Abstract
Background
Neoadjuvant chemotherapy (NAC) before radical cystectomy is associated with pathological downstaging (DS) and improved overall survival (OS) in patients with muscle-invasive bladder cancer (MIBC). Population-based studies have not unequivocally shown improved survival. The aim of this population-based study was to evaluate the effect of NAC on DS and OS in Norwegian patients with MIBC.
Methods
Patients in the Cancer Registry of Norway undergoing radical cystectomy (2008–2015) with or without NAC diagnosed with MIBC between 2008 and 2012 were included. Follow-up data were available until 31 December 2019. Logistic regression estimated the odds of DS with NAC, and a Cox model investigated the effect of DS on OS. Cox models, a mediator analysis and an instrumental variable approach were used to investigate the effect of NAC on OS.
Results
A total of 575 patients were included. NAC was administered to 82 (14%) patients. Compared to cystectomy only, NAC increased the proportion (43% vs. 22%) and the odds of DS (OR 2.51, CI 1.37–4.60, p = 0.003). Independent of NAC, the proportion of pN0 was higher in patients with DS (89% vs. 60%) and DS yielded a 78% mortality risk reduction (HR 0.22, CI 0.15–0.34, p = 1.9∙10–12), compared to patients without DS. We did not find an association between NAC and OS, neither by Cox regression (HR 1.16, CI 0.80–1.68, p = 0.417) nor by an instrumental variable approach (HR = 0.56, CI = 0.07–4.57, p = 0.586). The mediation analysis (p = 0.026) confirmed an indirect effect of NAC on OS through DS. Limitations include limited information of the primary tumour, details of NAC treatment and treatment indications.
Conclusions
NAC increases the probability of DS and is indirectly associated to OS. DS is related to the absence of regional lymph node metastases and is associated with an OS benefit. Improved staging and biomarkers are needed to identify patients most likely to achieve DS and to benefit from NAC.
Similar content being viewed by others
Background
In Europe [1] and in the USA [2], cisplatin-containing neoadjuvant chemotherapy (NAC) before radical cystectomy (RC) is recommended for patients with localized (T2-T4a, cN0, M0) muscle-invasive bladder cancer (MIBC) fit for cisplatin treatment. The European Association of Urology recommended NAC for MIBC in the 2008 guidelines [1] after several randomized controlled trials (RCT) [3,4,5,6] and meta-analyses [7, 8] had demonstrated a beneficial effect of NAC in MIBC. The survival benefit of NAC found in RCTs has been shown to be associated with pathologic downstaging (DS) of the primary tumour in the RC-specimen [6, 9, 10].
Meta-analyses based on results from RCTs have shown an absolute five-year overall survival (OS) benefit of 6–8% favouring NAC over RC alone [7, 8, 11]. Results from population-based studies have been inconclusive. Some authors did not find an association between NAC and improved survival [12, 13], while others did show a survival benefit for NAC relative to RC alone [14]. With this background, more data and analyses are warranted to establish the beneficial effect of NAC on a population-based level. Therefore, we aimed to describe the clinical characteristics of an unselected population of Norwegian patients with MIBC treated with NAC and RC (NAC group) vs. RC only (NoNAC group) and to evaluate the association between NAC and DS, the effect of DS on OS and the overall association between NAC and OS.
Methods
Material
The Cancer Registry of Norway (CRN) captures nearly 99% of new cancer diagnoses in Norway [15]. The collected data includes patient demographics, tumour characteristics, treatment codes (surgical, radiotherapy) and causes of death. For bladder cancer, histopathology of specimens from transurethral resection of bladder tumour (TURB), cystectomy and biopsies of metastases are registered, along with the corresponding dates for the procedures.
The Norwegian Patient Registry contains individual administrative, demographic, and coded medical information (diagnoses, procedures, chemotherapy) from all patients’ contacts with public hospitals. This data was linked to the CRN by the personal identification number assigned to all residents of Norway [16].
Study population
We included patients undergoing RC (2008–2015) with or without NAC who were diagnosed with MIBC (urothelial carcinoma) without known distant metastases between 2008 and 2012. The pre-RC status of regional lymph node metastases was unknown (cNx). Patients with a pre-RC histology verifying muscle-invasion and patients without such verification but treated with NAC were considered as having MIBC. We chose this period since we had quality ensured histopathological information from this period and to ensure sufficient follow-up time for survival analysis. Patients with pre-RC radiotherapy were excluded.
Measures
Muscle invasion
For the evaluable patients, the research team reviewed all available clinical notifications and histology reports at the CRN. The presence of MIBC was confirmed in the histology reports from TURB specimens. Muscle-invasion was defined as tumour invasion into the muscularis propria (≥T2). From the histology reports from cystectomy specimens, the histopathological T and N category (pT; pN) [17] without sub-classification into a and b for pT2-pT4 were confirmed. All MIBC are high-grade [18].
Neoadjuvant chemotherapy
We identified relevant specified intravenous chemotherapy codes (e.g., gemcitabine and cisplatin, methotrexate, vinblastine, doxorubicin and cisplatin (MVAC), carboplatin) and codes for intravenous administration of non-specified chemotherapy from the Norwegian Patient Registry. We excluded chemotherapy events concurrently registered with ICD-10 codes for a different cancer than C67. NAC was defined as any chemotherapy administered intravenously between diagnosis of bladder cancer and RC.
Downstaging
Based on the available data and definitions used in similar studies [12, 14, 19], we defined downstaging of the primary tumour (DS) as pT0/pTa/pTis/pT1 with the subunit of pT0 as complete response (CR), identified independent of the use of NAC. Patients without DS (non-DS) were characterized by having residual muscle-invasive disease (pT2-pT4) in the specimen. Downstaging can occur after TURB and NAC. Nodal downstaging could not be assessed because information about cN was not available.
Statistical analyses
The observation time started at the date of RC until death, emigration, or end of study (December 31st, 2019), whichever came first. Time in years from date of RC was used as timescale in all analyses.
We applied descriptive statistics (mean, median, interquartile range (IQR), proportions) to present pre- and post-operative characteristics in all patients as well as in the NAC and NoNAC group. The association between NAC and DS was estimated using logistic regression adjusted for all available pre-operative variables: age at diagnosis (≤59, 60–69, 70–79, ≥80), sex, type of hospital (academic vs. community), geographical health region (Southeast, West, Central, North) and the year of RC (2008–2009, 2010–2011, 2012–2015). OS was presented by Kaplan-Meier curves and the difference between them was evaluated with the log-rank test. The associations between DS with OS, as well as NAC with OS (total effect) were assessed with a Cox regression model adjusted for all available pre-operative variables.
The association between NAC and OS was additionally investigated by applying a mediation approach adjusted for available pre-operative variables. We applied a causal inference approach [20, 21] implemented in the R mediation package [22]. The total effect of NAC on OS (unadjusted for DS) evaluated with a Cox regression model was decomposed into two parts [23]: the indirect effect between NAC and OS mediated by DS, and the direct effect between NAC and OS (not through DS) This approach allowed us to assess the indirect effect of NAC on OS through DS.
In order to overcome the confounding by indication bias induced by missing information of factors leading to the decision of treatment, we applied an instrumental variable approach to estimate the causal effect of NAC on OS [24]. We used type of hospital as the instrumental variable and G-estimation [24,25,26] with adjustment for the remaining pre-operative variables.
Quantities reported from the model-based analyses are odds ratios (ORs) and hazard ratios (HRs) including 95% confidence intervals (CI) and p-values. The statistical significance level was set at 0.05. Statistical analyses were performed using Stata 17 (StataCorp, College Station, TX) and R (version 4.1.4).
Results
Patient characteristics
Between 2008 and 12, 5521 patients were diagnosed with first-time diagnosis of bladder cancer (urothelial carcinoma) and 917 of these patients underwent RC by the end of 2015. After exclusions, 575 patients were finally evaluable in our study (Supplementary Fig. S1): 82 (14%) patients in the NAC group and 493 (86%) patients in the NoNAC group. In the NAC group, 23 (28%) patients received gemcitabine and cisplatin, 10 (12%) patients MVAC and 1 (1%) patient Carboplatin. For 48 (59%) patients, the type of chemotherapy was unknown. The median follow-up time was 3.9 years.
The median age at diagnosis for the evaluable patients was 69 (IQR: 62,75) years and 124 (22%) of the patients were female (Table 1). Patients in the NAC group were younger (median 65 vs. 70 years), more frequently female (29% vs 20%) and more likely operated in an academic hospital (76% vs 61%) compared to the NoNAC group. Median time from the most recent TURB to cystectomy was 48 days for the patients undergoing cystectomy only, and 109 days for patients treated with NAC. The proportion of patients treated with NAC was increasing over time, with the largest proportions of patients (70%) treated between 2012 and 2015. Among the 82 patients in the NAC group, 47 (57%) patients died, compared to 301 (61%) patients out of 493 patients in the NoNAC group. The proportion of deaths due to other causes was larger in the NoNAC group (20% vs 12%) compared to the NAC group.
Out of 575 patients, pT was recorded in 514 (89%) patients and thus evaluable for DS, and pN was recorded for 433 (75%) of patients (Table 2). The proportions of pT3 (47% vs 27%) and pN+ (35% vs 25%) in the NoNAC group were larger compared to the NAC group, while the proportion of CR (9% vs 24%) was smaller. Out of 29 patients with DS in the NAC group, 16 (55%) patients had CR, whilst 38 (40%) out of 96 patients with DS in the NoNAC group had CR.
Out of 514 patients evaluable for DS, pN was recorded for 427(83%) patients (Supplementary table S1). The proportion of patients with pN0 among patients with DS (89% vs 60%) was larger compared to patients with non-DS without difference between patients treated with and without NAC (92% vs 88%).
Neoadjuvant chemotherapy and downstaging
Compared to patients in the NoNAC group, a larger proportion of patients achieved DS (43% vs. 22%) in the NAC group (Fig. 1). NAC significantly increased the odds for DS (OR 2.51, CI 1.37–4.60, p = 0.003) compared to NoNAC (Supplementary table S2).
Downstaging and overall survival
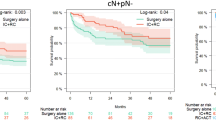
For patients with DS, the crude five-year OS was larger compared to patients with non-DS (80% vs. 38%, p < 0.001) (Fig. 2 a). The adjusted survival analysis revealed a 78% risk reduction of all-cause death (HR 0.22, CI 0.15–0.34, p = 1.9∙10− 12) in patients with DS compared to patients with non-DS (Supplementary table S2).
Kaplan-Meier curves of overall survival (OS). a OS in patients with downstaging (DS: ≤pT1) compared to patients without (non-DS) (n = 514) b OS in patients treated with neoadjuvant chemotherapy (NAC) compared to patients treated with cystectomy only (NoNAC) (n = 575); Cox regression results (hazard ratio HR, 95% confidence intervals CI and p-value)
Neoadjuvant chemotherapy and overall survival
The crude five-year OS for all patients (n = 575) was 47%: NAC 50% vs. NoNAC 47% (Fig. 2 b). NAC was not significantly associated with OS in the crude analysis (p = 0.552), in the Cox analysis (HR 1.16, CI 0.80–1.68, p = 0.417) nor when we applied the instrumental variable approach (HR 0.56, CI 0.07–4.57, p = 0.586) (Supplementary table S3).
The mediation analysis confirmed the above results by revealing an indirect effect of NAC on OS through DS (p = 0.026), but no total or direct effect of NAC on OS (Fig. 3, Supplementary table S4).
Summary of results. Summary of the results; logistic regression (1), Cox regression (2-3) and mediation analysis (4–5). (1) Neoadjuvant chemotherapy (NAC) is significantly associated with pathological downstaging (DS: ≤pT1), (2) DS is significantly associated with overall survival (OS), (3) NAC is not significantly associated with OS (unadjusted for DS) (Total effect), (4) NAC is not significantly associated with OS (not through DS) (Direct effect), (5) NAC is significantly associated with OS through DS (Indirect effect) (red arrow: significant; the thicker, the more significant; blue arrow: not significant). *All analyses were adjusted for age, sex, type of hospital, health region and cystectomy year
Discussion
In this population-based study, NAC increased the probability of achieving DS in patients with MIBC by a factor of 2.5. Independent of the means for obtaining DS (NAC or TURB), achievement of DS in MIBC patients was associated with a 78% risk reduction of all-cause mortality compared to non-DS and related to a decreased proportion of patients with regional node lymph node metastases verified in the RC specimen. NAC did not provide a beneficial survival over NoNAC, except for when the effect of NAC on OS went through DS.
Post-NAC DS was found in 43% of the patients in our study. In comparison, post-NAC DS was reported in 36% of patients in a US population-based study [14], in 51% of the patients in a large single-institution registry study [27] and in 61% in a Danish population-based study [19]. In two Nordic RCTs post-NAC DS was reported in 38% of the patients receiving cisplatin+doxyrubicin/methotrexate [9]. For the more modern chemotherapeutic regimens, the proportion of post-NAC DS was higher (Gemcitabine and cisplatin: 49%, dose dense MVAC: 63%) [28]. Different study designs have used different definition of DS. In our and other relevant population-based studies [12, 14, 19], DS was defined as downstaging of the primary tumour(<pT2) and independent of pN-status [12, 14, 19], whereas in selected clinical trials pN0 was included in the definition (<pT2pN0) [9, 28]. Notably DS can be the effect of NAC but can also be achieved after an extensive TURB.
We show that the proportions of pN0 was higher in patients with DS compared to patients with non-DS, although without any difference in downstaged patients treated with or without NAC. These results are in line with the corresponding combined results from two previous clinical trials [9]. Further, the demonstration of DS independent of the receipt of NAC revealed a beneficial survival, as patients with DS had a 78% risk reduction of all-cause death compared to patients without DS. These results indicate that independent of NAC, DS is related to the absence of regional lymph node metastases and indicates a more favourable prognosis compared to patients without DS. However, NAC significantly increased the odds of DS and possibly reflect the favourable effect of NAC on regional lymph node metastases and micrometastases.
Our findings of no survival benefit in the NAC group vs. NoNAC group is in agreement with the results from two other population-based studies from the US [12] and Sweden [13]. Despite efforts to account for selection bias and unrecognized confounders with statistical methods like propensity score weighting or the instrumental variable approach in our study, no OS benefit for NAC over NoNAC was found. However, we are the first to identify an indirect effect of NAC on survival through the demonstration of DS as we show that NAC has an effect on OS mediated by DS. We suggest the following explanations: The patients in the NAC group initially may have had a more advanced and aggressive disease compared to the patients in the NoNAC group, reducing the potential survival advantage gained by post-NAC DS when evaluating the total effect of NAC on OS. On the other hand, the population may consist of subgroups of patients who do not benefit from NAC, as the selection of patients treated with NAC in the real-world is most probably different from clinical trials [29]. Notably, in other population-based studies the proportions of cT2N0M0 (82–86%) [12, 13] were larger than in RCTs (34–40%) [5, 6, 30]. For this subgroup, RCTs have either not evaluated the mortality risk after NAC [5, 6] or found no survival benefit from NAC [30], and in two population-based studies no survival benefit over NoNAC was found [31, 32].
Our findings underline the necessity to determine which MIBC patients benefit from NAC in clinical practice. Identification of subgroups of patients most likely to achieve DS with or without NAC is necessary. The latter are of particular interest as they are possible candidates for bladder preserving strategies. Clinical staging by computed tomography is challenging with an estimated accuracy of 40–92% to predict pT and of 54–86% to predict pN [33]. Advances in image-guided approaches with multiparametric magnetic resonance imaging may reduce staging errors in the management of MIBC and aid in predicting treatment response to NAC [34]. Reliable biomarkers for chemotherapy sensitivity are needed.
Limitations of our study include the lack of pre-RC information about cT- and cN category and limited information about the primary tumour (lack of size, multiplicity, and widespread carcinoma in situ). To our knowledge, only cisplatin-based NAC was used in Norway in the study period. Although application details of NAC were not available to us, the results reflect the real-world situation where dosage reduction and uncompleted cycles often are necessary. We do not know why some patients received NAC and others did not (confounding by indication). For this reason, we applied the instrumental variable approach, although limited by a suboptimal instrumental variable and limited power. However, we had solid information on pT, and we were the first population-based study to apply a mediator analysis and identify an indirect effect of NAC on survival through DS.
Conclusion
In this nationwide population-based study of patients with MIBC, we found that on a population-based level DS demonstrated in the RC specimen is a good prognostic factor and provides a survival benefit over non-DS. NAC increases the odds of DS and is indirectly associated with an OS benefit. DS is related to absence of regional lymph nodes. Future perspectives include improvement of clinical staging, identification of patient subgroups most likely to achieve DS or non-DS, and identification of patients in whom NAC is necessary to achieve DS.
Availability of data and materials
The data that support the findings of this study are available from the Cancer Registry of Norway, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Cancer Registry of Norway.
Abbreviations
- CI:
-
Confidence interval
- CRN:
-
Cancer Registry of Norway
- DS:
-
Pathological downstaging of the primary tumour
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- NAC:
-
Neoadjuvant chemotherapy
- Non-DS:
-
Residual muscle-invasive bladder cancer
- MIBC:
-
Muscle-invasive bladder cancer
- MVAC:
-
Methotrexate, vinblastine, doxorubicin and cisplatin
- OR:
-
Odds ratio
- OS:
-
Overall survival
- RC:
-
Radical cystectomy
- RCT:
-
Randomized controlled trial
- TURB:
-
Transurethral resection of bladder tumour
References
J.A. Witjes HMB, R. Cathomas, E. Compérat, N.C. Cowan, J.A. Efstathiou, R. Fietkau, G. Gakis, V. Hernández, A. Lorch, M.I. Milowsky, M.J. Ribal , G. N Thalmann, A.G. van der Heijden, E. Veskimäe,E. Linares Espinós, M. Rouanne, Y. Neuzillet; members of the EAU guidelines panel for Muscle-invasive and Metastatic bladder cancer (MIBC). The EAU Guidelines on Muscle-invasive and Metastatic bladder cancer 2022 [Available from: https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/.
Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder Cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198(3):552–9. https://doi.org/10.1016/j.juro.2017.04.086.
Rintala E, Hannisdahl E, Fosså SD, Hellsten S, Sander S. Neoadjuvant chemotherapy in bladder cancer: a randomized study. Nordic cystectomy trial I. Scand J Urol Nephrol. 1993;27(3):355–62. https://doi.org/10.3109/00365599309180447.
Sherif A, Rintala E, Mestad O, Nilsson J, Holmberg L, Nilsson S, et al. Neoadjuvant cisplatin-methotrexate chemotherapy for invasive bladder Cancer - Nordic cystectomy trial 2. Scand J Urol Nephrol. 2002;36(6):419–25. https://doi.org/10.1080/003655902762467567.
International collaboration of trialists on behalf of the Medical Research Council Advanced Bladder Cancer Working PartyEORTC Genito-Urinary GroupAustralian Bladder Cancer Study GroupNational Cancer Institute of Canada Clinical Trials GroupFinnbladder, Norwegian Bladder Cancer Study GroupClub Urologico Espanol de Tratamiento Oncologico (CUETO) group. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. Lancet. 1999;354(9178):533–40.
Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder Cancer. N Engl J Med. 2003;349(9):859–66. https://doi.org/10.1056/NEJMoa022148.
Winquist E, Kirchner TS, Segal R, Chin J, Lukka H. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol. 2004;171(2 Pt 1):561–9. https://doi.org/10.1097/01.ju.0000090967.08622.33.
Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–5; discussion 5-6;. https://doi.org/10.1016/j.eururo.2005.04.006.
Rosenblatt R, Sherif A, Rintala E, Wahlqvist R, Ullen A, Nilsson S, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61(6):1229–38. https://doi.org/10.1016/j.eururo.2011.12.010.
Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after Neoadjuvant chemotherapy in bladder Cancer treated with cystectomy: a Meta-analysis. Eur Urol. 2014;65(2):350–7. https://doi.org/10.1016/j.eururo.2013.06.049.
Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. Neoadjuvant chemotherapy for muscle-invasive bladder Cancer: a systematic review and two-step Meta-analysis. Oncologist. 2016;21(6):708–15. https://doi.org/10.1634/theoncologist.2015-0440.
Hanna N, Trinh QD, Seisen T, Vetterlein MW, Sammon J, Preston MA, et al. Effectiveness of Neoadjuvant chemotherapy for muscle-invasive bladder Cancer in the current real world setting in the USA. Eur Urol Oncol. 2018;1(1):83–90. https://doi.org/10.1016/j.euo.2018.03.001.
Russell B, Sherif A, Häggström C, Josephs D, Kumar P, Malmström P-U, et al. Neoadjuvant chemotherapy for muscle invasive bladder cancer: a nationwide investigation on survival. Scand J Urol. 2019;53(4):206–12. https://doi.org/10.1080/21681805.2019.1624611.
Pfail JL, Audenet F, Martini A, Tomer N, Paranjpe I, Daza J, et al. Survival of patients with muscle-invasive Urothelial Cancer of the bladder with residual disease at time of cystectomy: a comparative survival analysis of treatment modalities in the National Cancer Database. Bladder Cancer. 2020;6:265–76. https://doi.org/10.3233/BLC-200303.
Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, et al. Data quality at the Cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–31. https://doi.org/10.1016/j.ejca.2008.10.037.
Bakken IJ, Ariansen AMS, Knudsen GP, Johansen KI, Vollset SE. The Norwegian patient registry and the Norwegian registry for primary health care: research potential of two nationwide health-care registries. Scand J Public Health. 2019;48(1):49–55. https://doi.org/10.1177/1403494819859737.
Sobin L, Wittekind C, editors. International Union against Cancer (UICC): TNM classification of malignant Tumours. 6th ed. New York: Wiley; 2002.
Jimenez RE, Gheiler E, Oskanian P, Tiguert R, Sakr W, Wood DP Jr, et al. Grading the invasive component of Urothelial carcinoma of the bladder and its relationship with progression-free survival. Am J Surg Pathol. 2000;24(7) https://journals.lww.com/ajsp/Fulltext/2000/07000/Grading_the_Invasive_Component_of_Urothelial.9.aspx.
Körner SK, Jensen JB. A population-based retrospective analysis on variation in use of neoadjuvant chemotherapy depending on comorbidity in patients with muscle-invasive bladder cancer undergoing cystectomy in Denmark in the period 2013-2019. Scand J Urol. 2022;56(1):34–8. https://doi.org/10.1080/21681805.2021.2002400.
Imai K, Keele LJ, Yamamoto T. Identification, inference and sensitivity analysis for causal mediation effects. Stat Sci. 2010;25:51–71.
Imai K, Tingley D, Yamamoto T. Experimental designs for identifying causal mechanisms. J Royal Stat Soc. 2013;176(1):5–51. https://doi.org/10.1111/j.1467-985X.2012.01032.x.
Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1–38. https://doi.org/10.18637/jss.v059.i05.
Agler R, De Boeck P. On the interpretation and use of mediation: multiple perspectives on mediation analysis. Front Psychol. 2017;8:1984. https://doi.org/10.3389/fpsyg.2017.01984.
Sjolander A, Martinussen T. Instrumental variable estimation with the R package ivtools. Epidemiologic. Methods. 2019;8(1). https://doi.org/10.1515/em-2018-0024.
Martinussen T, Nørbo Sørensen D, Vansteelandt S. Instrumental variables estimation under a structural Cox model. Biostatistics. 2017;20(1):65–79. https://doi.org/10.1093/biostatistics/kxx057.
Martinussen T, Vansteelandt S, Tchetgen Tchetgen EJ, Zucker DM. Instrumental variables estimation of exposure effects on a time-to-event endpoint using structural cumulative survival models. Biometrics. 2017;73(4):1140–9. https://doi.org/10.1111/biom.12699.
Bhindi B, Frank I, Mason RJ, Tarrell RF, Thapa P, Cheville JC, et al. Oncologic outcomes for patients with residual Cancer at cystectomy following Neoadjuvant chemotherapy: a pathologic stage-matched analysis. Eur Urol. 2017;72(5):660–4. https://doi.org/10.1016/j.eururo.2017.05.016.
Pfister C, Gravis G, Fléchon A, Soulié M, Guy L, Laguerre B, et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder Cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol. 2021;79(2):214–21. https://doi.org/10.1016/j.eururo.2020.08.024.
Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110(3):551–5. https://doi.org/10.1038/bjc.2013.725.
Sherif A, Holmberg L, Rintala E, Mestad O, Nilsson J, Nilsson S, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45(3):297–303. https://doi.org/10.1016/j.eururo.2003.09.019.
Mazzone E, Knipper S, Mistretta FA, Tian Z, Preisser F, Gallina A, et al. Is neoadjuvant chemotherapy for pT2 bladder cancer associated with a survival benefit in a population-based analysis? Cancer Epidemiol. 2019;58:83–8. https://doi.org/10.1016/j.canep.2018.11.007.
Hermans TJN, Voskuilen CS, Deelen M, Mertens LS, Horenblas S, Meijer RP, et al. Superior efficacy of neoadjuvant chemotherapy and radical cystectomy in cT3-4aN0M0 compared to cT2N0M0 bladder cancer. Int J Cancer. 2019;144(6):1453–9. https://doi.org/10.1002/ijc.31833.
Hensley PJ, Panebianco V, Pietzak E, Kutikov A, Vikram R, Galsky MD, et al. Contemporary staging for muscle-invasive bladder Cancer: accuracy and limitations. Eur Urol Oncol. 2022. https://doi.org/10.1016/j.euo.2022.04.008.
Woo S, Panebianco V, Narumi Y, Del Giudice F, Muglia VF, Takeuchi M, et al. Diagnostic performance of Vesical imaging reporting and data system for the prediction of muscle-invasive bladder Cancer: a systematic review and Meta-analysis. Eur Urol Oncol. 2020;3(3):306–15. https://doi.org/10.1016/j.euo.2020.02.007.
Acknowledgements
Not applicable.
Funding
The Dam Foundation (https://dam.no) has funded this project. The Dam Foundation had.
no role in the design of the study or data collection, analysis, interpretation of the data or manuscript writing.
Author information
Authors and Affiliations
Contributions
CTM, VB, GT, SDF and BKA contributed to the conception and design of the study; AB and BKA contributed to the acquisition of data; CTM, NCS, SDF and BKA contributed with the analysis, interpretation, and manuscript preparation. All authors contributed to drafting and revising the article. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approved by the Regional Committee for Medical and Health Research Ethics, Southeast Norway. Approval number: 2016/2286/REK sør-øst A. The study is also approved by the Cancer Registry of Norway and the Norwegian Patient Registry. All data management and analyses were conducted according to current legislation and regulation of privacy, without any possibilities for individual identification. The requirement for informed consent was waived by the Regional Committee for Medical and Health Research Ethics, Southeast Norway.
Consent for publication
The requirement for informed consent was waived by the Regional Committee for Medical and Health Research Ethics, Southeast Norway, approval number: 2016/2286/REK sør-øst A.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Study population.
Additional file 2: Table S1.
Pathological N-category after cystectomy with regards to downstaging of primary tumour and treatment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Møller, C.T., Støer, N.C., Blindheim, A. et al. Downstaging and survival after Neoadjuvant chemotherapy for bladder cancer in Norway; a population-based study. BMC Cancer 22, 1301 (2022). https://doi.org/10.1186/s12885-022-10394-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10394-w