Abstract
Background
Despite immunotherapy’s promise in oncology, its use for sarcoma remains challenging. There are no sarcoma-specific biomarkers for immune checkpoint inhibitors (ICI). Previously, we reported our institutional experience highlighting ICI activity in 29 patients with sarcoma. In this study, we explore responses to ICI based on ICI regimen and other covariates to identify significant clinical factors in advanced sarcoma outcomes.
Methods
Patients in The Ohio State University Sarcoma Clinics were enrolled in the Sarcoma Retrospective ICI database from January 1, 2015 through November 1, 2021. Data included treatment regimen (single-agent ICI or ICI + combination) along with clinical covariates. ICI + combination was further categorized into ICI + medication, ICI + radiation, ICI + surgery, or ICI + multiple (more than 2 modalities). Statistical analysis included log-rank tests and proportional hazard regression. The primary objective was to evaluate overall survival (OS) and progression-free survival (PFS).
Results
Of the patients in the database, 135 met inclusion criteria. We demonstrated improved OS in patients treated with ICI + combination (p = 0.014, median 64 weeks), but no effect on PFS (p = 0.471, median 31 weeks). Patients with a documented immune-related adverse event (irAE) of dermatitis had improved OS, but only in the ICI + combination cohort (p = 0.021). Patients who received single-agent ICI and whose change in the neutrophil-to-lymphocyte ratio (NLR) was less than 5 had an improved OS (p = 0.002); this was not seen in patients who received ICI + combination therapy (p = 0.441). There were no differences in OS based on age, gender, histology, or subcategories of ICI + combination. This was not the case for PFS; patients who received any ICI regimen and were younger than 70 had a worse PFS (p = 0.036) compared with their older counterparts in this dataset. Patients who developed an irAE, specifically colitis (p = 0.009), hepatitis (p = 0.048), or dermatitis (p = 0.003), had an improved PFS. There were no differences in PFS based on ICI regimen (or subcategories of ICI + combination), gender, histology, change in NLR, or grade of irAE.
Conclusions
This retrospective study demonstrates that ICI + combination therapy can improve OS in some patients with advanced sarcoma. This is consistent with our prior results of ICI in sarcoma.
Similar content being viewed by others
Background
Soft-tissue sarcomas (STSs) encompass a group of rare and heterogeneous malignant tumors that arise from the oncogenic transformation of mesenchymal tissue. These cancers account for less than 1% of all adult malignancies in the United States, with an estimated incidence of 13,130 new cases per year and approximately 5,350 deaths [1]. Over 100 distinct STS histological and molecular subtypes exist, each with unique clinical, therapeutic, and prognostic features [2]. Patients who develop metastatic disease have limited therapeutic options and median overall survival (OS) of about 18 months [1, 3,4,5,6]. Standard-of-care systemic chemotherapy agents include anthracyclines, ifosfamide, gemcitabine, taxanes, trabectedin, and pazopanib. The response rate remains low, and toxicities limit treatment dosing and length of therapy. This retrospective analysis will address the challenges and new development of immunotherapy for the treatment of sarcoma.
Two landmark trials (SARC028 and Alliance A091401) have demonstrated tumor response to immune checkpoint inhibitors (ICIs) as single-agent and combination regimens in certain sarcoma subtypes. However, there are no sarcoma-specific biomarkers for ICIs and programmed death-ligand 1 (PD-L1) expression did not correlate with clinical response [15, 16]. Indeed, a pooled analysis of clinical trials in STS demonstrated an inconsistent response to ICI based on the PD-L1 expression rate. Previously, we reported our institutional experience highlighting ICI activity in 29 patients with sarcoma [18]. We demonstrated a positive correlation between programmed death-1 (PD-1) expression and OS and progression-free survival (PFS) in sarcoma treated with PD-1 inhibitors, but not with PD-L1 expression as observed in other tumor types. Two other analyses of various sarcoma tissue samples showed a positive correlation between sarcomas that express PD-1/PD-L1 and T cell infiltration and activation [19, 20]. Patients with sarcomas containing increased copy numbers of the PD-1 gene have poorer survival outcomes [21]. Despite these observations, there are no reliable markers of response to immunotherapy in patients with sarcoma. In this updated study, we further explore OS and PFS outcomes of ICI-based therapy, focusing on the specific ICI regimen and clinical covariates to identify the significant clinical factors that could enlighten advanced sarcoma outcomes with immunotherapy.
Methods
Patients seen at The Ohio State University Comprehensive Cancer Center Sarcoma Clinics were enrolled in the Sarcoma Retrospective ICI database from January 1, 2015, through November 1, 2021. Patients were included if they had received any ICI therapy. Therapy was categorized as single-agent ICI or ICI + combination. ICI + combination was further categorized into ICI + medication, ICI + radiation, ICI + surgery, or ICI + multiple (more than 2 modalities). Primary outcomes were OS and PFS. Clinical covariates assessed via the medical record were stage, age, gender, histology, change in the neutrophil-to-lymphocyte ratio (NLR), number of cycles, and immune-related adverse events (irAEs). NLR was calculated as the change in the neutrophil-to-lymphocyte ratio between day 0 and day 14 after ICI treatment administration. A cutoff ratio of 5 was used based on prior studies [11], with < 5% known to be associated with ICI response. There were 9 irAE types assessed: pneumonitis, colitis/diarrhea, abnormal thyroid function, hepatitis, dermatitis/rash, myalgia/arthralgia, neurological abnormalities, hypophysitis, and others.
Statistical analysis
The primary objective was to evaluate overall survival and progression-free survival. We used log-rank tests and proportional hazard regression.
Results
Demographics
We screened 138 patients, and 135 met inclusion criteria (Table 1).
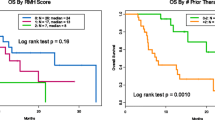
There were more male patients (53%) than females (47%). Most patients were younger than 70 years of age (71%). Most patients were metastatic/stage 4 (96%). Histology was categorized into undifferentiated pleomorphic sarcoma (UPS; 19%), leiomyosarcoma (LMS; 7%), liposarcoma (LPS; 10%), and other (68%). There were various other histologies, but each type had less than 6 patients; therefore, they were aggregated into the “other” category to facilitate statistical analysis (Table 2). The other histologies consisted of: angiosarcoma, chondrosarcoma, GIST, bone (osteosarcoma, Ewing), synovial, adenosarcoma, alveolar soft part sarcoma (ASPS), chordoma, clear cell, dendritic cell, epithelioid sarcoma, inflammatory myofibroblastic tumor, myxofibrosarcoma, PEComa, solitary fibrous tumor, and sarcoma not otherwise specified (NOS). 63% of patients were receiving ICI in the third-line setting or beyond, with only 10% receiving it as first-line therapy. 53% received single-agent ICI, and 47% received an ICI combined with another therapy (ICI + combination). ICI used were pembrolizumab, nivolumab, ipilumumab or combination ipilimumab and nivolumab. Of the patients that received ICI + combination, 59% received ICI + medications (either intravenous chemotherapy or targeted therapies: tyrosine kinase inhibitors, CDK4/6 inhibitors, mTOR inhibitors, estrogen receptor inhibitors, proteasome inhibitors, used either concurrently or sequentially), 15% received ICI + radiation (either concurrently or sequentially), 13% received ICI + surgery (either neoadjuvant or adjuvant), and 13% received ICI + multiple (a combination of 2 or more modalities). Radiation therapy and surgery were palliative in nature due to most patients having advanced stage disease at time of immunotherapy treatment. Most patients received 3 or more cycles of ICI therapy (any regimen; 56%). The NLR was less than 5 in 88% of patients and 12% had an NLR ≥ 5. The incidence of irAE was as expected for the ICI administered.
Overall survival
Patients treated with ICI + combination had an improved OS (median 64 weeks) compared to single-agent ICI (median 37 weeks) (p = 0.014) (Table 3).
Specifically, patients who received ICI in combination with 2 or more modalities (ICI + multiple) had the best outcome (Fig. 1).
Whether patients had received single-agent ICI or ICI + combination, those who received 3 or more cycles of ICI had an improved OS—a median of 74 weeks compared to 12 weeks for those patients who received less than 3 cycles (p < 0.001) (Table 2). Patients who received single-agent ICI and whose change in NLR was < 5 had an improved OS (median 42 weeks) compared to those whose NLR was ≥ 5 (median 9 weeks) (p = 0.002) (Fig. 2); however, this was not seen in patients who received ICI + combination therapy (p = 0.441).
Patients who had a documented irAE of colitis/diarrhea (p = 0.009), hepatitis (p = 0.042), or dermatitis/rash (p = 0.010) were associated with an improved OS across the whole cohort (Figs. 3 and 4).
More specifically, patients treated in the ICI + combination group and who developed dermatitis had an improved OS (p = 0.021) (Fig. 4).
There were no differences in OS based on age (p = 0.354), gender (p = 0.628), histology (p = 0.342), or subcategories of ICI + combination.
Progression-free survival
In this analysis, we demonstrated an improvement in PFS in patients with UPS (median 68 weeks) compared to the other histologies (p = 0.017) (Table 4).
This statistically significant signal was demonstrated in patients who received single-agent ICI (p = 0.009) but not those who received ICI + combination (p = 0.698). Patients who received any ICI regimen and were younger than 70 had a worse PFS—a median of 16 weeks compared to 42 weeks in patients 70 years and older (p = 0.036). There were no differences in PFS based on gender (p = 0.489). There was no effect on PFS between single-agent ICI and ICI + combination (regardless of subcategory) (p = 0.296). Patients who received 3 or more cycles of ICI—whether single-agent ICI or ICI + combination–had an improved PFS, a median of 27 weeks compared to 7 weeks (p < 0.001). There was no significant association between PFS and NLR (p = 0.649).
Patients who developed an irAEs, specifically colitis (p = 0.009), hepatitis (p = 0.048), and dermatitis (p = 0.003), had an improved PFS across the whole cohort. There was a higher PFS seen in patients who received ICI + combination and experienced dermatitis (p = 0.007) and irAEs in the “other” category (p = 0.033) compared to the single-agent ICI regimen. The development of any irAEs was positively associated with the number of doses of ICI administered (≥ 3) (p = 0.003), as expected. However, there was no correlation with the type of ICI agent (p = 0.134).
Discussion
We performed a retrospective study of patients with advanced sarcoma who received ICI therapy and evaluated OS/PFS outcomes based on the type of ICI regimen and clinical covariates. We demonstrated that patients who received ICI + combination therapy had an improved OS but saw no effect on PFS. Patients who received 2 or more therapy modalities with ICI (that is, chemotherapy/targeted therapy, radiation, or surgery) had improved outcomes compared to ICI alone or ICI with a single modality. Radiation therapy and surgery were palliative in nature and we demonstrated a potential overall survival benefit for patients with advanced sarcoma who received an ICI + combination therapy. This was also demonstrated in a systematic review where ICI, in combination with chemotherapy, had a significant overall response rate [12].
We demonstrated the importance of a low NLR, which is known to be associated with an improvement in OS, but only in patients who received single-agent ICI. This benefit was not demonstrated in patients who received ICI + combination, possibly because chemotherapy and radiation influence bone marrow function. Our data suggest that NLR can play a role as a prognostic biomarker in patients starting ICI treatment. This is consistent with prior studies that have shown an association between NLR and OS, where patients who had a moderate decrease in NLR during treatment with ICI were found to have the longest survival and patients with a significant decrease or increase in NLR had shorter OS [22]. NLR could be further evaluated as a biomarker of outcomes across a larger sample size of sarcoma populations receiving single-agent ICI therapy.
We found no statistically significant association between PFS and ICI regimen, except in patients with UPS. In this histologic group, we saw a longer median PFS compared to the other subtypes and our data is consistent with prior studies that patients with UPS have a positive response to ICI compared to other subtypes [7, 8, 13, 14]. Further, patients 70 years and older had a significantly improved PFS compared to younger patients. It is unclear at this time why older patients would have a better outcome. ICI therapy could be considered in elderly patients if indicated. However, this finding does not contradict current studies on PFS as a surrogate for OS. Our sample size is too limited to make conclusions on surrogate endpoints in soft tissue sarcoma.
NLR might not be helpful as a biomarker for PFS in sarcoma as there was no significant association. Further evaluation with a larger sample size is warranted given prior studies showing an association between NLR and PFS in non-small cell lung cancer, demonstrating that a lower ratio is associated with improved PFS [23].
We demonstrated an association between OS/PFS and the number of ICI cycles administered. However, this is a tautology and does not represent a novel finding in sarcoma management. We present the findings in the paper to highlight both the significant and clinically insignificant findings in this retrospective analysis.
The occurrence of certain irAEs were associated with improvements in OS and PFS, particularly dermatitis. Colitis and hepatitis were also associated with improved OS and PFS across the cohort. In those patients who received ICI + combination therapy, the “other” irAEs category was associated with an improved OS and PFS. As demonstrated in prior studies, the occurrence of certain irAEs is reflective of an immune system boost from ICI therapy and suggests increased activity against cancer. Our ICI cohort results are consistent with the historical data from our previous study [10]. It can also be suggested that patients who responded to ICI received more doses and therefore developed more irAEs.
There were limitations to this study. Our sample size of 135 patients is small; however, this was an improvement from our prior study looking at ICI outcomes in 26 patients with sarcoma. Some patients were censored during analyses due to limited clinical information. Due to the rarity of sarcoma, most subtypes were not included as separate histologies in these analyses as there were too few patients to reach statistically significant results. Therefore, less frequent histologies were categorized as the “other” group. Histologies that are associated with positive outcomes from ICI, like ASPS, were not adequately represented in this sample to demonstrate a meaningful outcome with ICI therapy. However, despite this arrangement, our data for PFS was consistent with prior studies demonstrating that certain subtypes, like UPS, have a more favorable response to ICI therapy compared to others, such as LMS.
Conclusions
This retrospective study demonstrates that ICI + combination therapy can improve OS in sarcoma. Benefits in OS/PFS were seen in patients who developed certain irAEs. Those with decreased changes in NLR also had improved OS, demonstrating a potential prognostic biomarker. Further studies will focus on evaluating larger sample sizes to evaluate more subtypes of sarcoma and to better understand the role of NLR in prognostication in sarcoma.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICI:
-
Immune checkpoint inhibitor
- irAE:
-
Immune-related adverse event
- LMS:
-
Leiomyosarcoma
- LPS:
-
Liposarcoma
- NLR:
-
Neutrophil-to-lymphocyte ratio
- OS:
-
Overall survival
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- PFS:
-
Progression-free survival
- STS:
-
Soft-tissue sarcoma
- UPS:
-
Undifferentiated pleomorphic sarcoma
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Kallen ME, Hornick JL. The 2020 WHO classification: what’s New in Soft tissue Tumor Pathology? Am J Surg Pathol. 2021;45(1):e1–e23.
von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, et al. NCCN Guidelines Insights: soft tissue sarcoma, Version 1.2021. J Natl Compr Canc Netw. 2020;18(12):1604–12.
ESMO. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii102–12.
Lochner J, Menge F, Vassos N, Hohenberger P, Kasper B. Prognosis of patients with metastatic soft tissue sarcoma: advances in recent years. Oncol Res Treat. 2020;43(11):613–9.
Younger E, Husson O, Asare B, Benson C, Judson I, Miah A, et al. Metastatic soft tissue sarcomas in adolescents and young adults: a specialist Center Experience. J Adolesc Young Adult Oncol. 2020;9(6):628–38.
Bramwell VH, Anderson D, Charette ML, Group SDS. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003(3):CD003293.
Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(12):1732–42.
Lorigan P, Verweij J, Papai Z, Rodenhuis S, Le Cesne A, Leahy MG, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a european Organisation for Research and Treatment of Cancer Soft tissue and bone Sarcoma Group Study. J Clin Oncol. 2007;25(21):3144–50.
Dileo P, Morgan JA, Zahrieh D, Desai J, Salesi JM, Harmon DC, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer. 2007;109(9):1863–9.
Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25(19):2755–63.
García-Del-Muro X, López-Pousa A, Maurel J, Martín J, Martínez-Trufero J, Casado A, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol. 2011;29(18):2528–33.
Blay JY, Leahy MG, Nguyen BB, Patel SR, Hohenberger P, Santoro A, et al. Randomised phase III trial of trabectedin versus doxorubicin-based chemotherapy as first-line therapy in translocation-related sarcomas. Eur J Cancer. 2014;50(6):1137–47.
Coens C, van der Graaf WT, Blay JY, Chawla SP, Judson I, Sanfilippo R, et al. Health-related quality-of-life results from PALETTE: a randomized, double-blind, phase 3 trial of pazopanib versus placebo in patients with soft tissue sarcoma whose disease has progressed during or after prior chemotherapy-a European Organization for research and treatment of cancer soft tissue and bone sarcoma group global network study (EORTC 62072). Cancer. 2015;121(17):2933–41.
Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–501.
D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19(3):416–26.
Italiano A, Mathoulin-Pelissier S, Cesne AL, Terrier P, Bonvalot S, Collin F, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049–54.
Quiroga D, Liebner DA, Philippon JS, Hoffman S, Tan Y, Chen JL, et al. Activity of PD1 inhibitor therapy in advanced sarcoma: a single-center retrospective analysis. BMC Cancer. 2020;20(1):527.
Kostine M, Cleven AH, de Miranda NF, Italiano A, Cleton-Jansen AM, Bovée JV. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod Pathol. 2016;29(9):1028–37.
Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017;123(17):3291–304.
Budczies J, Mechtersheimer G, Denkert C, Klauschen F, Mughal SS, Chudasama P, et al. PD-L1 (CD274) copy number gain, expression, and immune cell infiltration as candidate predictors for response to immune checkpoint inhibitors in soft-tissue sarcoma. Oncoimmunology. 2017;6(3):e1279777.
Li M, Spakowicz D, Burkart J, Patel S, Husain M, He K, et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol. 2019;145(10):2541–6.
Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23(4):634–40.
Acknowledgements
The authors would like to thank Sarah Hoffman, PharmD, and Thomas Schulz, PharmD, for their suggestions and help with this study. In addition, the authors wish to acknowledge the contributions of scientific editor Angela Dahlberg, BSJ, in the editing of this manuscript.
Funding
This study was supported by the National Institutes of Health (P30CA016058 and K12 CA133250).
Author information
Authors and Affiliations
Contributions
Acquisition of data: HGK, GT. Analysis, interpretation, and preparation of data: MH, SL, HI, MX, DQ, GT. Accrued patients to study: DAL, JLC, GT. Writing, review, and/or revision of the manuscript: MH, DAL, CV, GT. Provided funding: GT. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and ethical approval was obtained from The Ohio State University Comprehensive Cancer Center Institutional Review Board (IRB protocol No. 2021C0005). Individual consent from each subject was not required by the IRB as deidentified data was used. All research was carried out in accordance with these institutional and international guidelines and regulations. The need for informed consent was waived by The Ohio State University Comprehensive Cancer Center Institutional Review Board as deidentified data was used.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Husain, M., Quiroga, D., Kim, H.G. et al. Clinical markers of immunotherapy outcomes in advanced sarcoma. BMC Cancer 23, 326 (2023). https://doi.org/10.1186/s12885-023-10758-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10758-w