Abstract
NEDD4 family represent an important group of E3 ligases, which regulate various cellular pathways of cell proliferation, cell junction and inflammation. Emerging evidence suggested that NEDD4 family members participate in the initiation and development of tumor. In this study, we systematically investigated the molecular alterations as well as the clinical relevance regarding NEDD4 family genes in 33 cancer types. Finally, we found that NEDD4 members showed increased expression in pancreas cancer and decreased expression in thyroid cancer. NEDD4 E3 ligase family genes had an average mutation frequency in the range of 0-32.1%, of which HECW1 and HECW2 demonstrated relatively high mutation rate. Breast cancer harbors large amount of NEDD4 copy number amplification. NEDD4 family members interacted proteins were enriched in various pathways including p53, Akt, apoptosis and autophagy, which were confirmed by further western blot and flow cytometric analysis in A549 and H1299 lung cancer cells. In addition, expression of NEDD4 family genes were associated with survival of cancer patients. Our findings provide novel insight into the effect of NEDD4 E3 ligase genes on cancer progression and treatment in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ubiquitination is a key post-translational modification of proteins, which controls various aspects of protein functions including stability, location and signaling transduction [1, 2]. Ubiquitination is complex processes with multiple steps involving E1-ligases, E2-ligases and E3-ligases [3, 4]. E3 ligase is a distinct group of ligases that specifically recognize the substrates and link ubiquitin molecules to the substrates [5,6,7]. Subsequently, the ubiquitinated proteins are subjected to ubiquitin-proteasome system degradation or signaling transduction for functional pathways [8, 9].
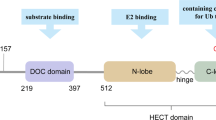
NEDD4 family (neuronally expressed developmentally downregulated 4) represent an important group of E3 ligases, including 9 factors of WWP1, WWP2, HECW1, HECW2, ITCH, RPF1, NEDD4L, SMURF1 and SMURF2 [10, 11]. NEDD4 E3 ligases recognize and modify proteins by mono-ubiquitination or poly-ubiquitination, thus participating in various cellular pathways of cell proliferation, cell junction, ion channel modulation and inflammation [12, 13]. Through ubiquitinating diverse substrates, NEDD4 family members regulate different biological processes, such as cardiovascular diseases, inflammatory disorders, nervous system disorders, immunity and cancer [14, 15].
An increasing number of studies have reported the implication of NEDD4 family members in the initiation, progression and survival of cancer. For instance, NEDD4 ubiquitinated VDAC2 and VDAC3 to inhibit ferroptosis of melanoma [16]. NEDD4 maintained the characteristics of stem cell in breast cancer and promoted the growth and migration of breast cancer cells [17]. SMURF1 has been found to induce invasion of ovarian cancer cells by ubiquitinating ARHGAP26 [18]. In addition, SMURF2 was found to suppress colorectal cancer via degradation of YY1 protein as well as modulating c-myc signaling [19].
Although emerging evidence suggest that NEDD4 E3 ligase family members play critical roles in different aspects of tumor, no comprehensive analysis has been conducted to demonstrate the entire landscape of NEDD4 family members in all types of cancers. In this study, we analyzed the data of different cancers at various levels of DNA, RNA and proteins to generate the expression, copy number variation, protein interactions in relation to different NEDD4 members of tumor in detail. Furthermore, subsequent molecular experiments were performed to confirm our findings that NEDD4 were closely implicated in many biological processes of different cancers.
Materials and methods
NEDD4 E3 ligase family genes collection
Nine NEDD4 E3 ligase family genes were collected from review papers that have been published recently. We converted related gene symbols into Ensemble gene IDs and HGNC symbols based on GeneCards (https://www.genecards.org/).
Genome-wide omics data over 33 cancer types from next-generation sequence data
We followed the methods of our previous study [20]. The analysis results considered the omics datasets from the TCGA Research Network (http://cancergenome.nih.gov/). 33 TCGA projects in total were analyzed, with each one representing a certain cancer type. These TCGA data that included the expression, the copy number alteration, the mutation, methylation as well as the clinical information (survival status and time, stage and grade) regarding Transcripts Per Kilobase Million (TPM) could be available on UCSC XENA(https://xenabrowser.net/).
Differentially expressed genes (DEGs) identification
The Deseq2 package in R assisted in identifying DEGs for identifying the gene expression change in various cancer types. DEGs refer to those of which the adjusted P-values are less than 0.05 and the expression undergoes at least two-fold changes.
Proteomics data regarding pan cancer from protein expression data.
We obtained the protein expression data regarding NEDD4 E3 ligase family genes from the protein atlas datasets (https://www.proteinatlas.org/). The protein expression regarding NEDD4 E3 ligase family genes from 20 cancer types were analyzed, including common cancers of breast cancer, lung cancer and colon cancer.
Enrichment analysis of NEDD4-related proteins
The NEDD4-related proteins were achieved from BioGRID (Biological General Repository for Interaction Datasets), a public database that archives and disseminates genetic and protein interaction data from model organisms and humans. Furthermore, enrichment analysis including BP (biological process), MF (molecular function), CC (Cellular Component) and KEGG (Kyoto Encyclopedia of Genes and Genomes) were analyzed by ClusterProfiler package of R language. Cytoscape was used to visualize the interaction network of NEDD4 members with interacted proteins. In addition, the Pearson Correlation Coefficient (PCC) between NEDD4 members and the pathway was calculated, aiming at identifying those that could affect specific pathway.
TIMER and CMAP analysis
TIMER (http://timer.cistrome.org) is a database used to analyze the relationship between gene expression and immune cells in tumor tissues. We used the TIMER database to analyze the relationship between NEDD4 family members and immune cells. Furthermore, we used the CMAP (https://clue.io/about) database to investigate which small molecule chemicals are associated with NEDD4 family members.
Clinical significance exhibited by NEDD4 E3 ligase family genes
For confirming the effect of NEDD4 E3 ligase family genes’ expression on patients’ survival, the median expression level regarding each hypoxia related gene was taken into account for dividing patients into 2 groups. The log-rank test assisted in examining their difference in the survival rate. The P-values < 0.05 reported statistical significance. Kaplan Meier method served for the prognosis analysis, which adopted the HR value. HR > 1 and HR < 1 indicate that high gene expression leads to poor and good prognosis, respectively.
Cell culture
H1299 and A549 cells were purchased from Cell Bank in Chinese Academy of Sciences Shanghai. H1299 cells were cultured in RPMI 1640 medium; A549 cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM), supplemented with10% fetal bovine serum (FBS)(CLARK, Australia), penicillin (100U),and streptomycin (100 g/ml).
Antibodies and reagents
Antibodies used in this study include β-actin (AC004, ABclonal), Flag (SG4110-16, Shanghai Genomics Technology), Myc (SG4110-18, Shanghai Genomics Technology), Smurf1(ab300408, Abcam), Smurf2(#12,024, Cell Signaling Technology (CST)), p62(#23,214, CST), p53(sc-126, Santa Cruz), Akt(#4691, CST), p-Akt(#4060, CST).
Western blot
In order to confirm the enrichment analysis results of NEDD4-interacted proteins, we performed transfection of Smurf1 and Smurf2 or siRNAs for Smurf1 and Smuf2 in A549 or H1299 cells, followed by western blot of p53, Akt, p-Akt (S473), p62 and LC3 proteins. Cells were lysed on ice with IP lysis buffer supplemented with protease inhibitor cocktails, then the lysed protein was harvested by centrifugation. Protein samples were separated on 10% SDS PAGE and transferred to PVDF membrane (Millipore, IPVH00010) for two hours at 80 V. After block in milk for one hour at room temperature, the membranes were probed with specific primary antibodies at 4 °C overnight. The membranes were then washed with TBST three times followed by incubation with HRP-conjugated secondary antibody at room temperature for two hours. After three washes, bands were detected by enhanced chemi-luminescence detection kit (Thermo Fisher Scientific, 32,106) and visualized via the DNR western blot detection system.
Flow cytometric analysis
To investigate cell apoptosis, H1299 or A549 cells were subjected to incubation with PI and FITC-Annexin V (BD Phamingen, 556,547). The percentage of apoptotic cells was then measured according to the manufacturer’s protocol.
Results
Expression profile regarding NEDD4 E3 ligase family genes of various cancer types
The study identified 9 NEDD4 E3 ligase family genes after searching the published review papers. The count data of TCGA were employed for confirming their differential expressions for various cancer types. NEDD4 E3 ligase family genes were distributed heterogeneously in various cancer types (Fig. 1A): NEDD4 E3 ligase family genes presented high expression in some tumors including pancreas cancer, esophagus cancer, gastric cancer and colon cancer; while NEDD4 E3 ligase family genes showed low expression in certain tumors of kidney, thyroid, and testis cancer. Table S1 gives the detailed LogFC and P value changes. The differential expression regarding HECW1 in each cancer was visualized (Fig. 1B). The immunohistochemistry results from the Protein Atlas database gave the protein expression regarding NEDD4 E3 ligase family genes of the 33 cancer types (Fig. 1C). Figure 1D illustrates the immunohistochemistry results regarding HECW1 that represents the protein expression. Finally, we analyzed the relationship between methylation of NEDD4 family members and tumor prognosis. We summarized the analyzing results in Table S2, and representative relationship between methylation of NEDD4 family genes and lung cancer prognosis was shown in Fig. 1E.
Expression profile of NEDD4 family members across different cancer types. A, Expression of NEDD4 family members in different cancer and normal samples. B, HECW1 expression in different cancers between cancer and normal tissues. C, NEDD4 family members protein expression across various cancer types. Each gene expression in one cancer were divided into four groups of high expression, medium expression, low expression and not detected. D, NEDD4 E3 ligase family protein expression in 16 cancer types based on immunohistochemistry staining. E, Association of NEDD4 E3 ligase family genes methylation with lung cancer prognosis
Pan-cancer genetic variation regarding NEDD4 E3 ligase family genes
Analysis results of the mutation frequency regarding NEDD4 E3 ligase family genes found their frequent mutation in UCEC (Fig. 2A). NEDD4 E3 ligase family genes had an average mutation frequency in the range of 0-32.1%, and that of the HECW1 and HECW2 were remarkably high (Table S3). For many cancers like TGCT, PCPG, THCA and UVM, NEDD4 E3 ligase family genes seldom reported mutation. For obtaining more data of NEDD4 E3 ligase family gene mutations, oncoplot was used for visualizing details of the mutation in UCEC (Fig. 2B). We also examined the copy number change regarding NEDD4 E3 ligase family genes (Fig. 2C) (Table S4). Specific to each cancer type, BRCA exhibited large copy number amplification while LAML nearly saw no CNV. Furthermore, we performed correlation analysis among the NEDD4 E3 ligase family genes, which was summarized in Fig. 2D and Table S5.
Pan-cancer genetic alternations of NEDD4 E3 ligase family genes. A, Pan-cancer mutation frequency of NEDD4 E3 ligase family genes. Red color represent high mutation frequency. B, Oncoplot for NEDD4 E3 ligase family genes in UCEC. NEDD4 E3 ligase family genes showed the most frequent mutation in UCEC. C, The copy number variations frequency of NEDD4 E3 ligase family genes in different cancers. Red color represents increased CNVs while blue color represents decreased CNVs. D, Correlation between NEDD4 E3 ligase family genes. Red color represent high correlation coefficient
Association between NEDD4 E3 ligase family genes and cancer-related pathways
For elucidating the molecular significance exhibited by NEDD4 E3 ligase family genes in cancer, we analyzed the relation between NEDD4 E3 ligase family genes and pathways related to cancer. NEDD4 E3 ligase family genes mainly affected biological processes (BP) including cytoplasmic translation, ribonucleoprotein complex and ribosome biogenesis (Fig. 3A). NEDD4 E3 ligase family genes enriched in molecular functions (MF) of ubiquitin-like protein transferase activity, WW domain binding, ribonucleoprotein complex binding (Fig. 3B). For Cellular Component (CC), NEDD4 E3 ligase family genes demonstrated enrichment in cell − substrate junction, focal adhesion and ribosome (Fig. 3C). KEGG pathway analysis showed that NEDD4 members participated in the regulation of multiple pathways including Akt, p53, autophagy and apoptosis (Fig. 3D) (Table S6), which were further visualized in Fig. 3E. In addition, the correlations between NEDD4 members and different pathways in various types of cancers were summarized in Fig. 3F.
Enrichment analysis of NEDD4 E3 ligase family genes in cancer. A, BP (biological process) Enrichment analysis of NEDD4 E3 ligase family genes in cancer. B, MF (molecular function) Enrichment analysis of NEDD4 E3 ligase family genes in cancer. C, CC (Cellular Component) Enrichment analysis of NEDD4 E3 ligase family genes in cancer. D, KEGG (Kyoto Encyclopedia of Genes and Genomes) Enrichment analysis of NEDD4 E3 ligase family genes in cancer. E, Visualization of NEDD4 E3 ligase family members related pathways. F, The correlations between NEDD4 members and different pathways in various types of cancers
Prognostic significance exhibited by NEDD4 E3 ligase family genes
The cox regression results showed the relation between NEDD4 E3 ligase family genes and the cancer prognosis (Fig. 4A) (Table S7). HECW1 expression correlated with worse survival in most of cancers. Besides, some NEDD4 E3 ligase family genes might affect the prognosis regarding different cancer types to different extents such as WWP2 and Smurf2. To be specific, Smurf2 affected the cancer prognosis in different cancer types, and forest plot was adopted for illustrating how they helped to predict cancer prognosis (Fig. 4B). In addition, the survival plot of relationship between Smurf2 and cancer prognosis in different types of cancers was shown (Fig. 4C).
Prognostic significance of NEDD4 E3 ligase family genes. A, Summary of the correlation between expression of NEDD4 E3 ligase family genes and survival of different cancers. Unfavorable survival is presented in red color, while favorable survival is presented in blue color. B, Forest plot for the prognostic analysis of Smurf2 across various cancer types. The HR and 95CI for each cancer were listed in the figure. C, The survival plot of relationship between Smurf2 and cancer prognosis in different types of cancers including COAD, KIRP, PAAD, STAD, HNSC, LIHC, PCPG, THCA. D, Correlation analysis of immune cells with NEDD4 members Smurf1 and Smurf2
TIMER and CMAP analysis
Finally, we analyzed the relationship of Smurf1 and Smurf 2 with immune cells in tumor tissues. The analysis revealed that Smurf1 is mainly associated with B cells and neutrophils. Smurf2, on the other hand, was mainly related to CD4+ and CD8+ T cells (Fig. 4D). In addition, to predict the potential relationship between NEDD4 gene families and small molecule chemicals, we used the CMAP database to analyze which small molecule substances might exert effect on NEDD4s. After analysis, we found that withaferin A, ellipticine, flupentixol and perphenazine were the most important small molecule chemical affecting NEDD4s (Table S8).
Association of NEDD4 family members with enrichment pathways of akt, p53 and autophagy in cancer
In order to confirm the results of enrichment analysis of NEDD4 family members-interacted proteins, we transfected plasmids of Smurf1, Smurf2 in A549 and H1299 lung cancer cells. The results suggested that NEDD4 members Smurf1 and Smurf2 suppresses p53 pathway (Fig. 5A, B) as p53 protein levels decreased. In addition, NEDD4 members Smurf1 and Smurf2 promote Akt pathway as overexpression of Smurf1 and Smurf2 increase p-Akt (S473) levels (Fig. 5C, D). Under the treatment of (Earle’s Balanced Salt Solution) EBSS (a stimulation of starvation for autophagy), autophagy was facilitated after transfection of NEDD4 members Smurf1 (Fig. 5E, F); autophagy was inhibited after transfection of NEDD4 members Smurf2 (Fig. 5H, I). Furthermore, under the treatment of EBSS (a stimulation of starvation for autophagy), autophagy was inhibited after transfection of Smurf1 siRNAs (Fig. 5G); autophagy was promoted after transfection of Smurf2 siRNAs (Fig. 5J).
Smurf1 and Smurf2 regulate p53, Akt and autophagy signaling pathway. A, Western blot analysis demonstrating p53 protein levels with myc-Smurf1 plasmids transfected in A549 cells. B, Western blot analysis demonstrating p53 protein levels with Flag-Smurf2 plasmids transfected in A549 cells. C, Western blot analysis demonstrating Akt, p-Akt (S473) protein levels with myc-Smurf1 plasmids transfected in A549 cells. D, Western blot analysis demonstrating Akt, p-Akt (S473) protein levels with Flag-Smurf2 plasmids transfected in A549 cells. E, Western blot analysis demonstrating p62 and LC3 protein levels with myc-Smurf1 plasmids transfected in A549 cells after treatment of EBSS for indicated time. F, Western blot analysis demonstrating p62 and LC3 protein levels with myc-Smurf1 plasmids transfected in H1299 cells after treatment of EBSS for indicated time. G, Western blot analysis demonstrating p62 and LC3 protein levels with si-Smurf1 transfected in H1299 cells after treatment of EBSS for indicated time. H, Western blot analysis demonstrating p62 and LC3 protein levels with Flag-Smurf2 plasmids transfected in A549 cells after treatment of EBSS for indicated time. I, Western blot analysis demonstrating p62 and LC3 protein levels with Flag-Smurf2 plasmids transfected in H1299 cells after treatment of EBSS for indicated time. J, Western blot analysis demonstrating p62 and LC3 protein levels with si-Smurf2 transfected in H1299 cells after treatment of EBSS for indicated time
Effect of NEDD4 family members on apoptosis in cancer
In order to confirm the effect of NEDD4 family members on apoptosis of cancer, we performed flow cytometric analysis. After transfection of NEDD4 family members Smurf1 and Smurf2, the lung cancer cells H1299 were subjected to cisplatin (a commonly used chemotherapy drug) treatment to detect the difference of apoptosis in relation to Smurf1 and Smurf2. Finally, we found that both Smurf1 and Smurf2 suppressed apoptosis in H1299 lung cancer cells (Fig. 6A-D). On contrast, transient silence of Smurf1 and Smurf2 by si-RNAs promoted apoptosis in H1299 lung cancer cells (Fig. 6E-H).
Smurf1 and Smurf2 regulate apoptosis. A-B, H1299 cells transfected with myc or myc-Smurf1 were treated with cisplatin (20µM) for 24 h followed by staining with PI and FITC-Annexin V, and analyzed by fluorescence-activated cell sorting (FACS). Scatter graph represents percentage of apoptotic cells from three independent experiments. C-D, H1299 cells transfected with Flag or Flag-Smurf2 were treated with cisplatin (20µM) for 24 h followed by staining with PI and FITC-Annexin V, and analyzed by fluorescence-activated cell sorting (FACS). Scatter graph represents percentage of apoptotic cells from three independent experiments. E-F, H1299 cells transfected with NC or si-Smurf1 were treated with cisplatin (20µM) for 24 h followed by staining with PI and FITC-Annexin V, and analyzed by fluorescence-activated cell sorting (FACS). Scatter graph represents percentage of apoptotic cells from three independent experiments. G-H, H1299 cells transfected with NC or si-Smurf2 were treated with cisplatin (20µM) for 24 h followed by staining with PI and FITC-Annexin V, and analyzed by fluorescence-activated cell sorting (FACS). Scatter graph represents percentage of apoptotic cells from three independent experiments
Discussion
E3 ligases is a distinct group of ligases that specifically recognize the substrates and link ubiquitin molecules to the substrates, of which NEDD4 E3 ligase family participates in various cellular pathways of cell proliferation, cell junction and inflammation. Although increasing evidence has reported the close implication of NEDD4 family members in multiple aspects of cancer. In this study, we systematically investigated the molecular alterations as well as the clinical relevance regarding NEDD4 family genes in 33 cancer types. The results described the landscape of NEDD4 expression, mutation and copy number variation as well as suggested the biological processes and pathways in relation to NEDD4-interacted proteins.
Analysis of the nine NEDD4 E3 ligase family genes of TCGA data suggested that NEDD4 E3 ligase family genes were distributed heterogeneously in various cancer types. NEDD4 E3 ligase family genes presented high expression in some tumors including pancreas cancer, esophagus cancer, gastric cancer and colon cancer; while NEDD4 E3 ligase family genes showed low expression in certain tumors of kidney, thyroid, and testis cancer. In addition, NEDD4 E3 ligase family genes had an average mutation frequency in the range of 0-32.1%, and that of the HECW1 and HECW2 were remarkably high. Furthermore, BRCA exhibited large copy number amplification while LAML nearly saw no CNV. We also analyzed the relationship between methylation of NEDD4 family members and tumor prognosis, which shows involvement of NEDD4s methylation in relation to tumor prognosis. The analysis of Smurfs with immune cells revealed that Smurf1 is mainly associated with B cells and neutrophils. Smurf2 was mainly related to CD4+ and CD8+ T cells. In addition, we found that withaferin A, ellipticine, flupentixol and perphenazine were the most important small molecule chemical affecting NEDD4s.
Next, we analyzed the relation between NEDD4 E3 ligase family genes and pathways related to cancer in order to elucidate the molecular significance. The enrichment analysis indicated biological processes including cytoplasmic translation, ribonucleoprotein complex and ribosome biogenesis as well as KEGG pathways including Akt, p53, autophagy and apoptosis. Our results in A549 lung cancer cells suggested that NEDD4 members Smurf1 and Smurf2 suppresses p53 pathway as p53 protein levels decreased. Smurf1 was found to promote p53 degradation via stabilizing its E3 ligase MDM2 [21]. In addition, NEDD4 members Smurf1 and Smurf2 promote Akt pathway as overexpression of Smurf1 and Smurf2 increase p-Akt levels. Previous study reported that Smurf1 overexpression activates PI3K/Akt signaling pathway [22]. Under the treatment of starvation, autophagy was facilitated after transfection of NEDD4 members Smurf1; autophagy was inhibited after transfection of NEDD4 members Smurf2. The analysis of si-Smurf1 and si-Smurf2 confirmed the effect of Smurf1 and Smurf2 in autophagy. The relationship between NEDD4s and autophagy still require further mechanism studies to clarify, as relevant reports were limited [23,24,25,26]. Furthermore, our results of flow cytometric analysis suggested that both Smurf1 and Smurf2 suppressed apoptosis in H1299 lung cancer cells, which were in consistence with previous reports of the involvement of Smurf1 and Smurf2 in apoptosis [27,28,29,30,31].
Finally, we explored the prognostic significance of NEDD4 E3 ligase family genes. HECW1 expression correlated with worse survival in most of cancers. Besides, some NEDD4 E3 ligase family genes might affect the prognosis regarding different cancer types to different extents such as WWP2 and Smurf2. NEDD4 expression has been reported to correlated with breast cancer development and worse survival [32]. In another study of primary breast cancer, NEDD4-1 expression were not significantly related with clinical outcomes of HER2-amplified breast cancer [33]. Low expression of NEDD4L was found to be associated with poor survival of gastric cancer patients [34]. In addition, overexpression of Nedd4-1 was found to associated with an extremely low survival rate of gastric cardia adenocarcinoma [35]. Further larger-scale studies are required to elucidate the exact correlation between NEDD4 family members and different cancers.
Conclusion
In summary, our study systematically demonstrated the expression, mutation, copy number variation, functional pathways and prognostic value of NEDD4 E3 ligase family genes across multiple cancers. The expression of NEDD4 E3 ligase family genes show significant association with pathways including MAPK, wnt, Akt, p53, autophagy and apoptosis as well as the potential to predict prognosis of cancer patients. These findings provide novel evidence for the investigation of NEDD4 E3 ligase family genes in the development and therapy of cancer.
Data Availability
Not Applicable.
Data Availability
The datasets generated and/or analysed during the current study are available in the TCGA Research Network repository, (http://cancergenome.nih.gov/).
References
Cockram PE, Kist M, Prakash S, Chen SH, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591–605. https://doi.org/10.1038/s41418-020-00708-5.
Mansour MA. Ubiquitination: friend and foe in cancer. Int J Biochem Cell Biol. 2018;101:80–93. https://doi.org/10.1016/j.biocel.2018.06.001.
Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53. https://doi.org/10.1038/nm.3739.
Toma-Fukai S, Shimizu T. Structural diversity of Ubiquitin E3 ligase. Molecules. 2021;26. https://doi.org/10.3390/molecules26216682.
Berndsen CE, Wolberger C. New insights into ubiquitin E3 ligase mechanism. Nat Struct Mol Biol. 2014;21:301–7. https://doi.org/10.1038/nsmb.2780.
van Wijk SJ, Fulda S, Dikic I, Heilemann M. Visualizing ubiquitination in mammalian cells. EMBO Rep. 2019;20. https://doi.org/10.15252/embr.201846520.
Zheng N, Shabek N. Ubiquitin ligases: structure, function, and Regulation. Annu Rev Biochem. 2017;86:129–57. https://doi.org/10.1146/annurev-biochem-060815-014922.
Swatek KN, Komander D. Ubiquitin modifications. Cell Res. 2016;26:399–422. https://doi.org/10.1038/cr.2016.39.
Varshavsky A. The Ubiquitin System, Autophagy, and regulated protein degradation. Annu Rev Biochem. 2017;86:123–8. https://doi.org/10.1146/annurev-biochem-061516-044859.
Boase NA, Kumar S. NEDD4: the founding member of a family of ubiquitin-protein ligases. Gene. 2015;557:113–22. https://doi.org/10.1016/j.gene.2014.12.020.
Wang ZW, Hu X, Ye M, Lin M, Chu M, Shen X. NEDD4 E3 ligase: functions and mechanism in human cancer. Semin Cancer Biol. 2020;67:92–101. https://doi.org/10.1016/j.semcancer.2020.03.006.
Huang X, Chen J, Cao W, Yang L, Chen Q, He J, Yi Q, Huang H, Zhang E, Cai Z. The many substrates and functions of NEDD4-1. Cell Death Dis. 2019;10:904. https://doi.org/10.1038/s41419-019-2142-8.
Zhang Y, Qian H, Wu B, You S, Wu S, Lu S, Wang P, Cao L, Zhang N, Sun Y. E3 ubiquitin ligase NEDD4 familyregulatory network in cardiovascular disease. Int J Biol Sci. 2020;16:2727–40. https://doi.org/10.7150/ijbs.48437.
Sicari D, Weber J, Maspero E, Polo S. The NEDD4 ubiquitin E3 ligase: a snapshot view of its functional activity and regulation. Biochem Soc Trans. 2022;50:473–85. https://doi.org/10.1042/BST20210731.
Ye X, Wang L, Shang B, Wang Z, Wei W. NEDD4: a promising target for cancer therapy. Curr Cancer Drug Targets. 2014;14:549–56. https://doi.org/10.2174/1568009614666140725092430.
Yang Y, Luo M, Zhang K, Zhang J, Gao T, Connell DO, Yao F, Mu C, Cai B, Shang Y, Chen W. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat Commun. 2020;11:433. https://doi.org/10.1038/s41467-020-14324-x.
Jeon SA, Kim DW, Lee DB, Cho JY. NEDD4 plays roles in the maintenance of breast Cancer stem cell characteristics. Front Oncol. 2020;10:1680. https://doi.org/10.3389/fonc.2020.01680.
Chen X, Chen S, Li Y, Gao Y, Huang S, Li H, Zhu Y. SMURF1-mediated ubiquitination of ARHGAP26 promotes ovarian cancer cell invasion and migration. Exp Mol Med. 2019;51:1–12. https://doi.org/10.1038/s12276-019-0236-0.
Gao Q, Wang S, Zhang Z. E3 ubiquitin ligase SMURF2 prevents colorectal cancer by reducing the stability of the YY1 protein and inhibiting the SENP1/c-myc axis. Gene Ther. 2021. https://doi.org/10.1038/s41434-021-00289-z.
Li H, Liu J, Shen S, Dai D, Cheng S, Dong X, Sun L, Guo X. Pan-cancer analysis of alternative splicing regulator heterogeneous nuclear ribonucleoproteins (hnRNPs) family and their prognostic potential. J Cell Mol Med. 2020;24:11111–9. https://doi.org/10.1111/jcmm.15558.
Nie J, Xie P, Liu L, Xing G, Chang Z, Yin Y, Tian C, He F, Zhang L. Smad ubiquitylation regulatory factor 1/2 (Smurf1/2) promotes p53 degradation by stabilizing the E3 ligase MDM2. J Biol Chem. 2010;285:22818–30. https://doi.org/10.1074/jbc.M110.126920.
Xia Q, Zhang H, Zhang P, Li Y, Xu M, Li X, Li X, Dong L. Oncogenic Smurf1 promotes PTEN wild-type glioblastoma growth by mediating PTEN ubiquitylation. Oncogene. 2020;39:5902–15. https://doi.org/10.1038/s41388-020-01400-1.
Borroni AP, Emanuelli A, Shah PA, Ilic N, Apel-Sarid L, Paolini B, Manikoth Ayyathan D, Koganti P, Levy-Cohen G, Blank M. Smurf2 regulates stability and the autophagic-lysosomal turnover of lamin A and its disease-associated form progerin. Aging Cell. 2018;17. https://doi.org/10.1111/acel.12732.
Feng X, Jia Y, Zhang Y, Ma F, Zhu Y, Hong X, Zhou Q, He R, Zhang H, Jin J, Piao D, Huang H, Li Q, Qiu X, Zhang Z. Ubiquitination of UVRAG by SMURF1 promotes autophagosome maturation and inhibits hepatocellular carcinoma growth. Autophagy. 2019;15:1130–49. https://doi.org/10.1080/15548627.2019.1570063.
Han D, Li S, Xia Q, Meng X, Dong L. Overexpressed Smurf1 is degraded in glioblastoma cells through autophagy in a p62-dependent manner. FEBS Open Bio. 2022;12:118–29. https://doi.org/10.1002/2211-5463.13310.
Kong D, Zhang Z, Chen L, Huang W, Zhang F, Wang L, Wang Y, Cao P, Zheng S. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 2020;36:101600. https://doi.org/10.1016/j.redox.2020.101600.
Hao M, Zhang J, Sun M, Diao K, Wang J, Li S, Cao Q, Dai S, Mi X. TRAF4 inhibits the apoptosis and promotes the proliferation of breast Cancer cells by inhibiting the ubiquitination of Spindle Assembly-Associated protein Eg5. Front Oncol. 2022;12:855139. https://doi.org/10.3389/fonc.2022.855139.
Liu H, Sun S, Liu B. Smurf2 exerts neuroprotective effects on cerebral ischemic injury. J Biol Chem. 2021;297:100537. https://doi.org/10.1016/j.jbc.2021.100537.
Nie J, Liu L, Wu M, Xing G, He S, Yin Y, Tian C, He F, Zhang L. HECT ubiquitin ligase Smurf1 targets the tumor suppressor ING2 for ubiquitination and degradation. FEBS Lett. 2010;584:3005–12. https://doi.org/10.1016/j.febslet.2010.05.033.
Wang M, Guo L, Wu Q, Zeng T, Lin Q, Qiao Y, Wang Q, Liu M, Zhang X, Ren L, Zhang S, Pei Y, Yin Z, Ding F, Wang HR. ATR/Chk1/Smurf1 pathway determines cell fate after DNA damage by controlling RhoB abundance. Nat Commun. 2014;5:4901. https://doi.org/10.1038/ncomms5901.
Xie P, Tang Y, Shen S, Wang Y, Xing G, Yin Y, He F, Zhang L. Smurf1 ubiquitin ligase targets Kruppel-like factor KLF2 for ubiquitination and degradation in human lung cancer H1299 cells. Biochem Biophys Res Commun. 2011;407:254–9. https://doi.org/10.1016/j.bbrc.2011.03.016.
Wan L, Liu T, Hong Z, Pan Y, Sizemore ST, Zhang J, Ma Z. NEDD4 expression is associated with breast cancer progression and is predictive of a poor prognosis. Breast Cancer Res. 2019;21:148. https://doi.org/10.1186/s13058-019-1236-7.
Luhtala S, Staff S, Kallioniemi A, Tanner M, Isola J. Clinicopathological and prognostic correlations of HER3 expression and its degradation regulators, NEDD4-1 and NRDP1, in primary breast cancer. BMC Cancer. 2018;18:1045. https://doi.org/10.1186/s12885-018-4917-1.
Gao C, Pang L, Ren C, Ma T. Decreased expression of Nedd4L correlates with poor prognosis in gastric cancer patient. Med Oncol. 2012;29:1733–8. https://doi.org/10.1007/s12032-011-0061-3.
Sun A, Yu G, Dou X, Yan X, Yang W, Lin Q. Nedd4-1 is an exceptional prognostic biomarker for gastric cardia adenocarcinoma and functionally associated with metastasis. Mol Cancer. 2014;13:248. https://doi.org/10.1186/1476-4598-13-248.
Acknowledgements
Not Applicable.
Funding
This study is supported by the National Natural Science Foundation of China (82102740) and the Natural Science Foundation of Liaoning Province (2020-MS-157).
Author information
Authors and Affiliations
Contributions
LJW, ZNJ and XCZ came up with ideas and refined the manuscript. CLZ, LXF and WYB drafted the manuscript. LH, CLZ and ZBW produced all the figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, L., Li, H., Liu, X. et al. Expression and regulatory network of E3 ubiquitin ligase NEDD4 family in cancers. BMC Cancer 23, 526 (2023). https://doi.org/10.1186/s12885-023-11007-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11007-w