Abstract
Background
Hypertension is a risk factor for cholangiocarcinoma (CCA). The effect of anti-hypertensive drugs on the prognosis of CCA is not clear.
Methods
This is a retrospective study of 102 patients (56.9% males, median age 66 years) diagnosed with CCA and hypertension concurrently and received radical surgery (R0), with a median follow-up of 36.7 months. Kaplan-Meier analysis, Cox regressions, and propensity score (PS) matching were applied for statistical analysis.
Results
Results of multivariable cox analysis showed that renin-angiotensin system inhibitors (RASis) usage was a protective factor for progression-free survival (PFS) (hazard ratio [HR] = 0.55, 95% confidence interval [95% CI]: 0.32–0.96) and overall survival (OS) (HR = 0.40, 95% CI: 0.20–0.79), respectively. Calcium channel blockers, diuretics, and β-blockers didn’t show significant associations. The association of RASis usage and PFS and OS was derived by PS matching, with a cohort of 28 RASis users and 56 RASis non-users. The median PFS and OS of RASis users (PFS, 17.6 months (9.2–34.4); OS, 24.8 months (16.5–42.3)) were longer than RASis non-users (PFS, 10.5 months (4.1–24.1); OS, 14.6 months (10.6–28.4)). The 1 year, 2 years, and 3 years’ survival rates of RASis users (89.1%, 77.0%, and 65.5%) were higher than RASis non-users (70.9%, 54.0%, and 40.0%).
Conclusions
RASis usage improves the survival of patients with CCA and hypertension concurrently.
Similar content being viewed by others
Background
Cholangiocarcinoma (CCA) is a highly fatal and heterogenous group of biliary malignancies arising from the biliary tree, comprising 15% of all primary liver cancers [1]. At the time of diagnosis, more than 80% of patients present with unresectable or metastatic disease. Even for a small subset of patients who are diagnosed with a localized, resectable tumor, the prognosis remains poor, with a recurrence rate of 32%, a median overall survival (OS) of 36.4 months, and a five-year survival of 25–45% after radical resection [2,3,4,5]. Combination of gemcitabine and cisplatin is the fist line adjuvant therapy for advanced CCA, but the median OS is only 11.7 months, with a five-year survival of 5–10%[6]. Strategies for adjuvant therapies should be improved from every aspect of life.
CCA is characterized by its hard consistency, due to its abundant fibrous tissues, when compared with other liver cancers. The association between CCA occurrence and cirrhosis had been confirmed by several studies [7, 8]. Angiotensin (ANG) II, the primary component of the renin-angiotensin system, was reported increasing in patients with cirrhosis and rats with active liver fibrogenesis [9,10,11,12]. ANG II also plays an integral role in the pathogenesis of hypertension. Renin-angiotensin system inhibitors (RASis), including angiotensin converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs), are generally applied for anti-hypertension. RASis were also reported to attenuate the fibrosis and improve the prognosis of various cancers [13, 14], but not well studied in CCA. In addition, hypertension is the most common comorbidity in CCA patients receiving adjuvant therapies (22.0-73.1%) [15,16,17,18,19,20]. But up to now, there is still no official guidelines for the usage strategies of anti-hypertensive drugs for patients with CCA and hypertension concurrently.
Herein, we conducted a retrospectively study to evaluate whether the use of RASis could improve the prognosis of CCA patients with hypertension.
Methods
Selection of patients
Patients who were histologically diagnosed with cholangiocarcinoma (CCA) and hypertension concurrently and received surgery from March 2017 to February 2022 in The First Affiliated Hospital of Sun Yat-sen University (FAHSYSU) were included retrospectively. The diagnosis of CCA was reviewed by three pathologists, including intrahepatic cholangiocarcinoma (iCCA), perihilar cholangiocarcinoma (pCCA) and distal cholangiocarcinoma (dCCA), according to the International Classification of Diseases 10 (ICD-10). Hypertension was diagnosed by patients’ medical history and patients’ systolic blood pressure (SBP, SBP ≥ 140mmHg) and/or diastolic blood pressure (DBP, DBP ≥ 90mmHg) measured before surgery. Patients’ demographics, blood pressures, laboratory parameters, surgical procedures, histological diagnosis and drug prescription and dispensing history were collected from Hospital’s Information System of FAHSYSU. Follow up results were collected by outpatient clinic visits and telephone follow up.
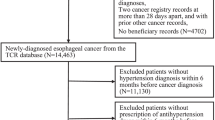
Figure 1 illustrated the process of patients selection. 157 patients (aged ≥ 18 years) were diagnosed with CCA and hypertension concurrently and received surgery from March 2017 to February 2022 in FAHSYSU. 29 patients received palliative or debulking surgery were excluded, and only patients received R0 resection could be included. 22 patients lost to follow up were excluded. 4 patients with non-cancer related mortality within 6 months after surgery were also excluded. Finally, 102 patients were enrolled in the study cohort.
Outcome definition
The primary outcome of interest was overall survival (OS),defined as the survival from surgical operation to cancer-related mortality. The secondary outcome was progression-free survival (PFS), defined as the survival from surgical operation to tumor recurrence or metastasis detected by imaging. We followed up patients till August 31, 2022.
Study variables
The exposure of interest was the administration of anti-hypertensive drugs, defined as any anti-hypertensive drugs taken at least 6 months continuously from 1 year before surgery to the outcome time or the last follow up time point. Four classes of anti-hypertensive drugs were included as follows: (1) renin-angiotensin system inhibitors (RASis), (2) calcium channel blockers (CCBs), (3) Diuretics, (4) β-blockers.
Other covariates included patients’ characteristics, blood pressures, tumor characteristics, laboratory parameters and adjuvant therapies. Patients’ characteristics were sex, age of receiving surgeries and comorbidity (biliary stone). Blood pressures were measured in the morning of the second day after patients admitted to hospital. Tumor characteristics included histological diagnosis, tumor size, tunor differentiation, perineural invasion, vascular cancer embolus, adjacent organ invasion, intrahepatic metastasis and lymph node metastasis. Laboratory parameters included TBIL, DBIL, IBIL, ALB, ALBI score/grade, urea, creatinine, serum potassium, CA19-9, CEA and CA125. Adjuvant therapies were just classified as yes or not, regardless of the therapeutic regimens.
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR). Categorical variables were expressed as percentages. Continuous variables were compared with Mann-Whitney U test. Categorical variables were compared with χ2 test or Fisher exact test as appropriate. Survival outcomes (OS and PFS) were assessed by the Kaplan-Meier method. The survival rates were compared by log-rank test. Cox regressions were expressed as hazard ratio [HR] with confidence interval [95% CI]. Prognostic factors associated with OS and PFS were assessed using univariable and multivariable Cox regressions. Variables, in univariable Cox regressions, with p < 0.200 were included in the multivariable Cox regression.
When compared the effect of anti-hypertensive drugs on outcome, propensity scores (PS) matching was used to control for confounding due to potential selection bias in the cohort study. The PS was estimated by multivariable logistic regression based on the variables included in this study. RASis users were matched to non-users in a 1:3 ratio without replacement using a greedy distance-based matching algorithm with the logit of the PS within 0.2 standard deviation. After PS matching, balance was evaluated using absolute standardized differences (ASD) for all covariates. An ASD of < 0.20 was considered an good balance.
All analyses were performed by IBM SPSS statistics 22.0 with PS matching plug-in. A two-tailed p < 0.05 was considered statistically significant for all tests unless indicated otherwise.
Results
Characteristics of patients with CCA
A total of 102 patients diagnosed with CCA and hypertension concurrently and received radical surgery were enrolled in this study. The main characteristics of the population were summarized in Table 1. There were 58 (56.9%) males in this cohort with a median age of 66.0 years (IQR: 58.0–72.0). Each patient’s blood pressure of the second morning after admission was collected, the median systolic blood pressure (SBP) and diastolic blood pressure (DBP) were 138 mmHg (IQR: 127–146) and 81 mmHg (IQR: 74–90) respectively. With respect to use of anti-hypertensive drugs, 39 (38.2%) patients took RASis, 69 (67.6%) took CCBs, 7 (6.9%) took diuretics, 20 (19.6%) took β-blockers. In terms of tumor features, there were 68 (66.7%) iCCA, 24 (23.5%) pCCA and 10 (9.8%) dCCA with the median tumor size of 55.0 mm (45.0-75.5), 20.0 mm (18.0–30.0), and 16.5 mm (14.0–22.0), respectively. Based on results of imaging and histology, 5 (4.9%) tumors were low differentiated, 93 (91.2%) tumors were moderate differentiated, 4 (3.9%) tumors were high differentiated, 37 (36.3%) had perineural invasion, 10 (9.8%) had vascular cancer embolus, 5 (4.9%) had adjacent organ invasion, 8 (7.8%) had intrahepatic metastasis, 25 (24.5%) had lymph node metastasis and 35 (34.3%) with biliary stone. 22 (21.6%) patients received adjuvant therapy. The median ALBI score was − 2.47 (IQR: -2.73–1.97) with 36 (35.3%), 56 (54.9%), and 10 (9.8%) of ALBI grade 1, 2, and 3, respectively. The median values of urea, creatinine, and serum potassium were 5.0 (4.2–6.5) mmol/L, 72 (59–82) µmol/L, and 3.98 (3.71–4.29) mmol/L, respectively. The median values of CA19-9, CEA and CA125 were 124.8 U/L (18.1-1116.9), 3.0 ng/ml (2.1–6.3), and 17.7 U/L (10.7–35.8), respectively.
Outcomes of patients
The median follow-up of the 102 patients was 36.7 months, with a median PFS of 12.4 months (6.2–24.5) and a median OS of 20.1 months (11.5–35.2). The 1 year, 2 years, and 3 years’ survival rates were 79.9%, 60.2%, and 49.4%, respectively. Results of univariable and multivariable cox analysis showed that RASis use (HR = 0.55, 95% CI: 0.32–0.96, p = 0.034), perineural invasion (HR = 2.09, 95% CI: 1.21–3.62, p = 0.009), and lymph node metastasis (HR = 1.80, 95% CI: 1.00-3.24, p = 0.050) were independently associated with PFS. RASis use (HR = 0.40, 95% CI: 0.20–0.79, p = 0.008), perineural invasion (HR = 2.25, 95% CI: 1.21–4.19, p = 0.011), and lymph node metastasis (HR = 2.10, 95% CI: 1.10–3.99, p = 0.024) were independently associated with OS (Tables 2 and 3). Kaplan-Meier curves of PFS and OS according to RASis use, perineural invasion, and lymph node metastasis were shown in Supplementary Fig. 1. These results indicated that RASis use was a protective factor for the survival of CCA.
Association between patients’ survival and RASis use
Anti-hypertensive drugs were classified to four groups based on their pharmacological mechanism, including RASis, CCBs, diuretics, and β-blockers. Supplementary Table 1 showed the detailed anti-hypertensive drugs.
According to the results of univariable and multivariable cox analysis, RASis use was associated with the survival (PFS and OS) of CCA patients. It’s a very interesting result and was not reported before. To verify this result, we performed PS matching to balance the bias between RASis users and non-users. The baseline characteristics were shown in Supplementary Table 2. There were a total of 102 patients, including 39 RASis users and 63 RASis non-users. Before PS matching, some covariates between RASis users and non-users were imbalanced (ASD > 0.20), including sex (male, ADS = 0.241), diuretics use (ASD = 0.377), β-blockers use (ASD = 0.305), DBP (ASD = -0.215), diagnosis (iCCA, ASD = -0.250; dCCA, ASD = 0.339), tumor size (ASD = 0.210), lymph node metastasis (ASD = -0.273), and adjuvant therapy (ASD = -0.274). After PS matching, 84 patients were enrolled in the cohort, including 28 RASis users and 56 RASis non-users. All covariates were well balanced (ASD < 0.20). The characteristics of the PS-matched cohort were shown in Table 4.
After PS matching, the median PFS and OS of RASis users (PFS, 17.6 months (9.2–34.4); OS, 24.8 months (16.5–42.3)) were longer than RASis non-users (PFS, 10.5 months (4.1–24.1); OS, 14.6 months (10.6–28.4)). RASis use was associated with a lower risk of PFS (HR = 0.72, 95% CI: 0.39–1.32, p = 0.283) and OS (HR = 0.42, 95% CI: 0.19–0.91, p = 0.028). Kaplan-Meier curves of PFS and OS according to RASis use were shown in Supplementary Fig. 2. The 1 year, 2 years, and 3 years’ survival rates of RASis users (89.1%, 77.0%, and 65.5%) were higher than RASis non-users (70.9%, 54.0%, and 40.0%).
As RASis could cause azotemia and hyperkalemia, we compared the level of urea, creatinine, and serum potassium between RASis users and non-users. Table 1 and Supplementary Table 3 showed the median values of urea, creatinine, and serum potassium of the entire cohort, RASis users, and RASis non-users, respectively. Results showed that even though RASis users had higher level of urea (5.7 vs. 4.7, mmol/L) and creatinine (76 vs. 69, mmol/L) than non-users (Supplementary Table 3), the values were still in normal range. Then we compared the proportion of patients with azotemia (urea and creatinine exceeded the normal range). There were no significant difference between RASis users and non-users (Supplementary Table 5). The level of serum potassium was not significantly different between RASis users and non-users (Supplementary Table 3) and only one RASis non-user patients in this study cohort got hyperkalemia (Supplementary Table 5). We also compared the level of urea, creatinine, and serum potassium in the cohort after PS matching and similar results were obtained (Supplementary Tables 4, 6). To clarify the correlation between azotemia and survival, we did cox analysis. Results of univariable cox analysis showed that urea and creatinine were not associated with PFS and OS (Table 2).
Discussion
As CCA commonly occurs aound 60 years old, there is a large proportion of patients are complicated with hypertension. But the anti-hypertensive guideline for patients with hypertension and CCA concurrently is blank. Up to now, the effect of anti-hypertensive drugs on the prognosis of CCA is still unclear. In this study, we described a retrospective cohort of 102 CCA patients with hypertension and CCA concurrently and received radical surgery. We have shown that RASis usage was independently associated with better PFS (HR = 0.55, 95% CI: 0.32–0.96, p = 0.034) and OS (HR = 0.40, 95% CI: 0.20–0.79, p = 0.008), but other types of anti-hypertensive drugs in this cohort, including CCBs, β-blockers, and diuretics, didn’t show the similar effect. After balancing other factors that could influence the PFS and OS by PS matching, 84 CCA patients were in the final cohort. Results shown that RASis usage improved the prognosis of patients with hypertension and CCA concurrently, with longer PFS (17.6 m vs. 10.5 m), OS (24.8 m vs. 14.6 m), and a higher 3 years’ survival rate (65.5% vs. 40.0%). These findings haven’t been reported before.
With the increase of aging population, the association between hypertension and CCA has been wildly studied. A hospital-based case-control study of 303 CCA patients, including 136 intrahepatic cholangiocarcinoma and 167 extrahepatic cholangiocarcinoma, showed that hypertension harbored strong association with CCA [21]. Other studies also proved that management of hypertension could improve the prognosis of CCA [22, 23]. These results indicated that hypertension is a risk factor for the survival of CCA. The renin-angiotensin system plays an important role in the regulation of blood pressure. Angiotensin-converting znzyme 2 (ACE2), a key component of the renin-angiotensin system, was reported wildly expressed in the biliary system [24] and increased in CCA [25, 26]. Angiotensin II (ANG II) was shown to facilitate fibrosis and tumor progression of CCA through an interaction with hepatic stellate cells [11, 12]. These researches proved that renin-angiotensin system was activated in CCA and could promote the progression of CCA. In addition, hypertension is also one of the common adverse events during the adjuvant therapy of CCA (22.0-57.7%) [17,18,19,20]. An open-label phase II prospective study of apatinib treatment for advanced CCA reported a 57.7% occurrence of hypertension [18], and hypertension was reported as the most common comorbidity in a radiation therapy of Yttrium-90 resin microspheres [20]. But up to now, there was no recommend of anti-hypertensive drugs for patients received adjuvant therapy. This is also a good point for further research.
The effect of anti-hypertensive drugs on malignancies is controversial. A total participants of 14,392 patients cohort showed that anti-hypertensive drugs were associated with lower all-cause mortality (hazard ratio [HR], 0.32; 95% CI, 0.25–0.42), cardiovascular mortality (HR, 0.33; 95% CI, 0.21–0.53), and cancer mortality (HR, 0.30; 95% CI, 0.19–0.47) [27]. A prospective cohort study of 3012 patients with gastric carcinoma undergoing radical gastrectomy showed that anti-hypertensive drugs were related to 42% (HR 0.58, 95% CI 0.47–0.73) reduced mortality risk relative to those without medications [28]. A large cohort of 90,708 lung cancer research demonstrated that the lower risk of lung cancer persisted with a longer follow-up period of anti-hypertensives usage [29]. Other studies on breast cancer [30, 31], pancreatic cancer [32], and colorectal cancer [33] also suggested that anti-hypertensive drugs could reduce the side effects of cancer treatment, and stop the reoccurrence of cancers in the survivors. But other researches have the opposite conclusion. A large-scale cohort study in Japan suggested that long-term use of antihypertensive drugs may be associated with an increased incidence of colorectal and renal cancer [34]. A population based study of 302,634 users of anti-hypertensive drugs and 605,268 non-users indicated that higher cumulative exposure to thiazides was associated with increased rates of incident skin cancer in people aged 66 years and older [35]. Other studies suggested that anti-hypertensive drugs increased the risk of kidney cancer [36] and squamous cell carcinoma [37]. These differences may due to the different types of cancers and population that enrolled in the studies. Up to now, the effect of anti-hypertensive drugs on the prognosis of CCA was not clarified.
As the renin-angiotensin system plays a key role in regulating the blood pressure. The effect of inhibitors of renin-angiotensin system on malignancies attracts lots of attention. A meta-analysis of the association between anti-hypertensive drugs use and breast cancer showed that only RASis were associated with a significantly lower breast cancer risk. β-blockers, CCBs and diuretics increased the risk of breast cancer [31]. A nationwide cohort study of 70,549 individuals from Korea showed that ARBs use was independently associated with a decreased risk of cancer overall compared to other antihypertensive drugs [38]. A Nationwide Cohort Study of 12,122 women identified from the Finnish Cancer Registry with ovarian cancer reported that ACEis confered survival benefits in women with ovarian cancer [39]. Other studies on breast cancer, colorectal cancer, lung cancer, stomach cancer, ovarian cancer, and melanoma also suggested that ACEis and/or ARBs could improve the survival [40,41,42,43,44].
With respect to the effect of renin-angiotensin system inhibitors on CCA, a study showed that ARBs attenuated CCA cell growth by inhibiting the oncogenic activity of Yes-associated protein [45]. Another study reported that telmisartan inhibited cell proliferation and tumor growth of CCA through cell cycle arrest [46]. But few studies on the effect of renin-angiotensin system inhibitors on CCA have been reported yet. This may due to a lower incidence of CCA compared with other cancers, including breast cancer and gastrointestinal cancer. Several studies explored the impact of RASis on patients with advanced, or recurrent, and/or metastatic CCA but got negative results [47, 48]. Herein, we described a PS matching cohort of 84 CCA patients and demonstrated that RASis usage could improve the survival of CCA patients. The possible reason was that the population of patients included in the present study was quite different from that in the previous studies. We included patients undergone radical surgery, while the previous studies included patients with unresectable tumors, which would be more difficult for RASis to yield effects.
Severe limitations of the current study should be acknowledged. First, this is not a prospective study, hence selection bias unavoidably existed and might influence the results. Secondly, this is single center study and the study cohort is not large enough, therefore large-scale prospective multicenter studies, ideally randomized controlled trials, are still warranted to verify the conclusion.
Conclusions
In this PS matching cohort study, we have demonstrated that RASis usage improved the survival of patients with CCA and hypertension concurrently.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Munugala N, Maithel SK, Shroff RT. Novel biomarkers and the future of targeted therapies in cholangiocarcinoma: a narrative review. Hepatobiliary Surg Nutr. 2022;11:253–66. https://doi.org/10.21037/hbsn-20-475.
Halder R, Amaraneni A, Shroff RT. Cholangiocarcinoma: a review of the literature and future directions in therapy. Hepatobiliary Surg Nutr. 2022;11:555–66. https://doi.org/10.21037/hbsn-20-396.
Hu LS, Zhang XF, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, et al. Recurrence patterns and timing courses following curative-intent resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2019;26:2549–57. https://doi.org/10.1245/s10434-019-07353-4.
Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018;105:848–56. https://doi.org/10.1002/bjs.10676.
Merath K, Mehta R, Hyer JM, Bagante F, Sahara K, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, et al. Impact of body mass index on tumor recurrence among patients undergoing curative-intent resection of intrahepatic cholangiocarcinoma- a multi-institutional international analysis. Eur J Surg Oncol. 2019;45:1084–91. https://doi.org/10.1016/j.ejso.2019.03.004.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. https://doi.org/10.1056/NEJMoa0908721.
Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. https://doi.org/10.1016/j.jhep.2012.02.022.
Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Hepatol. 2020;72:95–103. https://doi.org/10.1016/j.jhep.2019.09.007.
Helmy A, Jalan R, Newby DE, Hayes PC, Webb DJ. Role of angiotensin II in regulation of basal and sympathetically stimulated vascular tone in early and advanced cirrhosis. Gastroenterology. 2000;118:565–72. https://doi.org/10.1016/s0016-5085(00)70263-0.
Powell EE, Edwards-Smith CJ, Hay JL, Clouston AD, Crawford DH, Shorthouse C, Purdie DM, Jonsson JR. Host genetic factors influence disease progression in chronic hepatitis C. Volume 31. Hepatology; 2000. pp. 828–33. https://doi.org/10.1053/he.2000.6253.
Okamoto K, Tajima H, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi H, Nakamura K, Oyama K, Nakagawara H, et al. Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. Int J Oncol. 2012;41:573–82. https://doi.org/10.3892/ijo.2012.1499.
Okamoto K, Tajima H, Ohta T, Nakanuma S, Hayashi H, Nakagawara H, Onishi I, Takamura H, Ninomiya I, Kitagawa H, et al. Angiotensin II induces tumor progression and fibrosis in intrahepatic cholangiocarcinoma through an interaction with hepatic stellate cells. Int J Oncol. 2010;37:1251–9. https://doi.org/10.3892/ijo_00000776.
Zhang X, Wong GL, Yip TC, Tse YK, Liang LY, Hui VW, Lin H, Li GL, Lai JC, Chan HL, et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2022;76:469–82. https://doi.org/10.1002/hep.32294.
Wadsworth BJ, Cederberg RA, Lee CM, Firmino NS, Franks SE, Pan J, Colpo N, Lin KS, Benard F, Bennewith KL. Angiotensin II type 1 receptor blocker telmisartan inhibits the development of transient hypoxia and improves tumour response to radiation. Cancer Lett. 2020;493:31–40. https://doi.org/10.1016/j.canlet.2020.07.015.
Hafezi-, Nejad N, Bailey CR, Areda MA, Lafaro KJ, Liddell RP, Holly BP, Weiss CR. Characteristics and outcomes of percutaneous biliary interventions in the United States. J Am Coll Radiol. 2021;18:1059–68. https://doi.org/10.1016/j.jacr.2021.03.010.
Mao J, Yang X, Lin J, Yang X, Wang D, Zhang L, Bai Y, Bian J, Long J, Xie F, et al. Apatinib as non-first-line treatment in patients with Intrahepatic Cholangiocarcinoma. J Cancer. 2021;12:1555–62. https://doi.org/10.7150/jca.53482.
Lee S, Shroff RT, Makawita S, Xiao L, De Danner A, Bhosale P, Reddy K, Shalaby A, Raghav K, Pant S, et al. Phase II study of Ramucirumab in Advanced biliary tract Cancer previously treated by gemcitabine-based chemotherapy. Clin Cancer Res. 2022;28:2229–36. https://doi.org/10.1158/1078-0432.CCR-21-3548.
Zhang G, Gong S, Pang L, Hou L, He W. Efficacy and safety of Apatinib Treatment for Advanced Cholangiocarcinoma after failed gemcitabine-based chemotherapy: an open-label phase II prospective study. Front Oncol. 2021;11:659217. https://doi.org/10.3389/fonc.2021.659217.
Mei K, Qin S, Chen Z, Liu Y, Wang L, Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort a report in a multicenter phase Ib/II trial. J Immunother Cancer. 2021;9. https://doi.org/10.1136/jitc-2020-002191.
Pardo F, Sangro B, Lee RC, Manas D, Jeyarajah R, Donckier V, Maleux G, Pinna AD, Bester L, Morris DL, et al. The Post-SIR-Spheres surgery study (P4S): retrospective analysis of Safety following hepatic resection or transplantation in patients previously treated with Selective Internal Radiation Therapy with Yttrium-90 Resin Microspheres. Ann Surg Oncol. 2017;24:2465–73. https://doi.org/10.1245/s10434-017-5950-z.
Xiong J, Lu X, Xu W, Bai Y, Huang H, Bian J, Zhang L, Long J, Xu Y, Wang Z, et al. Metabolic syndrome and the risk of cholangiocarcinoma: a hospital-based case-control study in China. Cancer Manag Res. 2018;10:3849–55. https://doi.org/10.2147/CMAR.S175628.
Qu WF, Zhou PY, Liu WR, Tian MX, Jin L, Jiang XF, Wang H, Tao CY, Fang Y, Zhou YF, et al. Age-adjusted Charlson Comorbidity Index predicts survival in intrahepatic cholangiocarcinoma patients after curative resection. Ann Transl Med. 2020;8:487. https://doi.org/10.21037/atm.2020.03.23.
Nishioka T, Kubo S, Tanaka S, Wakasa K, Takemura S, Kinoshita M, Hamano G, Kuwae Y, Shibata T, Suehiro S. Outcomes of hepatic resection in Intrahepatic Cholangiocarcinoma patients with diabetes, hypertension, and Dyslipidemia: significance of Routine Follow-Up. Liver Cancer. 2016;5:107–20. https://doi.org/10.1159/000367752.
Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–40. https://doi.org/10.1016/j.jhep.2020.06.006.
Meiners J, Jansen K, Gorbokon N, Buscheck F, Luebke AM, Kluth M, Hube-Magg C, Hoflmayer D, Weidemann S, Fraune C, et al. Angiotensin-converting enzyme 2 protein is overexpressed in a wide range of human Tumour types: a systematic tissue microarray study on > 15,000 tumours. Biomedicines. 2021;9. https://doi.org/10.3390/biomedicines9121831.
Beyazit Y, Purnak T, Suvak B, Kurt M, Sayilir A, Turhan T, Tas A, Torun S, Celik T, Ibis M, et al. Increased ACE in extrahepatic cholangiocarcinoma as a clue for activated RAS in biliary neoplasms. Clin Res Hepatol Gastroenterol. 2011;35:644–9. https://doi.org/10.1016/j.clinre.2011.06.008.
Lu Q, Zhang Y, Geng T, Yang K, Guo K, Min X, He M, Guo H, Zhang X, Yang H, et al. Association of Lifestyle factors and antihypertensive medication Use with risk of all-cause and cause-specific mortality among adults with hypertension in China. JAMA Netw Open. 2022;5:e2146118. https://doi.org/10.1001/jamanetworkopen.2021.46118.
Wang L, Hu D, Fan Z, Yu J, Zhang S, Lin Y, Chen X, Lin X, Yan X, Lin J, et al. Prognostic value of long-term antidiabetic and antihypertensive therapy in postoperative gastric cancer patients: the FIESTA study. BMC Gastroenterol. 2022;22:429. https://doi.org/10.1186/s12876-022-02514-4.
Li J, Lam ASM, Yau STY, Yiu KKL, Tsoi KKF. Antihypertensive treatments and risks of lung Cancer: a large population-based cohort study in Hong Kong. BMC Cancer. 2021;21:1202. https://doi.org/10.1186/s12885-021-08971-6.
Fan Y, Khan NH, Farhan Ali Khan M, Ahammad MDF, Zulfiqar T, Virk R, Jiang E. Association of hypertension and breast Cancer: antihypertensive drugs as an effective adjunctive in breast Cancer therapy. Cancer Manag Res. 2022;14:1323–9. https://doi.org/10.2147/CMAR.S350854.
Xie Y, Wang M, Xu P, Deng Y, Zheng Y, Yang S, Wu Y, Zhai Z, Zhang D, Li N, et al. Association between antihypertensive medication use and breast Cancer: a systematic review and Meta-analysis. Front Pharmacol. 2021;12:609901. https://doi.org/10.3389/fphar.2021.609901.
Bian S, Dong H, Zhao L, Li Z, Chen J, Zhu X, Qiu N, Jia X, Song W, Li Z, et al. Antihypertension Nanoblockers increase Intratumoral Perfusion of Sequential cytotoxic nanoparticles to Enhance Chemotherapy Efficacy against Pancreatic Cancer. Adv Sci (Weinh). 2022;9:e2201931. https://doi.org/10.1002/advs.202201931.
Balkrishnan R, Desai RP, Narayan A, Camacho FT, Flausino LE, Chammas R. Associations between initiating antihypertensive regimens on stage I-III colorectal cancer outcomes: a Medicare SEER cohort analysis. Cancer Med. 2021;10:5347–57. https://doi.org/10.1002/cam4.4088.
Matsui S, Sobue T, Zha L, Kitamura T, Sawada N, Iwasaki M, Shimazu T, Tsugane S. Long-term antihypertensive drug use and risk of cancer: the Japan Public Health Center-based prospective study. Cancer Sci. 2021;112:1997–2005. https://doi.org/10.1111/cas.14870.
Drucker AM, Hollestein L, Na Y, Weinstock MA, Li WQ, Abdel-Qadir H, Chan AW. Association between antihypertensive medications and risk of skin cancer in people older than 65 years: a population-based study. CMAJ. 2021;193:E508–16. https://doi.org/10.1503/cmaj.201971.
Xie Y, Xu P, Wang M, Zheng Y, Tian T, Yang S, Deng Y, Wu Y, Zhai Z, Hao Q, et al. Antihypertensive medications are associated with the risk of kidney and bladder cancer: a systematic review and meta-analysis. Aging. 2020;12:1545–62. https://doi.org/10.18632/aging.102699.
Su KA, Habel LA, Achacoso NS, Friedman GD, Asgari MM. Photosensitizing antihypertensive drug use and risk of cutaneous squamous cell carcinoma. Br J Dermatol. 2018;179:1088–94. https://doi.org/10.1111/bjd.16713.
Cho IJ, Shin JH, Jung MH, Kang CY, Hwang J, Kwon CH, Kim W, Kim DH, Lee CJ, Kang SH, et al. Antihypertensive drugs and the risk of Cancer: a Nationwide Cohort Study. J Clin Med. 2021;10. https://doi.org/10.3390/jcm10040771.
Santala EEE, Artama M, Pukkala E, Visvanathan K, Staff S, Murtola TJ. Antihypertensive Drug Use and the risk of Ovarian Cancer Death among Finnish Ovarian Cancer Patients-A Nationwide Cohort Study. Cancers (Basel). 2021;13. https://doi.org/10.3390/cancers13092087.
Cui Y, Wen W, Zheng T, Li H, Gao YT, Cai H, You M, Gao J, Yang G, Zheng W, et al. Use of Antihypertensive Medications and Survival Rates for breast, colorectal, lung, or stomach Cancer. Am J Epidemiol. 2019;188:1512–28. https://doi.org/10.1093/aje/kwz106.
Harding BN, Delaney JA, Urban RR, Weiss NS. Post-diagnosis use of antihypertensive medications and the risk of death from ovarian cancer. Gynecol Oncol. 2019;154:426–31. https://doi.org/10.1016/j.ygyno.2019.05.030.
Kobara H, Fujihara S, Iwama H, Matsui T, Fujimori A, Chiyo T, Tingting S, Kobayashi N, Nishiyama N, Yachida T, et al. Antihypertensive drug telmisartan inhibits cell proliferation of gastrointestinal stromal tumor cells in vitro. Mol Med Rep. 2020;22:1063–71. https://doi.org/10.3892/mmr.2020.11144.
Fujita N, Fujita K, Iwama H, Kobara H, Fujihara S, Chiyo T, Namima D, Yamana H, Kono T, Takuma K, et al. Antihypertensive drug telmisartan suppresses the proliferation of gastric cancer cells in vitro and in vivo. Oncol Rep. 2020;44:339–48. https://doi.org/10.3892/or.2020.7607.
Williams NM, Vincent LT, Rodriguez GA, Nouri K. Antihypertensives and melanoma: an updated review. Pigment Cell Melanoma Res. 2020;33:806–13. https://doi.org/10.1111/pcmr.12918.
Saikawa S, Kaji K, Nishimura N, Seki K, Sato S, Nakanishi K, Kitagawa K, Kawaratani H, Kitade M, Moriya K, et al. Angiotensin receptor blockade attenuates cholangiocarcinoma cell growth by inhibiting the oncogenic activity of yes-associated protein. Cancer Lett. 2018;434:120–9. https://doi.org/10.1016/j.canlet.2018.07.021.
Samukawa E, Fujihara S, Oura K, Iwama H, Yamana Y, Tadokoro T, Chiyo T, Kobayashi K, Morishita A, Nakahara M, et al. Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int J Oncol. 2017;51:1674–84. https://doi.org/10.3892/ijo.2017.4177.
Nakai Y, Isayama H, Sasaki T, Takahara N, Saito K, Takeda T, Umefune G, Saito T, Takagi K, Watanabe T, et al. No Survival Benefit from the inhibition of renin-angiotensin system in biliary Tract Cancer. Anticancer Res. 2016;36:4965–70. https://doi.org/10.21873/anticanres.11065.
Gunchick V, McDevitt RL, Choi E, Winslow K, Zalupski MM, Sahai V. Survival analysis of 1140 patients with biliary Cancer and Benefit from Concurrent Renin-Angiotensin Antagonists, Statins, or aspirin with systemic therapy. Oncologist. 2023;28:531–41. https://doi.org/10.1093/oncolo/oyad063.
Acknowledgements
The authors would like to thank all the staff in the department of pancreatobiliary surgery, The First Affiliated Hospital of Sun Yat-Sen University for patient care and providing clinical data.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No.82072644).
Author information
Authors and Affiliations
Contributions
XY.Y. contributed to conception and design of the study. XX.Z. and JH.L. conducted the study and contributed equally. P.F. and XF.Q. organized the database. XY.Y., LJ.L. and JML provided the clinical data. XX.Z. wrote the first draft of the manuscript. JH.L., LJ.L., JM.L., and XY.Y. revised the manuscript. All authors read and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University in accordance with the declaration of Helsinki. The need for written informed consent was waived by the Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University due to retrospective nature of the study.
Consent for publication
NA.
Competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
. Kaplan-Meier curves of progression-free survival (A) and overall survival (B) according to RASis use, perineural invasion, and lymph node metastasis. RASis, renin-angiotensin system inhibitors. Supplementary Figure 2. Kaplan-Meier curves of progression-free survival (A) and overall survival (B) according to RASis use after propensity score matching. RASis, renin-angiotensin system inhibitors. Supplementary Tables: Supplementary Table 1. Anti-Hypertensive drugs. Supplementary Table 2. Characteristics of the study cohort before PS matching. Supplementary Table 3. Supplementary characteristics of patients in the whole study cohort. Supplementary Table 4. Supplementary characteristics of patients in the cohort after PS matching. Supplementary Table 5. The proportion of azotemia and hyperkalemia of the whole study cohort. Supplementary Table 6. The proportion of azotemia and hyperkalemia of the cohort after PS matching
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, XX., Li, JH., Fang, P. et al. Renin-angiotensin system inhibitors improve the survival of cholangiocarcinoma: a propensity score-matched cohort study. BMC Cancer 23, 826 (2023). https://doi.org/10.1186/s12885-023-11152-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11152-2