Abstract
Backgrounds
Tigecycline has a broad spectrum of antimicrobial activity and has been approved for the treatment of complicated intra-abdominal infections. However, it is debatable whether tigecycline should be used alone or in combination. This study aimed to investigate whether tigecycline plus β-lactam antibiotics (combination therapy [CT] group) are superior to tigecycline alone (monotherapy [MT] group) in non-critically ill intra-abdominal infection patients after tumor surgery.
Methods
This was a multicenter, retrospective cohort study. The primary outcome was mortality during the hospital stay. Secondary outcomes were clinical success rate, microbial eradication rate, relapse rate within one week, course of treatment, and adverse effects. Propensity score matching (PSM) was used to adjust the degree of infection before medication between the MT and CT groups. Univariate comparisons were performed using the chi-squared test for qualitative variables and Student’s t-test or the Mann-Whitney U-test for continuous variables, as appropriate. Multivariate logistic regression analysis was performed to examine the relationship between antimicrobial treatments and mortality during hospitalization. The paired samples Wilcoxon test was used to compare the parameters before and after medication.
Results
In total, 291 patients were included in the final analysis: 128 in MT group and 163 in CT group. Mortality rate was 6.25% in the MT group and 6.13% in the CT group (P = 0.97). Multivariate logistic regression model showed that carbapenem-resistant organisms (OR: 4.35, 95% CI: 2.36 ~ 61.70) and age > 65 (OR: 1.32, 95% CI:1.19 ~ 3.01) were independent risk factors for death. CT group had a shorter defervescence time (P < 0.05), with less likelihood of relapse (P < 0.05) but had a more significant effect on activated partial thromboplastin and prothrombin time.
Conclusions
Tigecycline plus β-lactam wasn’t superior to tigecycline monotherapy for the treatment of non-critically ill patients with intra-abdominal infection. But for advanced age patients with cancer, tigecycline combination therapy maybe a better choice in terms of mortality.
Similar content being viewed by others
Background
Intra-abdominal infection (IAI) is a common complication of abdominal surgeries. Postoperative intra-abdominal infection (PIAI) is an important type of IAI, accounting for approximately 8.5% of total IAIs, and its mortality rate is as high as 22%~55%[1]. PIAI refers to the clinical manifestations of IAI within 30 days of surgery, with laboratory tests and imaging confirming IAI or drainage fluid confirming the presence of an intra-abdominal abscess [1]. Appropriate empirical antimicrobial treatment can increase the success rate of clinical treatment, reduce hospital stay and hospitalization costs, and minimize antimicrobial resistance caused by selective pressure [2].
Multidrug-resistant (MDR) bacteria, such as methicillin-resistant Staphylococcus aureus, extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenemase-producing Klebsiella pneumoniae, and carbapenem-resistant Acinetobacter baumannii (CRAB), are common in intra-abdominal infections [3,4,5]. Cancer is the risk factor for MDR bacteria infection [6]. Therefore, it is challenging for physicians to choose an appropriate anti-infection regimen for IAI after tumor surgery.
In China and the US, tigecycline is approved for the treatment of complicated intra-abdominal infections, complicated skin and skin tissue infections, and community-acquired bacterial pneumonia. It is a minocycline derivative with antimicrobial activity against Gram-positive and Gram-negative bacteria, anaerobes, and atypical pathogens. Tigecycline is often used in combination with other antibiotics, such as β-lactam antibiotics, carbapenems, and aminoglycosides, owing to its heterogeneous resistance [7, 8]. The combination of tigecycline with β-lactam antibiotics has shown a good synergistic effect [9, 10], and tigecycline plus cefoperazone/sulbactam is the first-line treatment for CRAB in China. However, tigecycline has a higher concentration in the bile, gall bladder and colon [11, 12], which means that tigecycline is more effective against IAI compared to other site of infections. Furthermore, its unique pharmacological mechanism provides good antibacterial activity against various pathogenic bacteria that cause complicated intra-abdominal infections, especially MDR bacteria [13, 14]. Some studies suggested that tigecycline alone is not inferior to tigecycline-based combination regimens [15,16,17,18,19], while whether tigecycline should be used alone or in combination in PIAI cancer patients was remained unknown. This study aimed to investigate whether tigecycline combined with β-lactam antibiotics (combination therapy group, CT group) is superior to tigecycline alone (monotherapy group, MT group) in intra-abdominal infection after tumor surgery.
Methods
This was a five-center (in China), retrospective cohort study. The study design was based on a comparison of outcomes between two groups of patients with PIAIs. The primary outcome in this study was mortality during the hospital stay. The secondary outcomes were clinical success rate, microbial eradication rate, relapse rate within one week, course of treatment, and adverse effects.
Cohort description
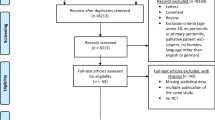
The sample groups for this cohort study were derived from individuals registered at the gastrointestinal surgery departments of Shanxi Provincial People’s Hospital, First Hospital of Shanxi Medical University, Second Hospital of Shanxi Medical University, Yuncheng Central Hospital and Shanxi Cancer Hospital; each of these hospitals was a tertiary hospital with over 2,200 inpatient beds. We retrospectively reviewed the clinical records of adult cancer patients (age > 18 years) with PIAI who were treated with tigecycline or tigecycline in combination with β-lactam antibiotics between January 2018 and November 2022. The study protocol was approved by the Ethics Committee of Shanxi Provincial People’s Hospital (2022 − 281). The need to obtain written informed consent was waived due to the retrospective nature of the study. A total of 336 participants who met the inclusion criteria were enrolled in the study (Fig. 1).
Definitions
Intra-abdominal infection must meet the following criteria: presence of organisms isolated from abdominal drainage, body temperature > 37.5 oC, and abdominal pain. Clinical success was defined as the absence of any sign of infection at the end of treatment, normal temperature, no indication for continuation of antibiotics treatment or a second operation, and no relapse within one week. Microbial eradication was defined as the absence of pathogens in the culture of specimens collected from the original site. Relapse was defined as the recurrence of abdominal pain within one week of the end of antibiotics treatment, with or without unexplained fever, increase in white blood cells, C-reactive protein, or procalcitonin (PCT), new radiographic findings (abscess or other infectious manifestations), and cannot be explained by infection at other sites.
Inclusion definition for the Monotherapy and Combination Groups
The MT group was defined as patients with PIAI treated with tigecycline alone: a loading dose of 100 mg, followed by 50 mg twice daily. The CT group was defined as patients with PIAI treated with tigecycline in combination with β-lactam antibiotics (ceftazidime, piperacillin/tazobactam, cefoperazone/sulbactam, ceftriaxone or cefoxitin); the dose of tigecycline in the CT group was the same as that in the MT group. The inclusion criteria were as follows: patients who had undergone abdominal surgery and had been diagnosed with IAI, male or female, older than 18 years, and treated with tigecycline or tigecycline in combination with β-lactam antibiotics. The exclusion criteria were as follows: missing data; use of tigecycline within the last month; the pathogen was resistant to tigecycline; bacteremia; intensive care unit admission; course of treatment was less than five days; use of immunosuppressants or immunodeficiencies; mechanical ventilation; renal replacement therapy; combination with other antibiotics; three antibiotics were used at the same time; and liver failure.
The patients’ baseline data (age, sex, underlying diseases, causative pathogens, course of treatment, type of surgery, and laboratory tests) were obtained from the Hospital Information System. Laboratory tests between 48 h before tigecycline administration and within 48 h after tigecycline discontinuation were recorded.
Statistical analysis
Propensity score matching (PSM) was used to adjust the degree of infection before medication (age, PCT) between the MT and CT groups. The Shapiro-Wilk test was used to test for normality. Univariate comparisons were performed using the chi-squared test for qualitative variables and Student’s t-test or the Mann-Whitney U-test for continuous variables, as appropriate. The paired samples Wilcoxon test was used to compare the parameters before and after medication. Conditional logistic regression model was used to investigate the correlation between the parameters and mortality. The significance level was set at P < 0.05. All statistical analyses were calculated using Extreme Smart Analysis platform. Retrieved from https://www.xsmartanalysis.com.
Results
After applying the inclusion and exclusion criteria, the study cohort included 336 patients. Statistical analysis revealed significant differences in age and PCT levels between the MT and CT groups. PSM was used to match the two groups to eliminate baseline differences. After PSM, 128 and 163 patients were included in the MT and CT groups, respectively. The baseline clinical characteristics are presented in Table 1.
The comparisons between the two groups are presented in Table 2. There was no significant difference in mortality during hospitalization between the two groups (6.25% vs., 6.13%, P = 0.97). Clinical efficacy was 80.47% (103/128) in the MT group and 86.50% (141/163) in the CT group (P = 0.17). Microbial eradication rates were 92.19% (118/128) in the MT group and 94.48% (154/163) in the CT group, respectively (P = 0.43). In addition, there was no significant difference in duration of microbial eradication (5.12 ± 1.38 vs. 4.93 ± 1.51, P = 0.78) and duration of clinical success (7 [6, 9] vs. 7 [6, 9], P = 0.76). However, the MT group needed a longer time for the temperature to return to normal (4.67 ± 0.32 vs. 3.89 ± 0.45, P < 0.05). In addition, the MT group was more likely to relapse within a week (14.06% vs. 6.13%, P < 0.05).
Multivariate analysis suggested that treatment regimen (OR: 1.57, 95% CI: 0.97–4.03) was not associated with mortality, but with age > 65 (OR: 1.32, 95% CI:1.19 ~ 3.01) and carbapenem-resistant organisms (CRO) [OR: 4.35, 95% CI: 2.36–61.70] (Table 3).
Furthermore, we compared changes in laboratory test results before and after tigecycline-based medication between the groups (Table 4). Coagulation function (activated partial thromboplastin time, APTT; prothrombin time, PT; fibrinogen, FIB) was significantly lower after tigecycline-based treatment than that before the treatment (P < 0.05) in each group. There was no difference in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels before and after medication for both groups. Total bilirubin (Tbil) in the MT group was significantly elevated after the treatment (14.93 ± 46.23 vs. 18.94 ± 40.55, P <0.05). The hemoglobin levels in the CT group were significantly reduced after treatment (100 (89, 112.75) vs. 96 (86, 112), P < 0.05). There was no significant change in platelet levels in both groups before and after treatment.
To compare the adverse effects after medication between the MT and CT groups, we first compared the parameters between the two groups before treatment to eliminate baseline differences and found that only total bilirubin was significantly different between the two groups before treatment. Afterward, we compared other parameters between the MT and CT groups after medication and found that only APTT, PT, and AST were statistically different between the two groups at the end of treatment.
Discussion
Several clinical studies have documented the effects of tigecycline as a single agent or in combination with other antimicrobials, and only three studies involved intra-abdominal infection [15,16,17,18, 20,21,22]. Cancer is the risk factor of MDR intra-abdominal infection. For cancer patients with IAI, whether tigecycline should be used alone or combination is still unknown. On searching PubMed and Web of Science, we believe that this is the first study to compare the efficacy and adverse effects of tigecycline monotherapy and tigecycline in combination with β-lactam antibiotics in IAI patients after tumor surgery.
It is debatable whether tigecycline should be used alone or in combination [23, 24]. Many studies have supported a combination regimen for hospital-acquired pneumonia [20, 24, 25]. For intra-abdominal infections, tigecycline alone has shown a success rate of up to 90%, with the in-hospital mortality and bacterial eradication rates not significantly different [16, 17]. In the present study, the addition of β-lactam antibiotics to tigecycline in PIAI patients was not associated with better clinical outcomes, including mortality during hospitalization, clinical success rate, microbial eradication rate, duration of microbial eradication, defervescence time, and duration of clinical success. We chose mortality during the hospital stay as the primary outcome instead of 28 days because the patients enrolled in this study had milder disease. Furthermore, at 28 days, mortality may have been influenced by underlying comorbidities.
Our findings were consistent with those of previous studies [16, 17, 26]. We observed that tigecycline plus β-lactam was not superior to tigecycline monotherapy for the treatment of non-critically ill patients with IAI after tumor surgery. We believe that this was due to the high concentration of tigecycline in the abdomen and its broad antimicrobial spectrum [12]. Therefore, tigecycline is effective in the treatment of mild abdominal infections, even when used alone. However, tigecycline concentration in the blood is very low, and therefore, should not be used for bacteremia [27]. As a result, we excluded patients with bacteremia and ICU admission, and our findings were limited to non-critically ill patients.
Although there was no difference in mortality during hospitalization between the two groups, the defervescence time of patients in the CT group was significantly lower than that of patients in the MT group. Tigecycline is a bacteriostatic agent, whereas β-lactam antibiotics are bactericidal agents; thus, more time is needed to lower the body temperature for tigecycline monotherapy [28]. The results of bacterial eradication rates may seem contradictory to relapse rates; however, given that the frequency of abdominal drainage culture performed for each patient was different and that there were many factors affecting bacterial culture, false negatives were likely to exist. Therefore, we believe that the results of relapse rates may be more reliable than those of bacterial eradication rates.
Multivariate analysis with a logistic regression model showed that mortality was not associated with tigecycline use alone, but with CRO and advanced age. First, tigecycline requires a high dose of CRO (200 mg loading dose, followed by 100 mg every 12 h)[8, 29, 30]; however, patients enrolled in this study were treated with a standard dose. Second, low doses of tigecycline have a higher risk of selecting drug-resistant isolates [23]. Third, advanced age and cancer were risk factors of MDR infection, which was related to treatment failure [6, 31]. Furthermore, superinfections from CRO secondary to tigecycline resistance were more common during monotherapy than during combination therapy [32]. Therefore, we suggest that even for non critical ill postoperative intra-abdominal infections, physicians should choose tigecycline combination therapy to reduce mortality for advanced age patients with cancer.
According to the manufacturer’s instructions, tigecycline may cause coagulopathy [11]. The effect of tigecycline on coagulation function mainly manifests as a prolongation of PT and APTT and a decrease in FIB [33,34,35,36], and our study supports this hypothesis. Antibiotics are generally associated with coagulation disorders, as they reduce the microflora of the colon and distal ileum, which synthesize vitamin K2[37, 38]. However, the mechanism underlying this effect remains unclear. Comparison of coagulation function between the two groups showed that the CT group had a more pronounced effect on APTT and PT than did the MT group. This may be because both tigecycline and β-lactam antibiotics may cause coagulopathy, and the combination of these two drugs exacerbates this adverse effect. Therefore, patients at risk of bleeding should be closely monitored during tigecycline treatment. Although tigecycline-based regimens have a significant effect on coagulation, patients usually recover spontaneously after drug discontinuation [36].
Regarding the effect on liver function, although AST was higher in the CT group, we did not think this result was meaningful considering that many drugs affect aminotransferases, and we cannot completely rule out the effect of other drugs on aminotransferases.
There are some limitations of this study. First, this was a retrospective study and was thus susceptible to selection bias. Although we have used PSM to reduce bias, some potential sources of biases may be neglected. Second, adequate drainage is critical for treatment of PIAI; however, we cannot confirm that each patient received adequate drainage. Third, the sample size is relatively small.
Conclusions
In conclusion, tigecycline alone or in combination with β-lactam antibiotics was not associated with mortality during hospitalization, clinical success, or microbial clearance. CRO infection and advanced age were independent risk factors for death. Tigecycline in combination with β-lactam antibiotics has a shorter defervescence time and is less likely to be associated with a relapse; however, it has a more significant effect on coagulation. But for advanced age patients with cancer, tigecycline combination therapy maybe a better choice in terms of mortality.
Data Availability
The data that support the findings of this study are available from the corresponding author (Professor Jinlin Guo) upon reasonable request.
Abbreviations
- CT group:
-
combination therapy group
- MT group:
-
monotherapy group
- IAI:
-
Intra-abdominal infection
- PIAI:
-
Postoperative intra-abdominal infection
- MDR:
-
Multidrug-resistant
- CRAB:
-
Carbapenem-resistant Acinetobacter baumannii
- PCT:
-
procalcitonin
- PSM:
-
Propensity score matching
- OR:
-
Odds ratio
- CI:
-
Confidence Interval
- CRO:
-
carbapenem-resistant organisms
- APTT:
-
activated partial thromboplastin time
- PT:
-
prothrombin time
- FIB:
-
fibrinogen
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- Tbil:
-
Total bilirubin
References
Liu CZ. Jing-yao;. New concept of diagnosis and treatment of intra-abdominal infections: consensus and controversies. Chin J Practical Surg. 2019;39(6):538–.
Sartelli M, Catena F, Ansaloni L, Coccolini F, Di Saverio S, Griffiths EA. Duration of Antimicrobial Therapy in Treating Complicated Intra-Abdominal Infections: a Comprehensive Review. Surg Infect (Larchmt). 2016;17(1):9–12.
Zhang S, Huang W. Epidemiological study of community- and hospital-acquired intraabdominal infections. Chin J Traumatol. 2015;18(2):84–9.
Yang Q, Wang H, Chen M, Ni Y, Yu Y, Hu B, et al. Surveillance of antimicrobial susceptibility of aerobic and facultative gram-negative bacilli isolated from patients with intra-abdominal infections in China: the 2002–2009 study for Monitoring Antimicrobial Resistance Trends (SMART). Int J Antimicrob Agents. 2010;36(6):507–12.
Takesue Y, Kusachi S, Mikamo H, Sato J, Watanabe A, Kiyota H, et al. Antimicrobial susceptibility of common pathogens isolated from postoperative intra-abdominal infections in Japan. J Infect Chemother. 2018;24(5):330–40.
Freire MP, de Oliveira Garcia D, Garcia CP, Campagnari Bueno MF, Camargo CH, Kono Magri ASG, et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infect. 2016;22(4):352–8.
Liu H, Jia X, Zou H, Sun S, Li S, Wang Y, et al. Detection and characterization of tigecycline heteroresistance in E. cloacae: clinical and microbiological findings. Emerg Microbes Infect. 2019;8(1):564–74.
Paul M, Carrara E, Retamar P, Tangden T, Bitterman R, Bonomo RA, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by european society of intensive care medicine). Clin Microbiol Infect. 2022;28(4):521–47.
Jin Jing WW-p. Synergy activity of tigecycline combined with other nine antimicrobials against carbapenem-resistant pathogens. Chin J Health Lab Technol. 2018;28(11):1281–4.
Liu B, Bai Y, Liu Y, Di X, Zhang X, Wang R, et al. In vitro activity of tigecycline in combination with cefoperazone-sulbactam against multidrug-resistant Acinetobacter baumannii. J Chemother. 2015;27(5):271–6.
Pfizer. Tygacil (tigecycline) for injection prescribing information. Philadelphia: PA; 2013 Oct.
Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58(6):1221–9.
Giammanco A, Cala C, Fasciana T, Dowzicky MJ. Global Assessment of the activity of Tigecycline against Multidrug-Resistant Gram-Negative pathogens between 2004 and 2014 as part of the Tigecycline evaluation and Surveillance Trial. mSphere. 2017;2(1).
Rodriguez-Bano J, Gutierrez-Gutierrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-Beta-Lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2).
Chang K, Wang H, Zhao J, Yang X, Wu B, Sun W, et al. Polymyxin B/Tigecycline combination vs. polymyxin B or tigecycline alone for the treatment of Hospital-Acquired Pneumonia caused by Carbapenem-Resistant Enterobacteriaceae or Carbapenem-Resistant Acinetobacter baumannii. Front Med. 2022;9:772372.
Chusri S, Singkhamanan K, Wanitsuwan W, Suphasynth Y, Kositpantawong N, Panthuwong S, et al. Adjunctive therapy of intravenous colistin to intravenous tigecycline for adult patients with non-bacteremic post-surgical intra-abdominal infection due to carbapenem-resistant Acinetobacter baumannii. J Infect chemotherapy: official J Japan Soc Chemother. 2019;25(9):681–6.
Heizmann WR, Loschmann PA, Eckmann C, von Eiff C, Bodmann KF, Petrik C. Clinical efficacy of tigecycline used as monotherapy or in combination regimens for complicated infections with documented involvement of multiresistant bacteria. Infection. 2015;43(1):37–43.
Lopez-Cortes LE, Cisneros JM, Fernandez-Cuenca F, Bou G, Tomas M, Garnacho-Montero J, et al. Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii: analysis of a multicentre prospective cohort. J Antimicrob Chemother. 2014;69(11):3119–26.
Seyman D, Berk H, Sepin-Ozen N, Kizilates F, Turk CC, Buyuktuna SA, et al. Successful use of tigecycline for treatment of culture-negative pyogenic vertebral osteomyelitis. Infect Dis. 2015;47(11):783–8.
Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56(4):2108–13.
Eckmann C, Heizmann W, Bodmann KF, von Eiff C, Petrik C, Loeschmann PA. Tigecycline in the treatment of patients with necrotizing skin and soft tissue infections due to multiresistant Bacteria. Surg Infect (Larchmt). 2015;16(5):618–25.
Wang HJ, Xing XZ, Qu SN, Huang CL, Zhang H, Wang H, et al. A randomized controlled trial comparing the efficacy of tigecycline versus meropenem in the treatment of postoperative complicated intra-abdominal infections. Ann Palliat Med. 2021;10(2):1262–75.
Cai Y, Bai N, Liu X, Liang B, Wang J, Wang R. Tigecycline: alone or in combination? Infect Dis (Lond). 2016;48(7):491–502.
Bai XR, Liu JM, Jiang DC, Yan SY. Efficacy and safety of tigecycline monotherapy versus combination therapy for the treatment of hospital-acquired pneumonia (HAP): a meta-analysis of cohort studies. J Chemother. 2018;30(3):172–8.
Qin Y, Zhang J, Wu L, Zhang D, Fu L, Xue X. Comparison of the treatment efficacy between tigecycline plus high-dose cefoperazone-sulbactam and tigecycline monotherapy against ventilator-associated pneumonia caused by extensively drug-resistant Acinetobacter baumannii. Int J Clin Pharmacol Ther. 2018;56(3):120–9.
Bodmann KF, Heizmann WR, von Eiff C, Petrik C, Loschmann PA, Eckmann C. Therapy of 1,025 severely ill patients with complicated infections in a german multicenter study: safety profile and efficacy of tigecycline in different treatment modalities. Chemotherapy. 2012;58(4):282–94.
Cunha BA, Baron J, Cunha CB. Once daily high dose tigecycline - pharmacokinetic/pharmacodynamic based dosing for optimal clinical effectiveness: dosing matters, revisited. Expert Rev Anti Infect Ther. 2017;15(3):257–67.
Yaghoubi S, Zekiy AO, Krutova M, Gholami M, Kouhsari E, Sholeh M, et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis. 2022;41(7):1003–22.
Hawkey PM, Warren RE, Livermore DM, McNulty CAM, Enoch DA, Otter JA, et al. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the british Society for Antimicrobial Chemotherapy/Healthcare infection Society/British infection Association Joint Working Party. J Antimicrob Chemother. 2018;73(suppl3):iii2–iii78.
Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother. 2013;57(4):1756–62.
Garnacho-Montero J, Dimopoulos G, Poulakou G, Akova M, Cisneros JM, De Waele J, et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 2015;41(12):2057–75.
Poulakou G, Kontopidou FV, Paramythiotou E, Kompoti M, Katsiari M, Mainas E, et al. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J Infect. 2009;58(4):273–84.
Hu J, Xiao YH, Zheng Y, Lai YX, Fang XL, Fang Q. Clinical characteristics and risk factors of tigecycline-associated hypofibrinogenaemia in critically ill patients. Eur J Clin Pharmacol. 2020;76(7):913–22.
Rossitto G, Piano S, Rosi S, Simioni P, Angeli P. Life-threatening coagulopathy and hypofibrinogenaemia induced by tigecycline in a patient with advanced liver cirrhosis. Eur J Gastroenterol Hepatol. 2014;26(6):681–4.
Yilmaz Duran F, Yildirim H, Sen EM. A lesser known side effect of Tigecycline: Hypofibrinogenemia. Turk J Haematol. 2018;35(1):83–4.
Liu J, Yan Y, Zhang F. Risk factors for Tigecycline-Associated Hypofibrinogenemia. Ther Clin Risk Manag. 2021;17:325–32.
Shirakawa H, Komai M, Kimura S. Antibiotic-induced vitamin K deficiency and the role of the presence of intestinal flora. Int J Vitam Nutr Res. 1990;60(3):245–51.
Guclu E, Kaya G, Ogutlu A, Karabay O. The effect of cefoperazone sulbactam and piperacillin tazobactam on mortality in Gram-negative nosocomial infections. J Chemother. 2020;32(3):118–23.
Acknowledgements
We thank the Extreme Smart Analysis platform (http://www.xsmartanalysis.com) for its analysis assistance.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: XinFeng Cai, HongXia Yan and JinLin Guo; Methodology: JinLin Guo and ZhiYing Hao; Data collection: HongXia Yan; Wei Zhao; WenJun Zhang; Lei Zhang; XinJing Wu; Formal analysis and investigation: JinLin Guo and Xu Wang; Writing—original draft preparation: XinFeng Cai, JinLin Guo and HongXia Yan; Writing—review and editing: JinLin Guo and ZhiYing Hao.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Shanxi Provincial People’s Hospital (2022 − 281) and was conducted following the legal requirements and tenets of the Declaration of Helsinki and its subsequent amendments. The need to obtain written informed consent was waived by the Ethics Committee of Shanxi Provincial People’s Hospital due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cai, X., Yan, H., Zhang, W. et al. Intra-abdominal infection after tumor surgery: tigecycline combined with β-lactam antibiotics versus tigecycline alone. BMC Cancer 23, 682 (2023). https://doi.org/10.1186/s12885-023-11169-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11169-7