer Res. 2020;26(24):6475–82. https://doi.org/10.1158/1078-0432.CCR-20-3134
Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25(31):4938–45. https://doi.org/10.1200/JCO.2007.11.6053
Motabi IH, Ghobadi A, Liu J, Schroeder M, Abboud CN, Cashen AF, et al. Chemotherapy versus Hypomethylating Agents for the treatment of relapsed Acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplant. Biol Blood Marrow Transplant. 2016;22(7):1324–9. https://doi.org/10.1016/j.bbmt.2016.03.023
Krakow EF, Walter RB, Nathe JM, Perez T, Ahmed A, Polissar N, et al. Intensive chemotherapy for acute myeloid leukemia relapse after allogeneic hematopoietic cell transplantation. Am J Hematol. 2022;97(6):E220–3. https://doi.org/10.1002/ajh.26540
Konuma T, Kato S, Ooi J, Ebihara Y, Mochizuki S, Ishii H, et al. Second allogeneic transplantation using unrelated cord blood for relapsed hematological malignancies after allogeneic transplantation. Leuk Lymphoma. 2016;57(1):103–9. https://doi.org/10.3109/10428194.2015.1045900
Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404–7. https://doi.org/10.3324/haematol.2018.188094
DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401–7. https://doi.org/10.1002/ajh.25000
DiNardo CD, Maiti A, Rausch CR, Pemmaraju N, Naqvi K, Daver NG, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7(10):e724–36. https://doi.org/10.1016/S2352-3026(20)30210-6
Gemici A, Ozkalemkas F, Dogu MH, Tekinalp A, Alacacioglu I, Guney T, et al. A real-life turkish experience of Venetoclax Treatment in High-risk Myelodysplastic Syndrome and Acute Myeloid Leukemia. Clin Lymphoma Myeloma Leuk. 2021;21(8):e686–92. https://doi.org/10.1016/j.clml.2021.04.004
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47. https://doi.org/10.1182/blood-2016-08-733196
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting Standards for therapeutic trials in Acute myeloid leukemia. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting Standards for therapeutic trials in Acute myeloid leukemia. J Clin Oncol. 2003;21(24):4642–9. https://doi.org/10.1200/JCO.2003.04.036
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25. https://doi.org/10.1182/blood-2005-10-4149
Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. (2010) US Department of Health and Human Services. National Institutes of Health National Cancer Institute. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557
Mittal V, Lo MM, Damon LE, Gaensler KL, Martin TG, Olin RL, et al. Efficacy of Venetoclax Combination Therapy with Hypomethylating Agents in patients with relapsed Acute myeloid leukemia after allogeneic hematopoietic cell transplantation. Blood. 2019;134(Supplement1):5089. https://doi.org/10.1182/blood-2019-129514
Byrne M, Danielson N, Sengsayadeth S, Rasche A, Culos K, Gatwood K, et al. The use of venetoclax-based salvage therapy for post-hematopoietic cell transplantation relapse of acute myeloid leukemia. Am J Hematol. 2020;95(9):1006–14. https://doi.org/10.1002/ajh.25859
Diab O, Abdelhakim H, McGuirk JP, Lin T. Outcomes of HMA and Venetoclax in Relapsed AML Post-Allogenic hematopoietic stem cell transplant. Blood. 2020;136(Supplement1):33. https://doi.org/10.1182/blood-2020-141715
Bewersdorf JP, Derkach A, Gowda L, Menghrajani K, DeWolf S, Ruiz JD, et al. Venetoclax-based combinations in AML and high-risk MDS prior to and following allogeneic hematopoietic cell transplantation. Leuk Lymphoma. 2021;62(14):3394–401. https://doi.org/10.1080/10428194.2021.1966788
Joshi M, Cook J, McCullough K, Nanaa A, Gangat N, Foran JM, et al. Salvage use of venetoclax-based therapy for relapsed AML post allogeneic hematopoietic cell transplantation. Blood Cancer J. 2021;11(3):49. https://doi.org/10.1038/s41408-021-00437-z
Schuler E, Wagner-Drouet EM, Ajib S, Bug G, Crysandt M, Dressler S, et al. Treatment of myeloid malignancies relapsing after allogeneic hematopoietic stem cell transplantation with venetoclax and hypomethylating agents-a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group. Ann Hematol. 2021;100(4):959–68. https://doi.org/10.1007/s00277-020-04321-x
Gao F, Gao Y, Luo Y, Yu J, Fu H, Lai X, et al. Venetoclax plus hypomethylating agent for the salvage treatment of relapsing myeloid malignancies after hematopoietic stem cell transplantation: a multicenter retrospective study on behalf of the Zhejiang Cooperative Group for blood and marrow transplantation. Am J Hematol. 2022;97(2):E44–7. https://doi.org/10.1002/ajh.26405
Ozturk C, Sahin U, Seval GC, Bozdag SC, Toprak SK, Yuksel MK, et al. Use of venetoclax in combination with hypomethylating agents in patients with acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022;57(supplement1):335–6.
Serpenti F, Mariarita S, Galassi G, Saporiti G, Grifoni F, Rampi N, et al. Off-label venetoclax in combination with hympomethylating agents for post-allogeneic HSCT AML relapse. Bone Marrow Transplant. 2022;57(1):120–1.
Zhao P, Ni M, Ma D, Fang Q, Zhang Y, Li Y, et al. Venetoclax plus azacitidine and donor lymphocyte infusion in treating acute myeloid leukemia patients who relapse after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2022;101(1):119–30. https://doi.org/10.1007/s00277-021-04674-x
Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348(6299):334–6. https://doi.org/10.1038/348334a0
Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25(1):65–80. https://doi.org/10.1038/cdd.2017.186
Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–56. https://doi.org/10.1038/nrc883
Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, Strasser A. BH3-Mimetic drugs: blazing the trail for New Cancer Medicines. Cancer Cell. 2018;34(6):879–91. https://doi.org/10.1016/j.ccell
Pihán P, Carreras-Sureda A, Hetz C. BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017;24(9):1478–87. https://doi.org/10.1038/cdd.2017.82
Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091–6.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8. https://doi.org/10.1038/nm.3048
Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362–75. https://doi.org/10.1158/2159-8290.CD-13-0609
Schroeder T, Rachlis E, Bug G, Stelljes M, Klein S, Steckel NK, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions–a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant. 2015;21(4):653–60. https://doi.org/10.1016/j.bbmt.2014.12.016
Karakulska-Prystupiuk E, Drozd-Sokołowska J, Waszczuk-Gajda A, Stefaniak A, Dwilewicz-Trojaczek J, Kulikowska A, et al. Azacitidine for Relapse after allogeneic stem cell transplantation-single-center study. Transpl Proc. 2018;50(7):2212–7. https://doi.org/10.1016/j.transproceed.2018.02.148
Rautenberg C, Bergmann A, Germing U, Fischermanns C, Pechtel S, Kaivers J, et al. Prediction of response and survival following treatment with azacitidine for Relapse of Acute myeloid leukemia and myelodysplastic syndromes after allogeneic hematopoietic stem cell transplantation. Cancers (Basel). 2020;12(8):2255. https://doi.org/10.3390/cancers12082255
Yoshimoto G, Mori Y, Kato K, Odawara J, Kuriyama T, Ueno T, et al. Azacitidine for the treatment of patients with relapsed acute myeloid leukemia after allogeneic stem cell transplantation. Leuk Lymphoma. 2021;62(12):2939–48. https://doi.org/10.1080/10428194.2021.1941937
Poiré X, Graux C, Ory A, Herman J, Baron F, Schoemans H, Lewalle P, et al. Sequential administration of low dose 5-azacytidine (AZA) and donor lymphocyte infusion (DLI) for patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) in relapse after allogeneic stem cell transplantation (SCT): a prospective study from the belgian Hematology Society (BHS). Bone Marrow Transplant. 2022;57(1):116–8. https://doi.org/10.1038/s41409-021-01464-x
Jin S, Cojocari D, Purkal JJ, Popovic R, Talaty NN, Xiao Y, et al. 5-Azacitidine induces NOXA to Prime AML cells for venetoclax-mediated apoptosis. Clin Cancer Res. 2020;26(13):3371–83. https://doi.org/10.1158/1078-0432.CCR-19-1900
Nguyen LXT, Troadec E, Kalvala A, Kumar B, Hoang DH, Viola D, et al. The Bcl-2 inhibitor venetoclax inhibits Nrf2 antioxidant pathway activation induced by hypomethylating agents in AML. J Cell Physiol. 2019;234(8):14040–9. https://doi.org/10.1002/jcp.28091
Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24(12):1859–66. https://doi.org/10.1038/s41591-018-0233-1
Yan CH, Wang JZ, Liu DH, Xu LP, Chen H, Liu KY, et al. Chemotherapy followed by modified donor lymphocyte infusion as a treatment for relapsed acute leukemia after haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: superior outcomes compared with chemotherapy alone and an analysis of prognostic factors. Eur J Haematol. 2013;91(4):304–14. https://doi.org/10.1111/ejh.12168
Sun W, Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, et al. Chemotherapy plus DLI for relapse after haploidentical HSCT: the biological characteristics of relapse influences clinical outcomes of acute leukemia patients. Bone Marrow Transplant. 2019;54(8):1198–207. https://doi.org/10.1038/s41409-018-0406-z
Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119(6):1599–606. https://doi.org/10.1182/blood-2011-08-375840
Maiti A, DiNardo CD, Qiao W, Kadia TM, Jabbour EJ, Rausch CR, et al. Ten-day decitabine with venetoclax versus intensive chemotherapy in relapsed or refractory acute myeloid leukemia: a propensity score-matched analysis. Cancer. 2021;127(22):4213–20. https://doi.org/10.1002/cncr.33814
Kwag D, Cho BS, Bang SY, Lee JH, Min GJ, Park SS, et al. Venetoclax with decitabine versus decitabine monotherapy in elderly acute myeloid leukemia: a propensity score-matched analysis. Blood Cancer J. 2022;12(12):169. https://doi.org/10.1038/s41408-022-00770-x
Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of Second Allogeneic hematopoietic cell transplant vs Donor lymphocyte infusion with overall survival in patients with Acute myeloid leukemia relapse. JAMA Oncol. 2018;4(9):1245–53. https://doi.org/10.1001/jamaoncol.2018.2091
Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26(3):381–9. https://doi.org/10.1038/leu.2011.234
Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol. 2018;19(12):1668–79. https://doi.org/10.1016/S1470-2045(18)30580-1
Schmid C, Labopin M, Schaap N, Veelken H, Brecht A, Stadler M, et al. Long-term results and GvHD after prophylactic and preemptive donor lymphocyte infusion after allogeneic stem cell transplantation for acute leukemia. Bone Marrow Transplant. 2022;57(2):215–23. https://doi.org/10.1038/s41409-021-01515-3
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. https://doi.org/10.1182/blood-2018-08-868752
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in previously untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383(7):617–29. https://doi.org/10.1056/NEJMoa2012971
Battiwalla M, Barrett AJ. Bone marrow mesenchymal stromal cells to treat complications following allogeneic stem cell transplantation. Tissue Eng Part B Rev. 2014;20(3):211–7. https://doi.org/10.1089/ten.TEB.2013.0566
Acknowledgements
The authors wish to thank Bullet Edits for the correction of the English style.
Funding
This study was supported by grants from the Key Project of the Educational Department of Liaoning Province (No. LZ2020003), and Central Guidance on Local Science and Technology Development Fund of Liaoning Province (No. 2023JH6/100100019).
Author information
Authors and Affiliations
Contributions
Conception and design: YFD, CHL, and JSY. Extraction and analysis of data: YFD, CHL, ZJZ, and YKL. Manuscript writing: YFD and CTZ. Revision of the manuscript: JSY. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplementary Material 1: Figure S1
. Forest plots of pooled CR/CRi rates with different races.

Supplementary Material 2: Figure S2
. Forest plots of pooled ORR rates with different races.

Supplementary Material 3: Figure S3
. Sensitivity analysis of pooled CR/CRi rates after treatment.

Supplementary Material 4: Figure S4
. Sensitivity analysis of pooled ORR rates after treatment.

Supplementary Material 5: Figure S5
. Sensitivity analysis of pooled six months OS rates after treatment.

Supplementary Material 6: Figure S6
. Sensitivity analysis of pooled one year OS rates after treatment.
Supplementary Material 7: Table S1
. The strategy of literature search (from inception to 23 February 2023)
Supplementary Material 8: Table S2
. The quality of the 10 included studies was assessed by MINORS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Du, Y., Li, C., Zhao, Z. et al. Efficacy and safety of venetoclax combined with hypomethylating agents for relapse of acute myeloid leukemia and myelodysplastic syndrome post allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. BMC Cancer 23, 764 (2023). https://doi.org/10.1186/s12885-023-11259-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11259-6