Abstract
Aims
To assess whether glycaemic control is associated with prognosis in people with cancer and pre-existing diabetes.
Methods
In this pre-registered systematic review (PROSPERO: CRD42020223956), PubMed and Web of Science were searched on 25th Nov 2021 for studies investigating associations between glycosylated haemoglobin (HbA1c) and prognosis in people with diabetes and cancer. Summary relative risks (RRs) and 95% Confidence Intervals (CIs) for associations between poorly controlled HbA1c or per 1-unit HbA1c increment and cancer outcomes were estimated using a random-effects meta-analysis. We also investigated the impact of potential small-study effects using the trim-and-fill method and potential sources of heterogeneity using subgroup analyses.
Results
Fifteen eligible observational studies, reporting data on 10,536 patients with cancer and pre-existing diabetes, were included. Random-effects meta-analyses indicated that HbA1c ≥ 7% (53 mmol/mol) was associated with increased risks of: all-cause mortality (14 studies; RR: 1.14 [95% CI: 1.03–1.27]; p-value: 0.012), cancer-specific mortality (5; 1.68 [1.13–2.49]; p-value: 0.011) and cancer recurrence (8; 1.68 [1.18–2.38; p-value: 0.004]), with moderate to high heterogeneity. Dose-response meta-analyses indicated that 1-unit increment of HbA1c (%) was associated with increased risks of all-cause mortality (13 studies; 1.04 [1.01–1.08]; p-value: 0.016) and cancer-specific mortality (4; 1.11 [1.04–1.20]; p-value: 0.003). All RRs were attenuated in trim-and-fill analyses.
Conclusions
Our findings suggested that glycaemic control might be a modifiable risk factor for mortality and cancer recurrence in people with cancer and pre-existing diabetes. High-quality studies with a larger sample size are warranted to confirm these findings due to heterogeneity and potential small-study effects. In the interim, it makes clinical sense to recommend continued optimal glycaemic control.
Highlights
• Diabetes is a common comorbidity in newly-diagnosed cancer patients.
• The impact of glycaemic control in people with both cancer and diabetes is unclear.
• In this meta-analysis, cancer prognosis is worse in those with poor HbA1c control.
• More studies with larger sample sizes are warranted to confirm these findings.
• Clinicians should continue to ensure HbA1c control in cancer patients with diabetes.
Similar content being viewed by others
Introduction
Cancer is an important cause of death worldwide. The Global Burden of Cancer Study reported an estimated 19 million new cancer cases and 10 million cancer deaths worldwide in 2020 [1]. Comorbidity, a potential determinant of cancer treatment, is becoming increasingly common in cancer patients, driven in part by an ageing population [2]. In particular, diabetes has become one of the most common comorbidities in cancer patients [3]. One Danish study reported that 7% of breast, 10% of prostate, 13% of colon and bladder, 25% of pancreatic, and 30% of liver cancer patients had diabetes at cancer diagnosis [4]. One of the reasons could be the shared risk factors (e.g., obesity, poor diet, physical inactivity) and common biological mechanisms between cancer and diabetes [5]; diabetes itself has been recognised as a potentially aetiological factor for many cancers [6, 7].
Several meta-analyses have shown that, compared to those without, cancer patients with pre-existing diabetes had a worse prognosis [8,9,10]. Among the proposed biological pathways, hyperglycaemia can stimulate tumour growth, thereby leading to disease progression [11]. A meta-analysis also reported that hyperglycaemia in solid tumours is associated with worse overall survival, regardless of the presence of diabetes diagnosis [12]. With the rising prevalence of diabetes globally, [13] the number of cancer patients with comorbid diabetes is expected to increase. While robust evidence from both randomised controlled trials and observational studies indicates a progressive association between glucose levels and risk of long-term cardiovascular diseases in people with diabetes, [14, 15] less is known about the relationship between glycaemic control, as measured by glycosylated haemoglobin (HbA1c), and prognosis in patients with cancer and pre-existing diabetes.
In this meta-analysis with dose-response analysis, we summarised the current evidence on the association between HbA1c and cancer prognosis in people with both cancer and diabetes.
Materials and methods
Data sources and search strategy
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline for this study [16] and registered the study protocol within PROSPERO (No. CRD42020223956). On 25th Nov 2021, we systematically searched MEDLINE (via PubMed) and Web of Science for observational cohort studies or post-hoc analyses of clinical trials in cancer patients with diabetes that reported the association between HbA1c and cancer prognosis, including: mortality, cancer recurrence, cancer progression, and hospitalisations. The search was limited to records in English. Keywords related to diabetes, HbA1c or glycaemia, cancer, and prognosis were used in the search. Bibliographies of relevant reviews were additionally sought to identify eligible studies. Details of the search strategy and the PRISMA checklist are shown in the Supplementary Material.
Study selection and data extraction
All titles and abstracts were independently screened by two reviewers (SL and UTK); articles with any disagreement at this stage were included for full-text assessment. Studies were eligible if they reported the relative risk (RR) estimate (hazard ratio, risk ratio, or odds ratio) with their standard errors (SEs), 95% confidence intervals (CIs), or p-values for the association between HbA1c and cancer prognosis; SEs were calculated from 95% CIs or p-values if not reported [17]. Studies were excluded if: (1) not all subjects had cancer and diabetes; (2) the exposure was not HbA1c (e.g., fasting glucose). If two or more articles included the same participants, the analysis with largest person-years was included. If a study was stratified by cancer, estimates for different sites were treated as different cohorts.
A standardised form was used to extract data on the study characteristics, participants, cancer sites, definitions and ascertainment of exposures and outcomes, mean/median of HbA1c, number of participants, events and person-years, duration of follow-up, methods of analysis, and most-adjusted estimates for each outcome. If no estimate was reported but Kaplan-Meier curves was available, we firstly extracted data from the curves using Engauge Digitizer and then used the “ipdfc” command in Stata to reconstruct individual-level time-to-event data from curves and applied Cox proportional hazard model to estimate the hazard ratio [18].
Risk of bias assessment
We used the Newcastle-Ottawa Scale (NOS) to assess the quality of included studies. This scale quantifies the risk of bias in observational studies based on three domains: selection of population, comparability, and ascertainment of outcomes. In the comparability domain, age and cancer characteristics were defined as the two most important factors that studies should adjust for. In the outcome domain, less than 20% loss to follow-up was deemed adequate; the sufficient length of follow-up was determined by the severity of cancer (e.g., 1 year is considered sufficient for pancreatic, 3 years for bladder, and 5 years for prostate cancer). Based on these criteria, each study was assigned a score ranging from 0 (lowest quality) to 9 (highest).
Statistical analysis
Our primary analyses sought to combine the RRs for the associations between poorly and well controlled HbA1c, with 7% (53 mmol/mol) as the glycaemic target according to current diabetes management guidelines [19]. Based on data availability, where possible we converted comparisons into poorly (≥ 7%) vs. well controlled HbA1c (< 7%); the flowchart for data conversion is reported in Supplementary Fig. 1. If a study reported associations between continuous HbA1c and outcomes, estimates were converted into comparisons above vs. below the cut-off (≥7% vs < 7%) as described in Chene et al. [20] If, conversely, a study reported the comparison across other cut-offs, the effect for per 1-unit increment was firstly estimated and then converted into comparisons ≥7% vs. < 7% [21, 22]. The secondary analysis aimed to quantify the dose-response relationship between HbA1c and outcomes. Some studies were not included in this analysis as means/medians of HbA1c were not reported and therefore it was not possible to convert estimates for categories to per 1-unit increment.
Due to inconsistent terminology and definitions of end-points, we classified cancer prognosis outcomes into: all-cause mortality, cancer-specific mortality, and cancer recurrence (including local, regional and distant recurrence, and/or metastasis) following guidelines for time-to-event end-point definitions in cancer studies [23].
Summary RRs and 95% CIs for poorly controlled HbA1c and per 1-unit increment of HbA1c (%) were combined using a random-effects model [24]. Heterogeneity across studies was quantified by the I2 statistics: we deemed an I2 value of lower than 50% as low, 50 to 75% as moderate, and larger than 75% as high [25]. Small-study effects (e.g., publication bias) were assessed by funnel plots and the Egger’s test [26]. We further investigated the impact of potential small-study effects using the trim-and-fill method and potential sources of heterogeneity using subgroup analyses. We used Stata/IC version 16.0 (Stata Corp, College Station, TX) for all analyses and considered a two tailed p-value < 0.05 as statistically significant.
Results
Characteristics of the included study
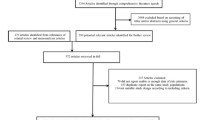
We identified 1179 papers in the systematic search; after screening of titles and abstracts, 65 records met the eligible criteria for full-text assessment: of these, 20 studies reported data on the associations between HbA1c and prognosis but five studies reported the outcome which could not be combined with other studies. Therefore, we included 15 studies with 10,536 participants with cancer and pre-existing diabetes in the meta-analysis. The flowchart of study selection is shown in Fig. 1; reasons and references for the excluded studies are presented in Supplementary Table 1.
The characteristics of the included studies are presented in Table 1. Overall, more than two-thirds of the studies were from Asian countries and the sample size was generally small (N < 500), except in two studies from UK and US (N > 1000). The most commonly investigated cancer site was bladder (n = 6), followed by pancreas (n = 3); most studies reported estimates for cut-offs of HbA1c, such as 7%, while some reported per 1-unit increment of HbA1c (details in Supplementary Table 2). The median follow-up ranged from 9 months to 6.8 years and only one-third of studies adjusted for at least one confounding factor. The overall quality of the included studies was moderate, with NOS scores ranging from 4 to 8 (median, 7; Supplementary Table 3).
Poorly versus well controlled HbA1c
For the comparison of HbA1c ≥ 7% vs < 7%, pooled meta-analytical estimates were obtained for all-cause mortality, cancer-specific mortality, and cancer recurrence. Random-effects meta-analyses suggested that, compared to HbA1c < 7%, patients with HbA1c ≥ 7% had an increased risk of all-cause mortality (14 studies; 9342 subjects and 3204 deaths; RR: 1.14; 95% CI: 1.03–1.27; p = 0.012), cancer-specific mortality (5 studies;1852 subjects and 116 cancer-specific deaths; 1.68; 1.13–2.49; p = 0.011) and cancer recurrence (8 studies; 1966 subjects, of which 658 with a cancer recurrence; 1.68; 1.18–2.38; p = 0.004), with moderate to high heterogeneity across studies for all three outcomes (I2 73.2% and p < 0.001; 71.3% and p = 0.008; and 75.8% and p < 0.001, respectively; Fig. 2). Results of Egger’s test and funnel plots are shown in Fig. 3, indicating small-study effects for all three outcomes (Egger’s test p = 0.005, p = 0.017, and p = 0.005, respectively). Pooled RRs for all-cause mortality, cancer-specific mortality, and cancer recurrence in trim-and-fill analyses were attenuated to 1.06 (95% CI: 0.94–1.20; p = 0.355), 1.20 (0.83–1.74; p = 0.340), and 1.24 (0.87–1.78 p = 0.241) after imputing potential unpublished studies (Supplementary Table 4). Funnel plots for trim-and-fill analyses are presented in Supplementary Fig. 2.
Secondary analysis: dose-response meta-analysis
Five studies reporting RRs of HbA1c across a cut-off could not be converted into effects per 1-unit change (Supplementary Table 2). As shown in Figs. 2, 1-unit increment of HbA1c (%) was associated with increased risks of all-cause mortality (13 studies; 9207 subjects and 3145 deaths; RR: 1.04; 95% CI: 1.01–1.08; p = 0.016) and cancer-specific mortality (4 studies; 1717 subjects and 86 cancer-specific deaths; 1.11; 1.04–1.20; p = 0.003) but not cancer recurrence (3 studies; 1524 subjects of which 443 with a cancer recurrence; 1.15; 0.97–1.37; p = 0.110). We observed moderate heterogeneity for all-cause mortality (I2 59.6%; p = 0.003; Fig. 2) and potential small-study effects for both all-cause mortality (Egger’s test, p = 0.040) and cancer-specific mortality (p = 0.018; Fig. 3). Taken potential unpublished studies into account, the trim-and-fill analyses showed pooled RRs of 1.02 (95% CI: 0.98–1.06; p = 0.374) and 1.08 (1.00–1.17; p = 0.053) for all-cause mortality and cancer-specific mortality, respectively (Supplementary Table 4); Supplementary Fig. 2 shows the corresponding funnel plots.
Subgroup analyses
We also conducted subgroup analysis by limiting the inclusion to studies of high quality (NOS score ≥ 6): results are shown in Supplementary Fig. 3. RRs were slightly attenuated for all-cause mortality but strengthened for cancer-specific mortality and cancer recurrence. For the comparison of poorly vs. well controlled HbA1c, the RR was 1.03 (95% CI: 1.00–1.07; p = 0.041) for all-cause mortality, 2.06 (1.11–3.82; p = 0.021) for cancer-specific mortality, and 1.71 (1.10–12.65; p = 0.018) for cancer recurrence. Heterogeneity across studies was slightly reduced for cancer-specific mortality but not for all-cause mortality or cancer recurrence.
Subgroup analyses by geographical areas were possible for all-cause mortality and cancer-specific mortality. We observed differences in the association of poorly vs. well controlled HbA1c with all-cause mortality and cancer-specific mortality between Asian and Western studies (Supplementary Fig. 4a): the RR for all-cause mortality was 1.04 (95% CI: 0.96–1.12; p = 0.348 in 7 studies, 8592 subjects, and 2902 deaths) in Western while it was 1.14 (1.03–1.27; p = 0.001 in 7 studies, 750 subjects, and 302 deaths) in Asian studies (p < 0.01 for difference by subgroups). The RR for cancer-specific mortality was 1.17 (0.98–1.41; p = 0.089 in 2 studies, 1564 subjects, and 65 deaths) in Western and 2.63 (1.61–4.28; p < 0.001 in 3 studies, 288 subjects, and 51 deaths) in Asian studies (p < 0.01 for difference by subgroups). Estimates for per 1-unit increment of HbA1c by geographical areas are shown in Supplementary Fig. 4b.
Analyses by cancer sites were possible for bladder, colorectal, pancreatic, and prostate cancer for all-cause mortality; and bladder cancer for recurrence (Supplementary Fig. 5). Moderate to high heterogeneity was observed in most subgroup analyses. A significant association between poorly vs. well controlled HbA1c and bladder cancer recurrence was found in five studies reporting this association (442 subjects of which 167 with a cancer recurrence; RR: 2.23; 1.18–4.21; p = 0.013; Supplementary Fig. 5).
Discussion
Our results, obtained from 15 studies with data on 10,536 patients with cancer and diabetes, showed that a poorly controlled diabetes and a progressively higher HbA1c were associated with a worse cancer prognosis. We observed moderate to high heterogeneities across the included studies and small-study effects for most outcomes which may have biased the estimates. Subgroup analyses suggested that differences in the quality of studies, cancer sites, and geographical areas might have contributed to such heterogeneities. Notably, geographical differences was likely attributable to the smaller study sample sizes in Asia than Western countries, though other unexamined factors may have also contributed to such differences, such as earlier onset of diabetes (i.e., at younger ages) and/or at lower body mass index in Asia [42]. Further original investigations with a lager sample size are needed to confirm current findings.
Previous meta-analyses have shown a poor survival associated with diabetes in patients with cancer, [8] including prostate, [9] pancreatic, [10] breast, [43] cervical, [44] colorectal, [45] lung, [46] and brain [47] cancer. Although the exact mechanisms underpinning worse outcomes in cancer patients with comorbid diabetes are unknown, hyperglycaemia and hyperinsulinemia have been proposed as possible biological pathways due to their roles in stimulating tumour growth [11]. Another meta-analysis suggested a positive association between hyperglycaemia and mortality in patients with cancer, regardless of the presence of diabetes diagnosis [12]. While most studies, original investigations, or systematic reviews included people without diabetes as the comparison group, to our knowledge there was no meta-analysis on the association between glycaemic control (or HbA1c levels) and survival in people with both cancer and diabetes. In particular, we found that HbA1c was associated with cancer recurrence in patients with bladder cancer and pre-existing diabetes.
Previous meta-analyses of randomised controlled trials indicated that improved glycaemic control or additional weight change achieved by current glucose-lowering medications was not associated with cancer incidence, [48, 49] suggesting that these biological pathways alone cannot fully explain the anti-tumour effect of glycaemic control and other collateral effects related to diabetes and cancer management may also have a part to play. Indeed, the presence of poorly controlled diabetes may affect the timing of cancer diagnosis in both directions, which may determine the stage at cancer diagnosis. Cancer stage is one of the most important determinants of prognosis, with long-term survival being much greater in early stages [50]. On the one hand, patients with poorly controlled diabetes might have a more frequent healthcare contact, which would possibly lead to an earlier cancer detection [51]. On the other hand, poorly controlled diabetes may represent a group of patients requiring a high burden of care, leading to a “competing demand” of diabetes care and less awareness of cancer symptoms [52]. A population-based study using electronic health records in Canada suggested that, among newly-diagnosed breast cancer patients, compared to those without diabetes, individuals with diabetes presented with a higher stage and were more likely to have metastases [53]. However, whether glycaemic control is related to stage at cancer diagnosis in people with diabetes and cancer was not investigated in the current meta-analysis due to lack of information, and further studies with individual-level data are needed.
The selection of cancer treatment is also important for prognosis while it may be delivered differently based on the glycaemic control and further contributed to disparities in prognosis. Diabetes is one of the risk factors for infection in cancer patients, [54] and a meta-analysis suggested that cancer patients with diabetes were also at greater risk of postoperative mortality [55]. Hence, surgery may be postponed if HbA1c is poorly controlled. Moreover, poorly controlled HbA1c may lead health care professionals to reduce the doses/regimens of some treatments, as the use of steroids (a common treatment for many cancers) increases glucose levels [56]. In addition, some common chemotherapeutic agents and newer targeted therapies for cancer may potentially cause cardiotoxic complications [57]. While diabetes, particularly uncontrolled, is a major risk factor of cardiovascular diseases, [14, 15] it is possible that hyperglycaemia would make these people with cancer and pre-existing diabetes more susceptible to such complications. However, in the current meta-analysis information related to diabetes medications (some of which may have antineoplastic effects independent of risk factor control [58]) or cancer treatment was not available in most of the included studies and therefore warrants further investigations.
It should be noted that the magnitude of the increased risks of cancer associated with diabetes varied by cancer types: for example, although diabetes was associated with both pancreatic and bladder cancer, the relative risks were over 2.0 for pancreatic and 1.2 for bladder cancer [7]. In light of the heterogeneous survival in people with different cancer types (e.g., 5-year survival rates for bladder and pancreatic were 52.6 and 6.5% in England during 2013–2017 [59]), the effect of glycaemic control on cancer survival may differ among diabetic people with different cancers similar to the variable effect of diabetes on cancer incidence. In fact, albeit with small numbers of studies, our subgroup analyses would suggest potential different relative risks of all-cause mortality in people with bladder and pancreatic cancer but no inference could be obtained due to the limited data; future research should focus on specific cancers to detail such differences.
Our study has important clinical implications. The impact of cancer diagnosis and treatment on diabetes management has drawn less attention, possibly because both clinicians and cancer patients may prioritise cancer over glycaemic control after a cancer diagnosis [60]. Based on current evidence, clinicians should continue to ensure glycaemic control in people with cancer and pre-existing diabetes, and it should be integral to clinical cancer care. This is also reflected in guidelines on glycaemic control in people with cancer recently issued by The Joint British Society for Inpatient Care and UK Chemotherapy Board, which emphasises the important of glucose monitoring in all patients with cancer, regardless of their diabetic status [61, 62]. While these guidelines are provided to reduce the acute hyperglycaemia-related complications during cancer treatment periods (in short-term), [61] our study fills the gap by suggesting that ameliorating pre-existing hyperglycaemia could improve survival also in the long-term, though future studies with large sample sizes are warranted to identify the optimal glycaemic goal and medications in people with cancer.
Our study has also some strengths and limitations. To our knowledge, this study is the first meta-analysis investigating the prognostic role of HbA1c in people with both cancer and diabetes; we also examined associations across a range of end-points, including all-cause mortality, cancer recurrence, and cancer-specific mortality, which are relevant in overall prognosis as well as in cancer epidemiology. To minimise the impact of publication bias, we extracted additional data from Kaplan-Meier curves if no estimates were reported. Yet, we still observed potential small-study effects for most outcomes, and RRs were attenuated to statistical non-significance in trim-and-fill analyses after imputing potentially unpublished studies. We have only included English articles which may have introduced language bias. The quality of included studies was moderate, particularly due to lack of adjustment for other important clinical factors (e.g., cancer characteristics, sex, body weight) which may have confounded the causal association between glucose control and the investigated outcomes. Age and cancer stage, for example, are the two most relevant confounders which have been adjusted for only in five studies; obesity itself is as an important risk factor for both diabetes and cancer, [5] and body weight may also affect the dose of chemotherapy. In addition, other diabetes related-factors, such as disease duration and treatment, may have also contributed to confounding. Furthermore, we included studies with heterogeneous prognosis and thus some of the statistical heterogeneity we observed was expected. Where possible, we performed subgroup analyses to detail cancer-specific associations. Nevertheless, due to limited data, we were not able to characterize the potentially diverse prognostic roles of HbA1c in different cancer populations. We have also conducted subgroup analyses by geographical areas and the quality of study to investigate sources of heterogeneity: our findings indicated that both factors may have contributed to the observed heterogeneities. In particular, the opposite directions in the of associations accounting for small-study effects (attenuated) vs the inclusion of only studies of higher quality (strengthened) for some outcomes would suggest the relevant impact of publication bias and study quality on the interpretation of our findings. Heterogeneity could also be related, however, to other factors, such as demographics of included participants, cancer characteristics, diabetes and cancer treatment, which were not investigated because of lack of relevant information in the included studies. In line with the current diabetes management guidelines, we converted all estimates into comparisons of above vs below a clinically relevant threshold (7%; 53 mmol/mol) but it should be noted that these conversions were based on two assumptions: a normal HbA1c distribution and a linear relationship with outcomes. Yet, we could not explore a potential non-linearity in the relationship between HbA1c and outcomes; therefore, further research is warranted to identify the optimal glycaemic target for cancer patients with diabetes.
In conclusion, our meta-analyses suggests that poor glycaemic control may be associated with worse outcomes in patients with cancer and diabetes. However, current findings are limited by evidence of potential bias in the published literature and more high-quality studies with a larger sample size are needed to confirm these conclusions; in the interim, it makes clinical sense to recommend continued optimal glycaemic control based on current evidence and guidelines. Further investigations are also warranted to identify the optimal goal for glycaemic control and characterise the effect of HbA1c in different cancer populations.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. Data extracted from included studies will be available online if accepted; analytic codes used in the meta-analysis are available from https://github.com/supingling/HbA1c_cancer_meta-analysis.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66:337–50.
Edwards BK, Noone A-M, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–314.
Loeppenthin K, Dalton SO, Johansen C, Andersen E, Christensen MB, Pappot H, et al. Total burden of disease in cancer patients at diagnosis-a Danish nationwide study of multimorbidity and redeemed medication. Br J Cancer. 2020;123:1033–40.
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21.
Ling S, Brown K, Miksza JK, Howells LM, Morrison A, Issa E, et al. Risk of cancer incidence and mortality associated with diabetes: a systematic review with trend analysis of 203 cohorts. Nutr Metab Cardiovasc Dis. 2021;31:14–22.
Ling S, Brown K, Miksza JK, Howells L, Morrison A, Issa E, et al. Association of type 2 diabetes with cancer: a meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care. 2020;43:2313–22.
Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–64.
Cai H, Xu Z, Xu T, Yu B, Zou Q. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes Metab Res Rev. 2015;31:336–43.
Ma J, Wang J, Ge L, Long B, Zhang J. The impact of diabetes mellitus on clinical outcomes following chemotherapy for the patients with pancreatic cancer: a meta-analysis. Acta Diabetol. 2019;56:1103–11.
Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46:595–607.
Barua R, Templeton AJ, Seruga B, Ocana A, Amir E, Ethier JL. Hyperglycaemia and survival in solid tumours: a systematic review and meta-analysis. Clin Oncol (R Coll Radiol). 2018;30:215–24.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:1–43.
The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. 2011;343:d2090.
Wei Y, Royston P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017;17:786–802.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S73–84.
Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144:610–21.
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9.
Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6:40–57.
Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (definition for the assessment of time-to-event endpoints in CANcer trials)†. Ann Oncol. 2015;26:873–9.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Ahn JH, Jung SI, Yim SU, Kim SW, Hwang EC, Kwon DD. Impact of glycemic control and metformin use on the recurrence and progression of non-muscle invasive bladder cancer in patients with diabetes mellitus. J Korean Med Sci. 2016;31:1464–71.
Boursi B, Giantonio BJ, Lewis JD, Haynes K, Mamtani R, Yang YX. Serum glucose and hemoglobin A1C levels at cancer diagnosis and disease outcome. Eur J Cancer. 2016;59:90–8.
Cheon YK, Koo JK, Lee YS, Lee TY, Shim CS. Elevated hemoglobin A1c levels are associated with worse survival in advanced pancreatic cancer patients with diabetes. Gut Liver. 2014;8:205–14.
Huang WL, Huang KH, Huang CY, Pu YS, Chang HC, Chow PM. Effect of diabetes mellitus and glycemic control on the prognosis of non-muscle invasive bladder cancer: a retrospective study. BMC Urol. 2020;20:117.
Hwang EC, Kim YJ, Hwang IS, Hwang JE, Jung SI, Kwon DD, et al. Impact of diabetes mellitus on recurrence and progression in patients with non-muscle invasive bladder carcinoma: a retrospective cohort study. Int J Urol. 2011;18:769–76.
Kaneda K, Uenishi T, Takemura S, Shinkawa H, Urata Y, Sakae M, et al. The influence of postoperative glycemic control on recurrence after curative resection in diabetics with hepatitis C virus-related hepatocellular carcinoma. J Surg Oncol. 2012;105:606–11.
Kang SG, Hwang EC, Jung SI, Yu HS, Chung HS, Kang TW, et al. Poor preoperative glycemic control is associated with dismal prognosis after radical nephroureterectomy for upper tract urothelial carcinoma: a korean multicenter study. Cancer Res Treat. 2016;48:1293–301.
Komatsu T, Chen-Yoshikawa TF, Ikeda M, Takahashi K, Nishimura A, Harashima SI, et al. Impact of diabetes mellitus on postoperative outcomes in individuals with non-small-cell lung cancer: A retrospective cohort study. PLoS One. 2020;15:e0241930.
Lee W, Yoon YS, Han HS, Cho JY, Choi Y, Jang JY, et al. Prognostic relevance of preoperative diabetes mellitus and the degree of hyperglycemia on the outcomes of resected pancreatic ductal adenocarcinoma. J Surg Oncol. 2016;113:203–8.
Lee SJ, Kim JH, Park SJ, Ock SY, Kwon SK, Choi YS, et al. Optimal glycemic target level for colon cancer patients with diabetes. Diabetes Res Clin Pract. 2017;124:66–71.
Li J, Ning NY, Rao QX, Chen R, Wang LJ, Lin ZQ. Pretreatment glycemic control status is an independent prognostic factor for cervical cancer patients receiving neoadjuvant chemotherapy for locally advanced disease. BMC Cancer. 2017;17:517.
Nik-Ahd F, Howard LE, Eisenberg AT, Aronson WJ, Terris MK, Cooperberg MR, et al. Poorly controlled diabetes increases the risk of metastases and castration-resistant prostate cancer in men undergoing radical prostatectomy: Results from the SEARCH database. Cancer. 2019;125:2861–7.
Okamura A, Watanabe M, Imamura Y, Hayami M, Yamashita K, Kurogochi T, et al. Glycemic status and prognosis of patients with squamous cell carcinoma of the esophagus. World J Surg. 2017;41:2591–7.
Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case-control study. Dig Dis Sci. 2008;53:2486–94.
Tai YS, Chen CH, Huang CY, Tai HC, Wang SM, Pu YS. Diabetes mellitus with poor glycemic control increases bladder cancer recurrence risk in patients with upper urinary tract urothelial carcinoma. Diabetes Metab Res Rev. 2015;31:307–14.
Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8.
Zhao XB, Ren GS. Diabetes mellitus and prognosis in women with breast cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e5602.
Chen S, Tao M, Zhao L, Zhang X. The association between diabetes/hyperglycemia and the prognosis of cervical cancer patients: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e7981.
Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum. 2013;56:1304–19.
Deng HY, Zheng X, Zha P, Peng L, Huang KL, Qiu XM. Diabetes mellitus and survival of non-small cell lung cancer patients after surgery: a comprehensive systematic review and meta-analysis. Thorac Cancer. 2019;10:571–8.
Liu H, Liu Z, Jiang B, Ding X, Huo L, Wan X, et al. Prognostic significance of hyperglycemia in patients with brain tumors: a meta-analysis. Mol Neurobiol. 2016;53:1654–60.
Lin C, Cai X, Yang W, Lv F, Nie L, Ji L. Glycemic control and the incidence of neoplasm in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Endocrine. 2020;70:232–42.
Lin C, Cai X, Yang W, Lv F, Nie L, Ji L. The body weight alteration and incidence of neoplasm in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Front Endocrinol. 2020;11:541699. https://doi.org/10.3389/fendo.2020.541699.
Hiom SC. Diagnosing cancer earlier: reviewing the evidence for improving cancer survival. Br J Cancer. 2015;112(Suppl 1):S1–5.
Colmers IN, Majumdar SR, Yasui Y, Bowker SL, Marra CA, Johnson JA. Detection bias and overestimation of bladder cancer risk in type 2 diabetes: a matched cohort study. Diabetes Care. 2013;36:3070–5.
Mounce LTA, Price S, Valderas JM, Hamilton W. Comorbid conditions delay diagnosis of colorectal cancer: a cohort study using electronic primary care records. Br J Cancer. 2017;116:1536–43.
Lipscombe LL, Fischer HD, Austin PC, Fu L, Jaakkimainen RL, Ginsburg O, et al. The association between diabetes and breast cancer stage at diagnosis: a population-based study. Breast Cancer Res Treat. 2015;150:613–20.
Park JH, Kim H-y, Lee H, Yun EK. A retrospective analysis to identify the factors affecting infection in patients undergoing chemotherapy. Eur J Oncol Nurs. 2015;19(6):597–603.
Barone BB, Yeh H-C, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care. 2010;33:931–9.
Parnham MJ, Nijkamp FP. Principles of Immunopharmacology. Basel: Birkhäuser Verlag; 2005.
Lenneman CG, Sawyer DB. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res. 2016;118:1008–20.
Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer—pathophysiology and clinical implications. Endocr Rev. 2019;41:33–52.
Office for National Statistics. One-year and five-year net survival for adults (15–99) in England diagnosed with one of 29 common cancers, by age and sex. 2019.
Pinheiro LC, Kaur H, Nilo D, Safford MM, DeRosa AP, Kern LM. Determining the impact of a cancer diagnosis on diabetes management: a systematic literature review. Am J Clin Oncol. 2019;42:870–83.
Giovannucci E. The critical need for guidance in managing glycaemic control in patients with cancer. Diabet Med. 2022;39(1):e14624.
Joharatnam-Hogan N, Chambers P, Dhatariya K, Board R; Joint British Diabetes Society for Inpatient Care (JBDS), UK Chemotherapy Board (UKCB). A guideline for the outpatient management of glycaemic control in people with cancer. Diabet Med. 2022;39(1):e14636.
Acknowledgements
We thank authors and participants of all included studies. FZ acknowledges the support of NIHR ARC.
Funding
SL was a Research Associate (University of Leicester) funded by British Heart Foundation Grant (PG/19/20/34284) during the study was conducted.
Author information
Authors and Affiliations
Contributions
SL contributed to concept, design, data extraction, data analysis, interpretation of the data and the first draft. FZ assisted in data analysis and contributed to critical revisions. MS and DA contributed to critical revisions. UTK contributed to concept, design, data extraction and critical revisions. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participants
The study protocol was pre-registered within PROSPERO (No. CRD42020223956). Since this study used only secondary data from publications, no ethical approval or consent to participants is required.
Consent to publication
Not applicable to seek consent to publish from participants, as this study used only secondary data from publications.
Competing interests
The preliminary results of this study were presented at European Diabetes Epidemiology Group meeting (EDEG) 2021. We have no conflict of interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
Search algorithms on 25th Nov 2021. Supplementary Table 1. Reasons of exclusion of studies following full-text review. Supplementary Table 2. Data conversions and references of included studies. Supplementary Table 3. Newcastle-Ottawa score for included studies. Supplementary Table 4. Trim-and-fill analyses results. Supplementary Fig. 1. Flowchart for data conversion. Supplementary Fig. 2. Funnel plots following trim-and-fill. Supplementary Fig. 3. Meta-analysis within studies of high quality (NOS score ≥ 6). Supplementary Fig. 4a. Subgroup analyses by geographical region for HbA1c ≥ 7% vs. HbA1c < 7%. Supplementary Fig. 4b. Subgroup analyses by geographical region per 1-unit increment of HbA1c. Supplementary Fig. 5. Subgroup analyses by cancer sites. PRISMA checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ling, S., Sweeting, M., Zaccardi, F. et al. Glycosylated haemoglobin and prognosis in 10,536 people with cancer and pre-existing diabetes: a meta-analysis with dose-response analysis. BMC Cancer 22, 1048 (2022). https://doi.org/10.1186/s12885-022-10144-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10144-y