Abstract
Background
Antiangiogenic drugs have shown initial efficacy in the treatment of advanced thymic carcinomas (TCs); however, data are limited. In this study, we provide real-world data relating to the efficacy of antiangiogenic drugs for the treatment of patients with TCs.
Methods
We retrospectively collected data on clinical progress after first-line chemotherapy in TCs patients who were treated with small molecule antiangiogenic drugs at our institution between January 2010 and December 2021. Tumor response was evaluated according to version 1.1 of the Response Evaluation Criteria in Solid Tumors. Progression free survival and overall survival were calculated using the Kaplan-Meier method.
Results
Of the 17 patients enrolled, 13 (76.5%) received apatinib and four (23.5%) anlotinib monotherapy with an objective response rate of 23.5%. Eleven (64.7%) patients had stable disease. The median follow-up period was 46.0 months (95% confidence interval [CI], 33.0–59.0 months). The median progression survival and overall survival were 7.9 months (95% CI, 6.5–9.3) and 47.0 months (95% CI, 35.4–58.6), respectively. In the 13 patients receiving apatinib, the median PFS was 7.0 months (95% CI, 5.0–9.0), compared with 8.0 months (95% CI, 2.7–13.3 months) for patients in the anlotinib group (P = 0.945). The most common grade 3 adverse events (AEs) were hypertension (n = 3, 23.1%), followed by proteinuria and hand-foot syndrome (HFS, n = 2, 15.4%). There were no grade 4 AEs although eight patients (47.1%) required mid-course discontinuation.
Conclusion
For refractory TCs, small molecule antiangiogenic drugs are efficacious as second- or post-line treatments. The toxicity of antiangiogenic therapy is manageable.
Similar content being viewed by others
Background
Thymic carcinomas (TCs) are aggressive mediastinal malignant tumors with an annual incidence of 1.3–3.2 per million [1, 2]. Patients with early-stage TCs often choose to undergo complete surgical resection and postoperative adjuvant radiotherapy. Paclitaxel combined with carboplatin is the recommended first-line treatment for patients with according to the National Comprehensive Cancer Network guideline with an overall response rate (ORR) of 22–36%. Unfortunately, advanced TCs show a limited response to chemotherapy and patients with TCs lack an effective second-line regimen after disease progression. Chemotherapy has shown a median progress-free survival time (mPFS) of 4.3–7.6 months as a second-line therapy in patients with recurrent TCs according to several prospective phase 2 studies [3,4,5,6]. In recent years, targeted drugs and immune checkpoint inhibitors (ICIs) have made some progress for the treatment of advanced thymic carcinomas. For previously treated TCs patients, the mPFS and ORR of ICIs monotherapy (pembrolizumab and nivolumab) were 3.8–6.1 months and 0–22.5%, respectively [7,8,9]. Clinical studies on second- and posterior treatment options for TCs are numerous, although most are limited to small samples and evidence from randomized controlled trial is lacking. As such, for patients with TCs who have progressed after first-line platinum-containing chemotherapy, standard second- or post-line treatment regimens have not been confirmed.
Over recent years, the efficacy of angiogenesis therapy in advanced non-small cell lung cancer, soft tissue sarcoma, and radioactive iodine-refractory differentiated thyroid cancer has been recognized. Clinical studies of antiangiogenic drugs in the treatment of metastatic TCs are gaining attention. For example, lenvatinib, sunitinib, bevacizumab and regorafenib have all showed some antitumor activity in patients with previously treated TCs [10,11,12,13,14,15]. Lenvatinib is known to exhibit the highest ORR of 38% as the second-line treatment in metastatic TCs patients [10].
Vascular endothelial growth factor receptor-2 (VEGFR2) and platelet-derived growth factor receptor-α (PDGFR-α) are known to be activated and involved in the growth and progression of TCs [16,17,18,19]. However, evidence on the efficacy of anlotinib and apatinib in the treatment of advanced TC is limited. Anlotinib is a newly developed oral small-molecule tyrosine kinase inhibitor (TKI) that targets VEGFR1, VEGFR2/KDR, VEGFR3, c-Kit, PDGFR-α, and the fibroblast growth factor receptor (FGFR1, FGFR2, and FGFR3). Furthermore, anlotinib can inhibit both tumor angiogenesis and tumor cell proliferation [20, 21]. Apatinib exerts its antigenic effects by inhibiting VEGFR-induced proliferation and migration of endothelial cells via the selectively targeting of VEGFR2 [22].
Based on this background, we explored the efficacy and toxicity of small molecule antiangiogenic drugs in previously treated patients with advanced TCs in the real-word.
Methods
Patients
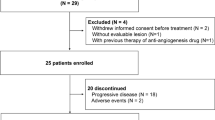
All patient information was obtained from the electronic medical records system of Zhejiang Cancer Hospital (Hangzhou, Zhejiang). Patients with pathologically documented metastatic TCs (stage IVA or IVB defined by the Masaoka-Koga classification) after failure of first-line or multiline chemotherapy who used small molecule antiangiogenic drugs in the next were included in this study from January 2010 to December 2021. The inclusion criteria were as follows: (1) Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, (2) at least one measurable target lesion defined by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and (3) received at least two cycles of small molecule antiangiogenic therapy after progression on a first-line platinum-based chemotherapy. The exclusion criteria were: (1) patients received immunotherapy or other targeted agents other than small molecule antiangiogenic drugs, (2) patients had an active autoimmune disease, and (3) patients had malignant tumors of other sites. Recurrence or metastases were performed evaluated by chest computed tomography (CT) scan, abdominal brain CT, or bone scans.
The study protocol was approved by the Institutional Ethics Committee at Zhejiang Cancer Hospital (No. IRB-2022-62). Individual patient consent was not required for this study.
Responses assessments
Patients received oral apatinib 250–500 mg per day or oral anlotinib 12 mg per day for 2 weeks, with 1 week off; each cycle was 3 weeks in length. Tumor responses were assessed every two cycles or evaluated early when significant signs of progression appeared. Objective tumor responses were according to RECIST 1.1, and include complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Treatment related adverse events (AEs) grade as assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
Data analyses were conducted using SPSS version 25 (SPSS Inc., Chicago, IL, USA) software. T test was used for normally distributed variables such as age between groups. The non-parametric rank sum test was used for continuous variables between groups that were not normally distributed. Other categorical variables were compared using Fisher’s exact Chi-squared test. P < 0.05 was considered significant. Survival analysis was calculated using the Kaplan-Meier method. PFS encompassed the time from the first cycle of antiangiogenic drugs therapy to documented progression or death from any cause. Overall survival (OS) was defined as the time diagnosed with advanced thymic carcinoma to death from any cause or last follow-up. COX regression was used for the univariate analysis of PFS and OS and significant variables (P < 0.05) were included in subsequent multivariate analysis. The median follow-up period was 46.0 months (95% confidence interval [CI], 33.0–59.0 months, adverse Kaplan-Meier estimated) and the last follow-up was the 17th of April 2022.
Results
Patients
A total of 17 patients were enrolled between January 2010 and December 2021, excluding those for whom PFS data were not available. The baseline characteristics of all patients were summarized in Table 1. The median age was 59 years (range, 35–73 years). Ten (58.8%) of the 17 patients were male. All subjects had an ECOG performance status of 0 or 1. Twelve patients had never smoked, and five patients had smoked before or were still smoking. Squamous cell thymic carcinoma was the most common type of pathology, accounting for 58.8% (10/17) of the subjects. Three cases had undifferentiated carcinoma, whereas the specific pathological type of the remaining four cases was unknown. Only one patient had a Masaoka-koga staging of IVA, whereas others were staged at IVB. The most common site of distant metastases was the lungs (76.5%, 13/17), followed by liver and bone (both 35.3%, 6/17). In addition, one (5.9%) patient had adrenal metastasis. Furthermore, of the 17 patients, ten (58.8%) had received one line of systemic chemotherapy at most, whereas seven (41.2%) had received two or more lines of chemotherapy. Thirteen patients (76.5%) were treated with apatinib and another four patients (23.5%) accepted anlotinib treatment.
Response and survival analysis
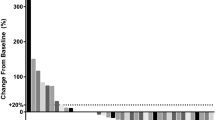
As shown in Table 2, four of the 17 patients had a partial response and 11 had stable disease, with an ORR of 23.5% and a disease control rate (DCR) of 88.2%. In the total population, the mPFS was 7.9 months (95% CI, 6.5–9.3 months) and the median OS (mOS) was 47.0 months (95% CI, 35.4–58.6 months) (Fig. 1). Of the patients who received apatinib treatment, four patients (4/13, 30.8%) achieved a partial response, seven patients (53.8%) had stable disease, and two patients (15.4%) showed disease progression. However, none of the patients who received anlotinib had an objective response, probably because of the small sample size. All patients (4/4,1005.0%) had stable disease in the anlotinib group. ORR did not differ significantly between subgroups (P = 0.519).
Figure 2 shows the time of discontinuation for apatinib and anlotinib. There were two patients (one apatinib and one anlotinib) still receiving treatment at the data cut-off point. The mPFS in the apatinib and alotinib groups were 7.0 months (95% CI, 5.0–9.0 months) and 8.0 months (95% CI, 2.7–13.3 months), respectively (P = 0.945). The mOS for patients receiving apatinib was 47.0 (95% CI, 43.7–50.2) months, whereas mOS for the group receiving anlotinib could not be determined due to the small sample size (P = 0.076). There was no significant difference in the therapeutic efficacy when compared between the two drugs. Eight patients were still alive at the data cut-off date (17th of April 2022). In the univariate COX regression analysis of PFS, only performance status score (0 vs. 1) was statistically significant (P = 0.026; HR = 4.531; 95% CI, 1.20–7.16) (see Supplementary Table 1).
In addition, all patients had previously received paclitaxel combined with platinum (paclitaxel plus carboplatin, paclitaxel plus cisplatin, or docetaxel plus cisplatin) as the first-line chemotherapy regimen. In total, 11 of the 17 (64.7%) patients received the paclitaxel plus cisplatin regimen, 4/17 (23.5%) patients received the paclitaxel plus cisplatin regimen and one patient (5.9%) received the docetaxel plus cisplatin regimen, respectively. Sixteen patients were evaluated. Of these, five patients had a partial response, ten patients had stable disease, and one patient showed disease progression. The mPFS for first-line platinum chemotherapy was 7.1 months (95% CI, 5.1–9.2 months).
Toxicity
The AEs observed in all patients at any grade was summarized in Table 3. There were four patients which could not be assessed due to incomplete information. The most common grade 1–2 AEs were hand-foot syndrome (HFS), blood toxicity, and proteinuria (both 5/13, 38.5%). The most common grade 3 AEs was hypertension (3/13, 23.1%), followed by HFS and blood toxicity (both 2/13, 15.4%). No grade 4 or 5 toxicities occurred. However, in the total population, eight patients (47.1%) required suspension of the drug during treatment due to intolerable toxic reactions. Fortunately, eventually all patients who needed to stop treatment resumed treatment. No patients died due to toxic reactions.
Discussion
To the best of our knowledge, this is the first real-world retrospective study of small molecule antiangiogenic drugs for previously treated thymic carcinoma in China. This research involved the largest sample size of small molecule antiangiogenic drugs for advanced TCs. Our analysis showed that apatinib and anlotinib exhibited considerable antitumor activity and durable responses in advanced TCs.
We summarized the phase II studies of small molecule antiangiogenic drugs in advanced thymic carcinomas (Table 4). Four phase II studies have explored the efficacy of antiangiogenic drugs (lenvatinib, sunitinib, apatinib, and regorafenib) for the treatment of advanced TCs. The National Comprehensive Cancer Network (NCCN) Guidelines Version 2.2022 Thymomas and Thymic Carcinomas recommended sunitinib with an ORR of 26.0% as the second-line treatment regimen for advanced TCs [11]. Another phase II trial of lenvatinib for 42 patients with advanced TCs, reported the best ORR of 38.0% (95% CI, 25.6–52.0, p < 0.0001), a mPFS of 9.3 (95% CI, 7.7–13.9) months, and a DCR of 95.0%. Similarly, the most common grade 3 AEs were hypertension (64.0%) and hand-foot syndrome (7.0%). Song et al. [23] previously evaluated the efficacy of apatinib in the treatment of advanced TCs, with an ORR, DCR, mPFS and mOS of 20.0 and 73.0%, 6.1 months (95%CI, 2.6–9.6 months) and 24.0 months (95%CI, 16.1–not evaluable (NE) months), respectively. Another phase II study [15] explored the efficacy of regorafenib monotherapy in eight patients with TCs as the second-line regimen. These authors reported a mPFS of 9.2 months (95% CI, 0.9–34.0 months) and a mOS of 20.1 months (95% CI, 0.9–NE) months, although the ORR was 0.0% according to the RECIST criteria.
In addition, Yudong et al. [24] (2018) reported a case of advanced thymic squamous cell carcinoma with EGFR exon 20 insertion that was effectively treated with apatinib after the failure of multiple lines of chemotherapy. The patient had received a partial response after 5 months of treatment and the PFS was 10.0 months. Similarly, a patient with advanced thymic squamous carcinoma with EGFR 20 exon insertion was successfully treated with apatinib and anlotinib after failure of previous multiline therapy [25]. This study showed that after reaching a PFS of 13.0 months with apatinib treatment, the patient was switched to anlotinib due to adverse effects. The PFS of anlotinib was 23.0 months and the OS was 6 years. After treatment with apatinib failed, anlotinib was able to control the patient’s mediastinal mass and all adverse effects were tolerated. He et al. [26] (2018) also reported a case of partial response following daily treatments with apatinib for advanced TC; the PFS was 6.3 months and drug-related toxicities were tolerable.
In contrast with previous studies, our present research included two small molecule TKI drugs for the treatment of TCs. Certainly, the ORR of this study was consistent with that of the four phase II studies. We observed a mOS of 47.0 (95%CI, 35.4–58.6 months) after a median follow-up of 46 months and there were eight (47.1%) surviving patients at the last follow-up date. In addition, we did not identify any significant difference in terms of efficacy between anlotinib and apatinib. Head-to-head studies of anlotinib and apatinib in advanced thymic carcinomas are now expected.
VEGF is involved in the development of normal blood vessel networks in the thymus [16]. Several previous studies have shown that VEGFR1 and VEGFR2 are highly expressed in thymic carcinomas. Angiopoietin 1 and VEGF are known to be the most up-regulated growth factors in TCs [17, 18, 27].
Our real-world data also reported the efficacy of antiangiogenic drugs for the treatment of advanced TCs. Consequently, small molecule angiogenesis therapy may represent a new treatment option for recurrent or metastatic advanced thymic carcinoma patients. TKIs have been observed to alter the tumor microenvironment by inducing immune microenvironment remodeling [28, 29]. Small molecule antiangiogenic agents combined with ICIs have shown good efficacy for a variety of solid tumors [30, 31]. Thus, novel combinations of small molecule antiangiogenic drugs with ICIs in patient with recurrent TCs need to be explored further. For example, a clinical trial of pembrolizumab in combination with sunitinib for advanced TCs (NCT03463460) is ongoing.
There are some limitations to our study that need to be considered. Firstly, this was a single-center, retrospective study and therefore retrospective nature of this study could have influenced some of the results. Second, only a limited number of cases were included in our study due to the fact that thymic tumors are relatively rare. Finally, this study was also limited by the small sample size in subgroup analysis.
Conclusion
In the real world, antiangiogenic drugs have shown some efficacy in advanced TCs. The mode of combination small molecule antiangiogenic drugs with chemotherapy or immunologic agents needs to be further explored in the future.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding authors on reasonable request.
Abbreviations
- TCs:
-
Thymic carcinomas
- WHO:
-
World Health Organization
- NCCN:
-
National Comprehensive Cancer Network
- ECOG:
-
Eastern Cooperative Oncology Group
- HFS:
-
Hand-foot syndrome
- VEGFR2:
-
Vascular endothelial growth factor receptor-2
- PDGFR-α:
-
Platelet-derived growth factor receptor-α
- TKI:
-
Tyrosine kinase inhibitor
- FGFR1:
-
Fibroblast growth factor receptor
- ICIs:
-
Immune checkpoint inhibitors
- mPFS:
-
median progression-free survival
- mOS:
-
median overall survival
- CI:
-
Confidence interval
- AEs:
-
Adverse events
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- DCR:
-
Disease control rate
- ORR:
-
Objective response rate
- HR:
-
Hazard ratio
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- CTCAE:
-
Common Terminology Criteria for Adverse Events
References
Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5:S260–5.
de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. 2008;44:123–30.
Inoue A, Sugawara S, Harada M, et al. Phase II study of Amrubicin combined with carboplatin for thymic carcinoma and invasive thymoma: North Japan lung Cancer group study 0803. J Thorac Oncol. 2014;9:1805–9.
Hellyer JA, Gubens MA, Cunanan KM, et al. Phase II trial of single agent amrubicin in patients with previously treated advanced thymic malignancies. Lung Cancer. 2019;137:71–5.
Okuma Y, Goto Y, Ohyanagi F, et al. Phase II trial of S-1 treatment as palliative-intent chemotherapy for previously treated advanced thymic carcinoma. Cancer Med. 2020;9:7418–27.
Tsukita Y, Inoue A, Sugawara S, et al. Phase II study of S-1 in patients with previously-treated invasive thymoma and thymic carcinoma: North Japan lung cancer study group trial 1203. Lung Cancer. 2020;139:89–93.
Cho J, Kim HS, Ku BM, et al. Pembrolizumab for patients with refractory or relapsed Thymic epithelial tumor: an open-label phase II trial. J Clin Oncol. 2019;37:2162–70.
Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-Centre, phase 2 study. Lancet Oncol. 2018;19:347–55.
Katsuya Y, Horinouchi H, Seto T, et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer. 2019;113:78–86.
Sato J, Satouchi M, Itoh S, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol. 2020;21:843–50.
Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 2015;16:177–86.
Remon J, Girard N, Mazieres J, et al. Sunitinib in patients with advanced thymic malignancies: cohort from the French RYTHMIC network. Lung Cancer. 2016;97:99–104.
Bedano PM, Perkins S, Burns M, et al. A phase II trial of erlotinib plus bevacizumab in patients with recurrent thymoma or thymic carcinoma. J Clin Oncol. 2008;26:753–4.
Perrino M, Bozzarelli S, et al. A phase II study of regorafenib in patients with thymic epithelial tumours previously treated with chemotherapy. J Clin Oncol. 2018;36:8579.
Perrino M, De Pas T, Bozzarelli S, et al. Resound trial: a phase 2 study of regorafenib in patients with thymoma (type B2-B3) and thymic carcinoma previously treated with chemotherapy. Cancer. 2022;128:719–26.
Cimpean AM, Raica M, Encica S, et al. Immunohistochemical expression of vascular endothelial growth factor a (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann Anat. 2008;190:238–45.
Sasaki H, Yukiue H, Kobayashi Y, et al. Elevated serum vascular endothelial growth factor and basic fibroblast growth factor levels in patients with thymic epithelial neoplasms. Surg Today. 2001;31:1038–40.
Lattanzio R, La Sorda R, Facciolo F, et al. Thymic epithelial tumors express vascular endothelial growth factors and their receptors as potential targets of antiangiogenic therapy: a tissue micro array-based multicenter study. Lung Cancer. 2014;85:191–6.
Meister M, Kahl P, Muley T, et al. Expression and mutational status of PDGFR in thymic tumours. Anticancer Res. 2009;29:4057–61.
Taurin S, Yang CH, Reyes M, et al. Endometrial cancers harboring mutated fibroblast growth factor receptor 2 protein are successfully treated with a new small tyrosine kinase inhibitor in an Orthotopic mouse model. Int J Gynecol Cancer. 2018;28:152–60.
Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109:1207–19.
Fathi Maroufi N, Rashidi MR, Vahedian V, et al. Therapeutic potentials of Apatinib in cancer treatment: possible mechanisms and clinical relevance. Life Sci. 2020;241:117106.
Song Z, Lou G, Wang Y, et al. Apatinib in patients with recurrent or metastatic thymic epithelial tumor: a single-arm, multicenter, open-label, phase II trial. BMC Med. 2022;20:154.
Yudong S, Zhaoting M, Xinyue W, et al. EGFR exon 20 insertion mutation in advanced thymic squamous cell carcinoma: response to apatinib and clinical outcomes. Thorac Cancer. 2018;9:885–91.
Zuo R, Zhang C, Lin L, et al. Durable efficacy of anlotinib in a patient with advanced thymic squamous cell carcinoma after multiline chemotherapy and apatinib: a case report and literature review. Thorac Cancer. 2020;11:3383–7.
He Y, Liu S, Wang C, Shi M, Liu G, et al. Apatinib treatment in extensive metastatic advanced thymic carcinoma. J Biol Regul Homeost Agents. 2018;32:693–7.
Tomita M, Matsuzaki Y, Edagawa M, et al. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J Thorac Cardiovasc Surg. 2002;124:493–8.
Fang Y, Wang Y, Zeng D, et al. Comprehensive analyses reveal TKI-induced remodeling of the tumor immune microenvironment in EGFR/ALK-positive non-small-cell lung cancer. Oncoimmunology. 2021;10:1951019.
Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40.
Gao G, Zhao J, Ren S, et al. Efficacy and safety of camrelizumab plus apatinib as second-line treatment for advanced squamous non-small cell lung cancer. Ann Transl Med. 2022;10:441.
Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1057–65.
Acknowledgements
The author would like to thank Mr. Zeguang Zhou for his encouragement to the medical career of Yelan Guan.
Funding
This study was supported by the Zhejiang Provincial Science and Technology Department Fund Project (No. LGF21H160006).
Author information
Authors and Affiliations
Contributions
YL.G. and XD.G. participated in the design of the study, performed the statistical analysis, and wrote the main text. JF.S. and J.X. assisted in data collection. JW.W. and Y.H. prepared the tables and figs. Y.S. and WX.W. are responsible for the integrity of the data and the accuracy of the data analysis. YL.G. and XD.G. contributed equally. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Ethics Committee of Zhejiang Cancer Hospital (approval number: IRB-2022-62). Due to the retrospective study design, the ethics committee of Ethics Committee of Zhejiang Cancer Hospital approved a waiver of written informed consent. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Univariate COX regression analysis of PFS and OS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guan, Y., Gu, X., Si, J. et al. The efficacy of small molecule anti-angiogenic drugs in previously treated Thymic carcinoma. BMC Cancer 23, 16 (2023). https://doi.org/10.1186/s12885-022-10448-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10448-z