Abstract
Background
Skin cutaneous melanoma (SKCM) is an extremely malignant tumor and accounts for the majority of skin cancer deaths. Aspartate beta-hydroxylase domain containing 1 (ASPHD1) may participate in cancer progression through controlling α-ketoglutarate-dependent dioxygenases. However, its role in skin cutaneous melanoma (SKCM) has not been well studied.
Methods
The gene expression data of ASPDH1 and differentially expressed genes (DEGs) from TCGA and GTEx were evaluated, and verified via the GEO database. Then, we performed GO/KEGG, GSEA, PPI network analysis to analyze the functional implications of the DEGs related to ASPHD1. Then, the association between the ASPHD1 expression and clinical parameters was investigated by Cox regression analysis. Subsequently, the survival time of SKCM patients was evaluated by plotting Kaplan-Meier curves. Moreover, we investigated the correlation between the ASPHD1 expression and lymphocytic infiltration by using the data from TISIDB and TIMER 2.0. Next, we explored the association between ASPHD1 expression and drug sensitivity. Finally, we validate the expression differences by analyzing the results of qPCR, Western blot from human normal epidermal melanocytes and melanoma cells, and immunohistochemistry (IHC) from non-tumor skin as well as melanoma tissues.
Results
The ASPHD1 expression level was significantly upregulated in several cancers, including SKCM especially SKCM-metastasis tissues, and patients with an increased ASPHD1 expression had longer overall survival time than low expression ones. The functional enrichment analysis of ASPHD1-related DEGs showed an association with cell development regulation and tumorigenic pathways. Furthermore, the increased ASPHD1 expression level was associated with the level of immunostimulors, immunoinhibitors, chemokines, and TILs, such as CD4+, CD8+ T cell, mast cell, Th2 cell, and dendritic cell. More interesting, we found that ASPHD1 expression was tightly associated with CTLA4 and CD276 which are immune checkpoint markers. Moreover, the upregulated expression of ASPHD1 exhibited higher IC50 values for 24 chemotherapy drugs, including doxorubicin, and masitinib. Finally, the differential expression of ASPHD1 in SKCM was validated by the results of qPCR, Western blot, and IHC.
Conclusion
The expression of ASPHD1 in SKCM patients is closely related to patient survival. ASPHD1 may participate in the regulation of tumor immune microenvironment. Additionally, it may serve as a prognostic biomarker for SKCM and future in-depth studies are necessary to explore its value.
Similar content being viewed by others
Background
Skin cutaneous melanoma (SKCM) is the most prevalent subtype of melanoma, and it is also the most lethal, accounting for most skin cancer deaths [1]. Although melanoma is a rare tumor compared to lung, breast, and gastrointestinal cancers, its incidence has increased rapidly in the past 40 years [2]. Even though there have been numerous new therapeutic agents introduced in recent years for unresectable melanoma and its overall survival rate has been significantly improved, the proportion of beneficiaries remains low and the treatment cost has increased significantly [2,3,4,5]. In most cases, the occurrence of skin cutaneous melanoma is related to high-level mutations caused by ultraviolet radiation [2]. Known genomic mutations in BRAF and NRAS are involved in melanoma formation [3, 6,7,8]. In addition, other pathways such as histone modification, methylation, and cell cycle change are also related to the occurrence of melanoma [9,10,11]. Although several biomarkers related to melanoma prognosis have been proposed [12], they are still controversial and have not been recognized. Therefore, it is urgently needed to discover new biomarkers for predicting melanoma recurrence, metastasis, and long-term survival.
ASPHD1 is a protein-coding gene whose related diseases include schizophrenia 3 and chromosome 16P11.2 Deletion Syndrome [13, 14]. The related gene annotation of Gene Ontology is dioxygenase activity. ASPHD1 is an important paralog of aspartate beta-hydroxylase (ASPH) whose gene coding protein is one of the type 2 transmembrane proteins, belongs to the family of α-ketoglutarate (αKG)-dependent dioxygenases [15]. The protein sequences and three dimensional structures from AlphaFold (predicted) for ASPH and ASPHD1 genes were showed in Figure S1. αKG-dependent dioxygenases can regulate tricarboxylic acid (TCA) cycle metabolites including αKG, 2-hydroxyglutarate, and fumarate, which are associated with chromatin demethylation [16]. ASPH participants in regulating calcium homeostasis and the malignant transformation of tumor cells [15]. The overexpression of ASPH in several human solid tumors, such as pancreatic ductal adenocarcinoma [17], breast cancer [18], colorectal cancer [19], cholangiocarcinoma [20], and hepatocarcinoma [21], has been observed. ASPH protein could catalyze posttranslational hydroxylation in the cbEGF-like domains of numerous proteins that are associated with cell motility and invasiveness [18]. Overexpressed ASPH proteins are transported from the endoplasmic reticulum to the plasma membrane, where their C-terminal region is exposed to the extracellular environment, and then it is possible to bind an antibody [21]. In addition, the antigenic epitopes of the ASPH protein can stimulate the cellular immune response by activating the Notch and SRC signaling pathways [15, 17, 18, 21]. Thus, ASPH is a potential candidate for molecular targeted therapy and immunotherapy. However, as a critical paralog of ASPH, the relationship between ASPHD1 and cancer is less studied, and its role in the occurrence and progression of neoplasm is not clear. Therefore, it is of significant importance to investigate the ASPHD1 expression level in different stages of SKCM and their correlations.
We investigated the association between ASPHD1 expression and the prognosis of SKCM patients by using several public databases in this study. Moreover, the relationship between ASPHD1 expression and lymphocytic infiltration in tumor immune microenvironments was also evaluated.
Methods
Patient data files
The original mRNA sequence profiles and clinical information of SKCM and non-tumor samples were obtained from TCGA (https://portal.gdc.cancer.gov/), GTEx (https://xena.ucsc.edu/), and GEO database (GSE114445, GSE15605, and GSE46517 datasets, https://www.ncbi.nlm.nih.gov/geo/). Then, sample data were subjected to normalization to minimize potential batch effects. In this study, gene expression profiles of 471 SKCM tissues and 558 nontumor tissues were analyzed using the TCGA and GTEx data. For validation, 30 normal skin and 93 primary melanoma gene expression profiles were obtained from three GEO datasets. Then, immunohistochemistry (IHC) of 4 normal human skin tissues and 2 melanoma tissues was obtained from the HPA database (http://www.proteinatlas.org/). 471 SKCM patient data from TCGA were used for statistical analysis including clinicopathological characteristics and overall survival. Table 1 provided details about the SKCM patients.
Identification of differentially expressed genes (DEGs)
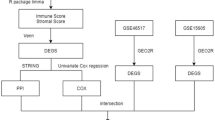
471 SKCM patients were categorized into two groups based on median ASPHD1 expression levels. Then, DEGs were identified by using the “DESeq2” R package and ∣Log2 FC∣>1.5 as well as FDR < 0.05 were set. Then the heatmap map was performed by applying the “ggplot2” package.
Functional enrichment analysis and gene set enrichment analysis (GSEA) of ASPHD1-related DEGs
The “limma” and “corrplot” R packages were used to identify the top 30 related DEGs. ASPHD1-related DEGs were analyzed using the “clusterProfiler” and “ggplot2” packages to investigate GO and KEGG functions. Subsequently, the ASPHD1-related pathways were assessed by using GSEA (version 4.1.0) in SKCM. An annotated gene set (C5. all. v7.1. symbols. gmt) was chosen as the reference. Gene expression enrichment analysis was carried out on ASPHD1 data sets with high or low expression. For each analysis, 1000 gene sets were set to identify significantly different pathways.
Three major parameters,∣standardized enrichment score∣>1, nominal p-value < 0.05 as well as error detection rate (FDR) q-value < 0.25 were regarded as statistically significant.
Protein-protein interaction network (PPI) and gene co-expression network analysis of ASPHD1-associated DEGs
The genes related to ASPHD1 were input into the STRING database (http://string-db.org) (version 11.5) to evaluate the interaction between all the known proteins and explore potential mechanisms. As a minimum interaction fraction, medium confidence (0.400) was set to exclude irrelevant protein nodes. we used the Coexpedia database (http://www.coexpedia.org/) to analyze ASPHD1 co-expression genes in SKCM. Then, co-expressed genes were sorted and screened by neighbor’s local linear selection scores with a p-value < 0.05.
Clinicopathological features associated with ASPHD1 expression in SKCM
We used Perl (Version 5.34.0) and R (Version 4.1.0) to sort, merge, and visualize the data derived from the TCGA and GTEx databases. Data from three GEO datasets were processed using similar methods. The SKCM patients were classified based on 50% ASPHD1 expression level. Then, an analysis of the relationship between ASPHD1 expression, survival status, and clinical features was performed. We applied Cox regression analyses to identify factors that affect overall survival (OS) in patients with SKCM by using R software.
Association of ASPHD1 expression and immune-related factors in SKCM tumors
Integrated repository portals, TIMER 2.0 (http://timer.cistrome.org/) and TISIDB (http://cis.hku.hk/TISIDB/index.php) were used to evaluate the interactions between the tumor and immune system. They were used to investigate the ASPHD1 expression in different tumors and the relationship between the expression level of ASPHD1 and gene markers of TILs including CD4+/CD8+ T cell, T helper cell, dendritic cell (DC), B cell, and mast cell in this study. In addition, we studied the relationship of ASPHD1 with immunoinhibitors, immunostimulors, and chemokines in SKCM.
Drug sensitivity prediction
IC50 of chemotherapy agent represents the half amount of an agent inhibiting some specific biological procedures. We used the “pRRophetic” R package to predict the IC50 of various chemotherapy drugs.
Cell culture
Three human melanoma cells (A375, SK-MEL-28, and A875) as well as human normal epidermal melanocyte (HEMn) were provided by iCell Bioscience Co., Ltd (Shanghai, China). The absence of contamination of the three cell lines was confirmed by short tandem repeat analysis (Biowing applied biotechnology Co., Ltd, Shanghai, China). Routine examinations confirmed that the cells did not contain mycoplasma. DMEM, MEM, RPMI 1640, and DMEM F12 medium with adding 10% FBS and 1% penicillin-streptomycin were used to culture them under the conditions with 5% CO2 and 90% humidity at 37℃, respectively. All cell culture products were provided by Thermo Fisher Scientific Inc. (Waltham, MA, USA).
Quantitative real-time PCR analysis
The processes of extracting the total RNA (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA), the reverse transcription (Takara Bio Inc., Kusatsu, Japan), and real-time quantitative polymerase chain reaction analyses (Yeasen Biotechnology Co., Ltd., Shanghai, China) for mRNA of ASPHD1 and actin-beta were performed as previously described [22]. The 2−ΔΔCt method was used to determine the relative expression levels of the target genes.
Primers for qPCR were as follows
Human ASPHD1-F: 5′−CCAGAGTGGAATGTGGAAGGGAAAC − 3′.
Human ASPHD1-R: 5′−AGCAGTACCAGAGGAACAGGGAAG − 3′.
Human actin-beta-F: 5′−TCGTGCGTGACATTAAGGAGAAGC − 3′.
Human actin-beta-R: 5′− GGCGTACAGGTCTTTGCGGATG − 3′.
Western blot
A lysis buffer was applied to extract the total protein of cells, then a BCA assay kit was used to evaluate the protein concentrations. All kits were provided by Beyotime Biotechnology Co., Ltd (Shanghai, China). Western blot was performed as previously described [22]. Anti-ASPHD1 (Abcam, cat#ab197301, Waltham, MA, USA) and anti-β-Tubulin (Abbkine Scientific Co., Ltd, cat#A01030, Wuhan, China) were used with the recommended concentrations. The blots were cut prior to hybridisation with antibodies.
Statistical analysis
Mann–Whitney U test was used to compare the difference in ASPHD1 expression between SKCM and nontumor samples. The relationship between ASPHD1 expression and clinicopathological parameters was evaluated by chi-square test. The difference in survival time of SKCM patients was evaluated by plotting Kaplan-Meier curves. Univariate and multivariate analyses were performed by cox proportional hazard regression model. R software (version 4.1.0) was applied to conduct statistical analyses and p < 0.05 was considered significant in all trials.
Results
Patient characteristics and ASPHD1 expression in SKCM tissues
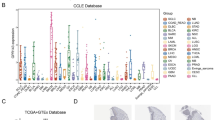
According to the TIMER 2.0 database, the differential expression of ASPHD1 in various cancers was showing in Fig. 1A. The ASPHD1 expression levels were significantly upregulated in 15 kinds of cancers, including gastrointestinal cancer, hepatobiliary carcinoma, urological tumor, lung cancer, and breast invasive carcinoma, but decreased in glioblastoma multiforme compared with normal tissues.
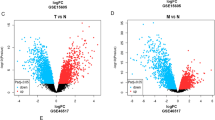
We could also find that ASPHD1 expressed higher in SKCM, especially in SKCM metastasis tissues, but the compared result could be hardly obtained because there were not enough paired normal tissues with SKCM tissues in the TCGA database. To verify these findings in SKCM, the RNA-seq data of 471 SKCM samples were derived from TCGA and 558 nontumor samples including 1 from TCGA and 557 from GTEx were analyzed. The result showed that ASPHD1 expression in SKCM tissues was significantly elevated compared to tissues from non-tumors (Fig. 1B). A similar result was also verified by the GEO database (Fig. 1C). Meanwhile, mRNA and protein levels of ASPHD1 from three human melanoma cell lines were much higher than that of human normal epidermal melanocytes according to qRT-PCR (Fig. 1D) and western blot (Fig. 1E).
We divided 471 patients with SKCM into low and high groups based on 50% ASPHD1 expression. Then, we compared the mRNA levels between the two groups. We identified a total of 82 DEGs (Fig. 1F), including 49 upregulated genes and 33 downregulated genes. Then we plotted a heatmap displaying 10 representative DEGs (Fig. 1G).
Differential mRNA expression profiles in skin cutaneous melanoma (SKCM) patients according to the ASPHD1 expression. (A) The expression of ASPHD1 in different tumors from the Tumor Immune Estimation Resource (TIMER 2.0). ASPHD1 expression was higher in SKCM tissues than in nontumor tissues based on TCGA and GTEx databases (B) as well as the GEO database (C). The results of qPCR (D) and western blot (E) from human normal epidermal melanocyte (HEMn) and three human melanoma cell lines (A375, SK-MEL-28, and A875). 471 SKCM patients were divided into low and high expression groups according to the median ASPHD1 level, the differentially expressed genes (DEGs) were recognized and plotted by volcano (F) and heatmaps (G). *p < 0.05; ***p < 0.001; ****p < 0.0001
Functional enrichment analysis of ASPHD1-related DEGs
We performed the GO (Fig. 2A) and KEGG (Fig. 2B) functional enrichment analysis to reveal the functional implication of ASPHD1-related DEGs. The relationship with the biological process (BP) included epidermal cell differentiation, skin development, and epidermis development; cellular components (CC) contained cell − cell junction, external side of plasma membrane and collagen-containing; molecular function (MF) involved peptidase regulator activity, antigen binding, and actin binding. KEGG included chemical carcinogenesis-receptor activation, Ras signaling pathway, and coronavirus disease [23,24,25]. Then, the 10 most correlated DEGs were plotted by the “limma” R package (Fig. 2C). The result of GSEA showed that the most enriched signaling pathways were peptidyl proline hydroxylation, disulfide oxidoreductase activity, regulation of protein targeting to membrane, L-ascorbic acid binding, pentose metabolic process, protein N-linked glycosylation, abnormal protein glycosylation, endoplasmic reticulum golgi intermediate compartment, zinc ion homestasis, response to copper ion. (Fig. 2D).
Functional enrichment analysis of ASPHD1-related DEGs in SKCM. (A) Annotations of GO enrichment analysis include biological process (BP), cellular components (CC), and molecular function (MF) categories. (B) KEGG pathway annotations, copyright permission of KEGG pathway maps was obtained from Kanehisa Laboratories. (C) Top 10 correlation genes of ASPHD1. Red means positive regulation and green means negative regulation. (D) Merged enrichment plots from the gene set enrichment analysis (GSEA).
Protein-protein interaction network (PPI) and gene co-expression network analysis
We used the STRING database to carry out PPI analysis to further investigate the mechanisms, and the results showed that 10 genes interacted with ASPHD1 including GDPD3, CDIPT, INO80E, FAM57B, PA1, DOC2A, KIF22, PRRT2, ENSG00000263136, and SEZ6L2 (Fig. 3A). Then, we identified the co-expression genes of ASPHD1 by the Coexpedia database, and it was found that 78 genes were associated with ASPHD1 (Fig. 3B).
Correlation of ASPHD1 expression and clinicopathological characteristics
The relationship between the expression of ASPHD1 and clinicopathological characteristics, including AJCC stage (stage0, I-IV), tumor stage (T0-T4), lymph node stage (N0-3), and immune subtypes (C1-6) was investigated. The results were shown in Fig. 4A-D. The results indicated that ASPHD1 expression was related to the pathological stage (III vs. 0, p = 0.047); T stage (II vs. 0, p = 0.033) as well as N stage (I vs. 0, p = 0.009) (Table 2).
Correlation between ASPHD1 expression level and clinicopathological characteristics in SKCM patients. (A) Pathological stage. (B) Tumor stage (T). (C) Lymph node stage (N). (D) Immune subtype: C1 – wound healing, n = 41; C2 – IFN-gamma dominant, n = 27; C3 – inflammatory, n = 14; C4 – lymphocyte depleted, n = 19; C6 – TGF-b dominant, n = 2
Association of ASPHD1 expression and overall survival time in SKCM patients
According to the median of the ASPHD1 expression level, high and low expression was grouped. The Kaplan-Meier curves revealed that the patients in the high group had better overall survival (OS) than the ones in the low group (Fig. 5A). The median OS in the high group was 42.2 months, while that in the low group was 37.1 months. The 5-year survival rates in the high and low groups were 66.1% and 54.6%, respectively. The result from the TISIDB database had a similar result (Fig. 5B). Moreover, the survival of metastatic patients with upregulated ASPHD1 had a better OS than the low ones (Fig. 5C).
Then, univariate analysis revealed that age, pathological stage, T stage, N stage, and ASPHD1 expression were potential survival predictors (Table 3). Multivariate analysis showed that age, T stage, N stage, and ASPHD1 expression were independent predictors of OS (Table 3; Fig. 5D).
Kaplan-Meier curves comparing the low and high ASPHD1 expression in SKCM patients. Survival curves of overall survival (OS) in all SKCM patients (A) and metastatic patients (B) from TCGA. (C) Survival curves of OS in SKCM from TISIDB. (D) Forest plot for the multivariate Cox proportional hazard regression model. * p < 0.05, ** p < 0.01, *** p < 0.001
Relationship of tumor-infiltrating lymphocytes and ASPHD1 in SKCM
The TISIDB platform was applied to evaluate the association between the ASPHD1 expression level and TILs in SKCM. High-expression level of ASPHD1 had a positive relationship with central memory CD4+ T cell (p < 0.001), central memory CD8+ T cell (p = 0.026), CD56 bright natural killer cell (p < 0.001), CD56 dim natural killer cell (p < 0.001), mast cell (p = 0.018), activated dendritic cell (p = 0.007), plasmacytoid dendritic cell (p < 0.001) and type 17 T helper cell (p < 0.001) as shown in Fig. 6A. Moreover, increased ASPHD1 expression was positively correlated with immune checkpoint CTLA4 (p < 0.001) and CD276 (p < 0.001) in SKCM (Fig. 6B-D) according to the TIMER 2.0 database.
Correlation of ASPHD1 expression with immune infiltration and immune checkpoint in SKCM patients. (A) Correlation of the expression of ASPHD1 with infiltration levels of central memory CD4+ T cell, central memory CD8+ T cell, CD56 bright natural killer cell, CD56 dim natural killer cell, mast cell, activated dendritic cell, plasmacytoid dendritic cell and type 17 T helper cell. (B) ASPHD1 expression correlated with immune checkpoint CTLA4 and CD276. (C) Relation of the expression of ASPHD1 with CD276. (D) Correlation of the expression of ASPHD1 with CTLA4.
Association of immunomodulators and ASPHD1 in SKCM
Immunoinhibitors and immunostimulors are clearly related to the function of the immune cell of the tumor immune microenvironment. The results in Fig. 7A showed that ASPHD1 was associated with immunoinhibitors, including PVRLL2 (p < 0.001), CTLA4 (p < 0.001), TGFB1(p < 0.001), TGFBR1 (p < 0.001) and CD160 (p = 0.001). The high-expressed ASPHD1 had a close association with immunostimulors, such as C10orf54 (p = 0.008), CD70 (p = 0.016), CD276 (p < 0.001), ICOSLG (p < 0.001), IL6 (p = 0.011), NTSE (p < 0.001), PVR (p < 0.001), TNFRSR8 (p = 0.001), TNFRSR18 (p < 0.001), TNFRSF25 (p = 0.007), CD28 (p = 0.020), TNFRSF4 (p = 0.004), TNFSF15 (p < 0.001) (Fig. 7B).
The ASPHD1 expression is related to chemokines in SKCM
Chemokines play an important role in TILs infiltration and affect their function. This study uncovered the association between ASPHD1 expression and chemokines. As shown in Fig. 8, ASPHD1 was associated with CCL20 (p = 0.001), CXCL1 (p < 0.001), CXCL3 (p = 0.002), CXCL6 (p = 0.015) and CXCL16 (p = 0.002), CCL17 (p = 0.016), CCL23 (p = 0.048), CCL28 (p = 0.037), CXCL10 (p = 0.004), CXCL11 (p = 0.006), XCR1 (p = 0.010).
Association of ASPHD1 expression and drug sensitivity in SKCM
The IC50 values of chemotherapy drugs were compared to investigate the difference in the drug resistance potential in low and high ASPHD1 expression groups. Figure 9 showed that 24 drugs or inhibitors, including doxorubicin and masitinib, were identified to be potential drugs for ASPHD1-downregulated SKCM patients.
Validation of ASPHD1 expression
The IHC results indicated that ASPHD1 was not detected or low expressed in normal skin tissues and cells including Langerhans, fibroblasts, keratinocytes, melanocytes, and epidermal cells (Fig. 10A-D), but increased significantly the expression in melanoma (Fig. 10E-F).
Discussion
An increasing number of studies have been published and have shown that ASPH is related to the malignant transformation and invasiveness of tumor cells [15, 17,18,19,20,21]. In addition, small-molecule inhibitors aimed at protecting against ASPH have been developed [15]. Therefore, ASPH is one of the most important biological targets to control tumor cells’ migration and invasion. However, the ASPHD1 expression level and its value in SKCM tissues are still unclear. In this work, we comprehensively analyzed the relationship between ASPHD1 expression and the clinical parameters, overall survival, and tumor immune microenvironment in SKCM. Analyzed results showed that ASPHD1 expression in SKCM tissues was upregulated, and patients with increased expression of ASPHD1 had a better prognosis, and the ASPHD1 expression level was an independent prognostic index along with age, T stage, and N stage on survival. Furthermore, the ASPHD1 expression was correlated with TILs, immunostimulors, immunoinhibitors, and chemokines as well as the sensitivity of several chemotherapy drugs. Therefore, the results of our study suggested that ASPHD1 might be a biomarker of prognosis that was related to the regulation of the immune microenvironment and a potential therapy target for SKCM.
Then, we investigated the biological functions of ASPHD1 in SKCM by functional enrichment analysis. ASPHD1 is closely related to αKG-dependent dioxygenases, which participates in maintaining stem cell properties to control cell fate by regulating tricarboxylic acid (TCA) cycle metabolites [26, 27]. The results from the functional annotation of ASPHD1-related DEGs showed that the ASPHD1 expression was related to epidermis development, skin development, and epidermal cell differentiation. Next, GSEA was conducted to identify the closely related signaling pathways of ASPHD1 in the development of SKCM. Proline hydroxylation is one of post-translational modifications and is related to 2-oxoglutarate (2OG) oxygenases which could catalyze the hydroxylation of proteins then regulate the basic cellular processes. Moreover, they are in-volved in many kinds of diseases, especially cancer [28]. N-linked glycosylation is a post-translational modification of proteins and is associated with ligand-receptor interactions [29]. Some studies have shown that programmed death-ligand 1 (PD-L1) in human tumors is glycosylated with heavy N-linked glycan moieties to maintain its protein stability and interaction with programmed cell death protein 1 (PD-1) [29]. Ascorbate could activate αKG-dependent dioxygenases to regulate cancer cell fate [30]. Zinc and copper are two kinds of metal elements that are essential for human physi-ology by regulating cell proliferation, catalysis, gene expression [31]. In addition, zinc is crucial for the immune system [32, 33] and copper is related to the synthesis of melanin pigment [34]. Their metabolic homeostasis is closely regulated in the body, so abnormal homeostasis has been reported in lots of cancers including melanoma [31,32,33,34]. Evidence showed that melanoma cells (A375) released fibroblast growth factor (FGF)-1 and FGF-2 and this process required copper ions by activation of phosphati-dylinositol 3-kinase (PI3K)/Akt pathway [35]. In conclusion, ASPHD1 is associated with different signaling pathways which involve in the occurrence and progression of SKCM.
Furthermore, we performed PPI and gene co-expression analyses to explore the related proteins and genes. The results showed that most of them are cancer-related. GDPD3 is necessary to maintain the stem cells of chronic myelogenous leukemia (CML). In addition, GDPD3 loss leads to the activation of AKT/mTORC1 signal and the progression of cell cycle [36]. In gastric cancer, CDIPT plays a crucial role in tumorigenesis and progression [37]. Some studies have suggested the functions of PA1 and INO80E in DNA damage repair and transcription regulation [38, 39]. KIF22, PRRT2, and SEZ6L2 are also related to several kinds of human solid tumors through different signaling pathways [40,41,42]. Therefore, we could hypothesize that ASPHD1 played a vital role in tumorigenesis and development.
Subsequently, we revealed that ASPHD1 was associated with the TILs infiltration in SKCM, and ASPHD1 participated in regulating TME. The results of this study showed that there was a strong correlation between ASPHD1 expression and the infiltration of CD4+ T cell, CD8+ T cell, natural killer cell, dendritic cell, mast cell, and Th17 cell. Moreover, increased ASPHD1 expression had a positive association with immune checkpoints CTLA4 and CD276 in SKCM, and upregulated ASPHD1 was associated with immunostimulors, immunoinhibitors, and chemokines. As a homolog of ASPHD1, ASPH contains both HLA I- and II- restriction epitopes, therefore it can induce ASPH antigen-specific CD4+ and CD8+ T cell responses, resulting in antitumor effects [16, 43]. To date, the relationship between ASPHD1 and cancers has been less studied, and its role in the occurrence and development of cancers is not clear. Therefore, it is of significant importance to explore the expression level and effect of ASPHD1 on prognosis in SKCM. When it was overexpressed in SKCM tissues along with the progression of SKCM, ASPHD1 protein could be transported to the cell surface and recognized by the immune system to stimulate antitumor immunity. This explains why the ASPHD1 expression level in SKCM tissues is much higher, and the prognosis of the high ASPHD1 expressed patients is better than that of low ASPHD1 expression ones.
Furthermore, the results of qPCR, western blot, and IHC validated that ASPHD1 expressed higher in SKCM cells and tissues compared to normal skin cells and tissues. Despite its consistency with our previous analysis, this study still has certain limitations. First, because partial clinical data are incomplete and perhaps related to the prognosis of SKCM, it is difficult to assess the relationship between ASPHD1 and these clinical characteristics of SKCM. Next, cases with incomplete clinicopathological data were filtered to obtain complete cases, which may lead to unsatisfactory results. For example, the relationship between the M stage and prognosis was inconsistent with the current clinical guidelines, probably due to the small number of patients with metastasis in the TCGA database. Finally, as far as we know, there have been a few studies focusing on ASPH and cancers, such as pancreatic ductal adenocarcinoma, hepatocellular carcinoma, and cholangiocarcinoma, but this is the first report on the relationship between ASPHD1 and melanoma. Hence, further research should be carried out on the role of ASPHD1 in cancer.
Conclusion
The increased ASPHD1 expression is associated with better survival in SKCM, which is perhaps related to the higher level of TILs infiltration, immunostimulatory factors, and chemokines. Together, our study suggests ASPHD1 may be a potential biomarker for SKCM. Targeting ASPHD1 to regulate the progress of SKCM may be one of the potential directions, which could provide an essential theoretical basis for the precise treatment of SKCM. In the future, more biological experiments are needed to explore the function of ASPHD1, and it is necessary to carry out clinical trials to verify its value in the diagnosis and treatment of SKCM.
Data Availability
Publicly available datasets were analyzed in this study, and these can be found in the Cancer Genome Atlas (https://portal.gdc.cancer.gov/), the Genotype-Tissue Expression (GTEx) (https://xena.ucsc.edu/), the Gene Expression Omnibus (GEO) database (GSE114445, GSE15605, and GSE46517 datasets, https://www.ncbi.nlm.nih.gov/geo/), and the Human Protein Atlas (http://www.proteinatlas.org/).
Abbreviations
- ASPHD1:
-
Aspartate Beta-Hydroxylase Domain Containing 1
- SKCM:
-
Skin cutaneous melanoma
- TCGA:
-
The Cancer Genome Atlas
- GTEx:
-
Genotype-Tissue Expression
- GEO:
-
Gene Expression Omnibus
- DEGs:
-
Differentially expressed genes
- GSEA:
-
Gene set enrichment analysis
- PPI:
-
Protein-protein interaction
- qPCR:
-
Quantitative real-time polymerase chain reaction
- IHC:
-
Immunohistochemistry
- OS:
-
Overall survival
- TILs:
-
Tumor-infiltrating lymphocytes
- HEMn:
-
Human normal epidermal melanocytes
- BRAF:
-
V-raf murine sarcoma viral oncogene homolog B1
- NRAS:
-
Neuroblastoma Ras virus oncogene homolog
- ASPH:
-
Aspartate beta-hydroxylase
- αKG:
-
α-ketoglutarate
- TCA:
-
Tricarboxylic acid
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- IC50:
-
Half maximal inhibitory concentration
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death-ligand 1
- CML:
-
Chronic myelogenous leukemia.
References
Swetter SM, Tsao H, Bichakjian CK, Curiel-Lewandrowski C, Elder DE, Gershenwald JE, Guild V, Grant-Kels JM, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80(1):208–50.
Welch HG, Mazer BL, Adamson AS. The Rapid rise in cutaneous melanoma diagnoses. N Engl J Med. 2021;384(1):72–9.
Leonardi GC, Falzone L, Salemi R, Zanghì A, Spandidos DA, McCubrey JA, Candido S, Libra M. Cutaneous melanoma: from pathogenesis to therapy (review). Int J Oncol. 2018;52(4):1071–80.
Hartman RI, Lin JY. Cutaneous Melanoma-A review in detection, staging, and management. Hematol Oncol Clin North Am. 2019;33(1):25–38.
Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(12):1884–901.
Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with pten loss to induce metastatic melanoma. Nat Genet. 2009;41(5):544–52.
Nissan MH, Pratilas CA, Jones AM, Ramirez R, Won H, Liu C, Tiwari S, Kong L, Hanrahan AJ, Yao Z, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res. 2014;74(8):2340–50.
Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob JJ, Halpern A, Herlyn M, Marchetti MA, McArthur G, Ribas A et al. Melanoma. Nat Rev Dis Primers 2015, 1:15003.
Sarkar D, Leung EY, Baguley BC, Finlay GJ, Askarian-Amiri ME. Epigenetic regulation in human melanoma: past and future. Epigenetics. 2015;10(2):103–21.
Moran B, Silva R, Perry AS, Gallagher WM. Epigenetics of malignant melanoma. Semin Cancer Biol. 2018;51:80–8.
Scatozza F, Moschella F, D’Arcangelo D, Rossi S, Tabolacci C, Giampietri C, Proietti E, Facchiano F, Facchiano A. Nicotinamide inhibits melanoma in vitro and in vivo. J Exp Clin Cancer Res. 2020;39(1):211.
Ross CL, Kaushik S, Valdes-Rodriguez R, Anvekar R. MicroRNAs in cutaneous melanoma: role as diagnostic and prognostic biomarkers. J Cell Physiol. 2018;233(7):5133–41.
Guha S, Rees E, Darvasi A, Ivanov D, Ikeda M, Bergen SE, Magnusson PK, Cormican P, Morris D, Gill M, et al. Implication of a rare deletion at distal 16p11.2 in schizophrenia. JAMA Psychiatry. 2013;70(3):253–60.
Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, Guzzo F, English DP, Varughese J, Gasparrini S, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110(8):2916–21.
Kanwal M, Smahel M, Olsen M, Smahelova J, Tachezy R. Aspartate β-hydroxylase as a target for cancer therapy. J Exp Clin Cancer Res. 2020;39(1):163.
Baksh SC, Finley LWS. Metabolic coordination of cell fate by α-Ketoglutarate-dependent dioxygenases. Trends Cell Biol. 2021;31(1):24–36.
Ogawa K, Lin Q, Li L, Bai X, Chen X, Chen H, Kong R, Wang Y, Zhu H, He F, et al. Aspartate β-hydroxylase promotes pancreatic ductal adenocarcinoma metastasis through activation of SRC signaling pathway. J Hematol Oncol. 2019;12(1):144.
Lin Q, Chen X, Meng F, Ogawa K, Li M, Song R, Zhang S, Zhang Z, Kong X, Xu Q, et al. ASPH-notch Axis guided Exosomal delivery of Prometastatic Secretome renders breast Cancer multi-organ metastasis. Mol Cancer. 2019;18(1):156.
Benelli R, Costa D, Mastracci L, Grillo F, Olsen MJ, Barboro P, Poggi A, Ferrari N. Aspartate-β-Hydroxylase: A Promising Target to Limit the Local Invasiveness of Colorectal Cancer.Cancers (Basel)2020, 12(4).
Huang CK, Iwagami Y, Zou J, Casulli S, Lu S, Nagaoka K, Ji C, Ogawa K, Cao KY, Gao JS, et al. Aspartate beta-hydroxylase promotes cholangiocarcinoma progression by modulating RB1 phosphorylation. Cancer Lett. 2018;429:1–10.
Aihara A, Huang CK, Olsen MJ, Lin Q, Chung W, Tang Q, Dong X, Wands JR. A cell-surface β-hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60(4):1302–13.
Liu Y, Wan HH, Tian DM, Xu XJ, Bi CL, Zhan XY, Huang BH, Xu YS, Yan LP. Development and Characterization of High Efficacy Cell-Penetrating Peptide via Modulation of the Histidine and Arginine Ratio for Gene Therapy. Materials (Basel) 2021, 14(16).
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–51.
Kanehisa MGS. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587–92.
Bedzhov I, Graham SJ, Leung CY, Zernicka-Goetz M. Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo.Philos Trans R Soc Lond B Biol Sci2014, 369(1657).
Ge Y, Fuchs E. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet. 2018;19(5):311–25.
Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–28.
Wang YN, Lee HH, Hsu JL, Yu D, Hung MC. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J Biomed Sci. 2020;27(1):77.
Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549(7673):476–81.
Ressnerova A, Raudenska M, Holubova M, Svobodova M, Polanska H, Babula P, Masarik M, Gumulec J. Zinc and copper homeostasis in Head and Neck Cancer: review and Meta-analysis. Curr Med Chem. 2016;23(13):1304–30.
Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000;59(4):541–52.
Skrajnowska D, Bobrowska-Korczak B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms.Nutrients2019, 11(10).
Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ, et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509(7501):492–6.
Di Serio C, Doria L, Pellerito S, Prudovsky I, Micucci I, Massi D, Landriscina M, Marchionni N, Masotti G, Tarantini F. The release of fibroblast growth factor-1 from melanoma cells requires copper ions and is mediated by phosphatidylinositol 3-kinase/Akt intracellular signaling pathway. Cancer Lett. 2008;267(1):67–74.
Naka K, Ochiai R, Matsubara E, Kondo C, Yang KM, Hoshii T, Araki M, Araki K, Sotomaru Y, Sasaki K, et al. The lysophospholipase D enzyme Gdpd3 is required to maintain chronic myelogenous leukaemia stem cells. Nat Commun. 2020;11(1):4681.
Wei XL, Luo TQ, Li JN, Xue ZC, Wang Y, Zhang Y, Chen YB, Peng C. Development and validation of a Prognostic Classifier based on lipid metabolism-related genes in gastric Cancer. Front Mol Biosci. 2021;8:691143.
Gong Z, Cho YW, Kim JE, Ge K, Chen J. Accumulation of Pax2 transactivation domain interaction protein (PTIP) at sites of DNA breaks via RNF8-dependent pathway is required for cell survival after DNA damage. J Biol Chem. 2009;284(11):7284–93.
Cox E, Hwang W, Uzoma I, Hu J, Guzzo CM, Jeong J, Matunis MJ, Qian J, Zhu H, Blackshaw S. Global analysis of SUMO-Binding proteins identifies SUMOylation as a Key Regulator of the INO80 chromatin remodeling complex. Mol Cell Proteomics. 2017;16(5):812–23.
An N, Zhao Y, Lan H, Zhang M, Yin Y, Yi C. SEZ6L2 knockdown impairs tumour growth by promoting caspase-dependent apoptosis in colorectal cancer. J Cell Mol Med. 2020;24(7):4223–32.
Liu Y, Li RH, Ren G, Jiang J. Suppression of KIF22 Inhibits Cell Proliferation and Xenograft Tumor Growth in Tongue Squamous Cell Carcinoma. Biomed Res Int 2020, 2020:6387545.
Wang L, He M, Fu L, Jin Y. Exosomal release of microRNA-454 by breast cancer cells sustains biological properties of cancer stem cells via the PRRT2/Wnt axis in ovarian cancer. Life Sci. 2020;257:118024.
Tomimaru Y, Mishra S, Safran H, Charpentier KP, Martin W, De Groot AS, Gregory SH, Wands JR. Aspartate-β-hydroxylase induces epitope-specific T cell responses in hepatocellular carcinoma. Vaccine. 2015;33(10):1256–66.
Acknowledgements
We acknowledge the TCGA, GTEx, GEO, and the Human Protein Atlas database for providing its platform and contributors for uploading their meaningful datasets. We also acknowledge Figurer 2D was obtained by KEGG and copyright permission of KEGG was obtained from Kanehisa Laboratories.
Funding
This work was financially supported by the National Natural Science Foundation of China (82072862; 82272949), the Starting Grant from The Seventh Affiliated Hospital Sun Yat-sen University (ZSQYRSF0008), and the Guangdong Provincial Key Laboratory of Digestive Cancer Research (No. 2021B1212040006).
Author information
Authors and Affiliations
Contributions
SQS, MD, JW, and YH designed the study, performed the experiments, and wrote the manuscript; XYC, JQX, YL, HHW, and JW analyzed the data, and performed the experiment; LPY and YSX supervised the study, analyzed the data and revised the manuscript. All authors read and approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Ethics statement
Not applicable.
Consent for publication
Not applicable.
Competing interests
All the authors declare that no conflicts of interest exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, S., Deng, M., Wen, J. et al. Aspartate beta-hydroxylase domain containing 1 as a prognostic marker associated with immune infiltration in skin cutaneous melanoma. BMC Cancer 23, 292 (2023). https://doi.org/10.1186/s12885-023-10625-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10625-8