Abstract
Purpose
To explore the diagnostic value of integrated positron emission tomography/magnetic resonance imaging (PET/MRI) for the staging of endometrial carcinoma and to investigate the associations between quantitative parameters derived from PET/MRI and clinicopathological characteristics of endometrial carcinoma.
Methods
Altogether, 57 patients with endometrial carcinoma who underwent PET/MRI and PET/computed tomography (PET/CT) preoperatively were included. Diagnostic performance of PET/MRI and PET/CT for staging was compared by three readers. Associations between PET/MRI quantitative parameters of primary tumor lesions and clinicopathological characteristics of endometrial carcinoma were analyzed. Histopathological results were used as the standard.
Results
The overall accuracy of the International Federation of Gynecology and Obstetrics (FIGO) staging for PET/MRI and PET/CT was 86.0% and 77.2%, respectively. PET/MRI had higher accuracy in diagnosing myometrial invasion and cervical invasion and an equivalent accuracy in diagnosing pelvic lymph node metastasis against PET/CT, although without significance. All PET/MRI quantitative parameters were significantly different between stage I and stage III tumors. Only SUVmax/ADCmin were significantly different between stage I and II tumors. No parameters were significantly different between stage II and III tumors. The SUVmax/ADCmin in the receiving operating characteristic (ROC) curve had a higher area under the ROC curve for differentiating stage I tumors and other stages of endometrial carcinoma.
Conclusions
PET/MRI had a higher accuracy for the staging of endometrial carcinoma, mainly for FIGO stage I tumors compared to PET/CT. PET/MRI quantitative parameters, especially SUVmax/ADCmin, were associated with tumor stage and other clinicopathological characteristics. Hence, PET/MRI may be a valuable imaging diagnostic tool for preoperative staging of endometrial carcinoma.
Similar content being viewed by others
Introduction
In 2020, 417,367 new endometrial carcinoma cases and 97,370 endometrial carcinoma-related deaths were reported worldwide [1]. The treatment modalities for endometrial carcinoma include surgery, radiotherapy, chemotherapy, and hormone therapy, and the choice of treatment depends on the staging. In 1971, the International Federation of Gynecology and Obstetrics (FIGO) Committee sought to improve the clinical staging of endometrial carcinoma. These staging criteria were last revised in 2009 [2]. Preoperative imaging techniques have exceptional advantages for staging endometrial carcinoma because of their non-invasiveness and relative accuracy. Compared with clinical staging, these imaging techniques can accurately evaluate staging before treatment, which plays a significant role in guiding clinical treatment and judging prognosis.
Magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT) are two standard imaging techniques that are clinically applied for the preoperative staging of endometrial carcinoma. Compared with MRI, PET/CT plays a key role in exploring lymph node metastasis. PET/CT has been reported to have high sensitivity, specificity, and positive predictive value for endometrial carcinoma metastasis [3]. However, PET/CT has a relatively low resolution for soft tissues and thus, lower efficiency in detecting the primary tumor of the endometrial carcinoma compared with MRI [4]. Recently, PET/MRI has attracted attention as a new multi-modality molecular imaging technique. PET/MRI integrates the high precision of MRI and high sensitivity of PET by generating complete integrated images at the structural, functional, and molecular levels [5]. PET/MRI is reported to have value in evaluating gynecological tumor staging, assessing treatment response, detecting recurrent lesions, and predicting prognosis [6,7,8]. Moreover, previous studies have demonstrated the relationship between MR quantitative parameters and endometrial carcinoma staging and pathological features [9, 10]. However, the value of PET/MRI parameters in endometrial carcinoma staging has not yet been analyzed.
Thus, this study aimed to evaluate the applicability of integrated PET/MRI in diagnosing and staging endometrial carcinoma by comparing PET/MRI and PET/CT imaging results with postoperative pathological data. Furthermore, we extracted quantitative parameters from PET/MRI to explore their relationship with the staging and clinicopathological characteristics of endometrial carcinoma.
Material and methods
Patients
Data of patients with pathologically proven endometrial carcinoma, who underwent PET/CT and PET/MRI from December 2017 to January 2021 were retrospectively analyzed. All procedures were approved by the ethics committee of the hospital. Written informed consent was obtained from all patients.
We screened the patients based on the following inclusion criteria: (1) patients who underwent PET/CT and PET/MRI before treatment, (2) patients who received surgical treatment that confirmed endometrial carcinoma by postoperative pathology, (3) patients with complete postoperative pathological data, and (4) patients who had < 2 weeks between imaging and surgery. The exclusion criteria were as follows: (1) patients who received other treatments (e.g., radiotherapy and chemotherapy) before imaging, (2) patients who had other types of malignant tumor history before endometrial carcinoma diagnosis, (3) patients in whom the measurement of parameters was affected by artifacts in PET/MRI images, and (4) patients who belonged to the FIGO stage IIIC2 or IV. Finally, 57 patients were enrolled in this study (Fig. 1). All 57 patients underwent total abdominal or laparoscopic hysterectomy with bilateral salpingo-oophorectomy, and 41 patients underwent pelvic lymphadenectomy.
PET/CT scanning and image acquisition
Before non-contrast-enhanced 18F-fluorodeoxyglucose (18F-FDG) PET/CT scanning, all patients fasted for ≥ 6 h and had blood glucose levels of ≤ 7 mmol/L. They were injected with 3.70–5.55 MBq/kg 18F-FDG in the resting state. After 60 min, 18F-FDG PET/CT scanning was performed with a Discovery PET/CT 690 scanner (GE Healthcare, Waukesha, WI, USA) while the patients were lying on the scan bed. The scan ranged from the calvarium to the middle thigh (120 s/bed). The slice thickness, tube voltage, and tube current for CT scans were 3.75 mm, 120–140 keV, and 80 mA, respectively.
PET/MRI scanning and image acquisition
Pelvic 18F-FDG PET/MRI scanning was performed 33 ± 12 min after PET/CT scanning. All images were acquired from the scans that were performed using Signa PET/MRI (GE Healthcare, Waukesha, WI, USA), integrating time-of-flight–PET, and 3.0 T MRI (GE Signa 750w) scanners. PET images and MRI images were acquired simultaneously. A 32-channel coil (upper anterior array) served as the cavitary scanning coil. The pelvic axial scan ranged from the vaginal level to the superior iliac boundary. For pelvic MRI scanning, T2-weighted images (T2WI) were acquired in the axial, sagittal, and coronal planes using the following T2WI parameters: repetition time (TR), 2600–3400 ms; echo time (TE), 60–90 ms; section thickness, 5 mm; interval, 1 mm; matrix, 384 × 384; field of view (FOV), 240 × 240 mm. Axial T1-weighted images parameters were as follows: TR, 500 ms; TE, 8 ms; section thickness, 5 mm; interval, 1 mm; matrix, 384 × 384; FOV, 240 × 240 mm. Axial diffusion-weighted imaging (DWI) with b-values of 0 and 800 s/mm2 were obtained. For PET scanning, the correction for γ-ray attenuation was performed with the Dixon MRI sequence. PET scanning was conducted under list mode. Images were reconstructed by iterative ordered subset expectation maximization. The pelvic scan time for PET/MRI was approximately 24 min.
Image analysis
All 18F-FDG PET/CT and PET/MRI images were uploaded to a GE AW4.6 workstation (GE Healthcare, Waukesha, WI, USA) and then post-processed using PET volume computer-assisted reading (VCAR) software. Three radiologists/nuclear medicine physicians, each with double board certifications and with > 5 years of medical imaging diagnosis experience, evaluated the PET/CT and PET/MRI images independently and sequentially at two time points. PET/MRI image evaluation was performed 4–5 weeks after PET/CT image evaluation, thereby eliminating subjective image bias. The readers were unaware of the other readers’ evaluation results. To achieve a final summary of the results, diagnosis was determined through negotiation and consensus when their opinions were inconsistent.
For maximum standardized uptake value (SUVmax) measurements of PET/MRI, by applying the iterative adaptive algorithm, PET VCAR enables automatic segmentation. SUVmax was defined as the maximum value of SUV.
IMAgenGINE MRToolbox (Vision Tech, Hefei, Anhui, China) software was used for apparent diffusion coefficient (ADC) measurements. Regions of interest (ROIs) were drawn on the ADC maps with T1WI and T2WI sequences as references. ROIs were manually delineated on the slices containing the largest diameter of the tumors as much as possible, avoiding necrotic and hemorrhage tissue. Necrotic areas were defined as areas with relatively low signal intensity on DWI and T1WI, and high signal intensity on T2WI compared with solid tumors. Hemorrhage areas were defined as areas with relatively high signal intensity on DWI and T1WI compared with solid tumors. ADCmean and ADCmin were defined as the average and the lowest ADC values in ROI, respectively. Three readers independently measured all parameters, and the average was calculated.
Diagnostic criteria
PET/CT and PET/MRI diagnostic criteria for staging endometrial carcinoma were based on the FIGO staging system. Postoperative pathological staging was applied as the gold standard.
To diagnose myometrial invasion, the proportion of the tumor thickness that invaded the myometrial layer was used to calculate the myometrial invasion. The superficial myometrial invasion ratio was < 50%. The deep myometrial invasion ratio was ≥ 50%. The diagnosis of cervical invasion was based on high uptake tumor invasion at the cervix in the PET/CT image, low signal disappearance of the cervical interstitial layer under the T2WI sequence, and high signal in the DWI image at the cervix. On PET/MRI images, tumor invasion of adjacent structures was determined primarily on the basis of MRI findings, with reference to PET findings. On PET/CT images, tumor invasion of adjacent structures was determined primarily on the basis of PET findings, with reference to CT findings.
On PET/CT and PET/MRI images, lymph node metastasis diagnosis was based on the abnormally high uptake of FDG in the pelvic lymph nodes (i.e., exceeding normal muscle or exceeding normal lymph nodes at the same level in the contralateral pelvis that corresponded to the lymph node chains), regardless of whether their short-axis diameter was higher than 1 cm. In addition, abnormally high signal intensity on DWI was also considered as a positive sign of lymph node metastasis.
Histological examination
Tumor staging was performed according to the 2018 FIGO criteria. All surgical specimens were examined and reported by a gynecologic pathologist. A comprehensive histological evaluation was performed for each lesion, including histological type, tumor grade, myometrial and cervical stromal invasion, and lymph node status. For patients who did not undergo pelvic lymph node dissection, lymph node metastasis was confirmed at follow-up (at least 12 months). A decrease in lymph node size after treatment was considered a sign of malignancy.
Statistical analysis
The McNemar test was used to determine the statistical significance of differences in the accuracy of staging as determined by PET/MRI and PET/CT. The inter-reader agreement in PET/CT and PET/MR evaluation from the three readers was assessed using Kendall’s concordance coefficient (W). The associations between PET/MRI parameters and clinicopathological characteristics were analyzed using independent sample t-test (two sets of variables) and one-way analysis of variance, followed by Bonferroni posthoc test (three sets of variables). The correlations between PET/MRI parameters were assessed using Pearson’s correlation coefficient test. Receiver operating characteristic (ROC) curve was used to evaluate the value of PET/MRI quantitative parameters to differentiate FIGO stage I and FIGO stage II + III endometrioid carcinoma. The Youden index was used to obtain the cut-off value. The SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Statistical significance was set at p < 0.05.
Results
Patient characteristics
Altogether, 57 patients were enrolled in the study. The participants’ clinicopathological characteristics are summarized in Table 1.
Comparison between PET/MRI and PET/CT staging
The diagnostic results of PET/MRI and PET/CT staging for endometrial carcinoma are presented in Tables 2 and 3. The overall accuracy of FIGO staging for PET/MRI and PET/CT was 86.0% (49/57) and 77.2% (44/57), respectively (no significant difference, P = 0.180). PET/MRI overstaged the actual FIGO stage in five (8.8%) patients, whereas PET/CT overstaged the actual FIGO stage in nine (15.8%) patients. PET/MRI incorrectly classified two IA tumors as IB, one IA tumor as II, and one IA tumor and one II tumor as IIIC1. PET/CT incorrectly classified six IA tumors as IB, one IA tumor as II, and one IA tumor and one II tumor as IIIC1. PET/MRI understaged the actual FIGO stage in three (5.3%) patients, whereas PET/CT understaged the actual FIGO stage in four (7.0%) patients. PET/MRI incorrectly classified one IB tumor as IA, one II tumor as IB, and one IIIC1 tumor as II. PET/CT incorrectly classified two IB tumors as IA, one II tumor as IB, and one IIIC1 tumor as II. Kendall’s concordance coefficient showed that evaluation of PET/CT and PET/MR images was highly concordant among the three readers (W = 0.883, p < 0.001; W = 0.915, p < 0.001, respectively).
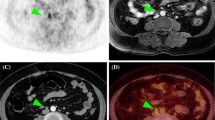
The sensitivity, specificity, and accuracy for detecting myometrial invasion were 88.9%, 94.9%, and 93.0% for PET/MRI and 61.1%, 79.5%, and 73.7% for PET/CT, respectively (Table 4; no significant difference, P = 1). PET/MRI incorrectly estimated the depth of myometrial invasion in four tumors, whereas PET/CT incorrectly estimated the depth of myometrial invasion in 10 tumors. Figure 2 depicts the detection of myometrial invasion. The sensitivity, specificity, and accuracy for detecting cervical invasion were 81.3%, 95.1%, and 91.2% for PET/MRI, and 81.3%, 92.7%, and 89.5% for PET/CT, respectively (Table 4; no significant difference, P = 1). PET/MRI incorrectly estimated cervical invasion in five tumors, whereas PET/CT incorrectly estimated cervical invasion in six tumors. For one case of stage IIIC1 tumor, PET/CT incorrectly classified the absence of cervical invasion as the presence of cervical invasion. Meanwhile, PET/MRI classification was correct, although this did not affect the FIGO stage of the tumor. Figure 3 depicts the detection of cervical invasion. In the patient-based analysis, the sensitivity, specificity, and accuracy for detecting pelvic lymph node metastasis were 87.5%, 95.9%, and 94.7% for both PET/MRI and PET/CT, respectively (Table 4; no significant difference, P = 1). Both PET/MRI and PET/CT incorrectly diagnosed pelvic lymph node metastasis in three tumors. For one case of stage IA tumor and one case of stage II tumor, both PET/MRI and PET/CT had false-positive results, with abnormally high uptake values in pelvic lymph nodes, although the pathological results were negative. For one case of stage IIIC1 tumor, both PET/MRI and PET/CT had a false-negative result. Figure 4 depicts the detection of pelvic lymph node metastasis.
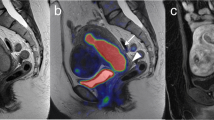
FIGO stage IB, pathologically confirmed as highly differentiated adenocarcinoma in a 64-year-old female patient. A PET/MR images. B MR T2-weighted images. C PET/CT images. D CT images. PET/MRI and MRI show the endometrial carcinoma invading > 50% of the myometrium. PET/CT shows the endometrial carcinoma invading < 50% of the myometrium, while CT is difficult to show the border of the tumor. The PET/MRI staging was consistent with the pathological stage, namely FIGO stage IB. The PET/CT staging was IA. The white arrow indicates endometrial carcinoma. FIGO, International Federation of Gynecology and Obstetrics; PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography
FIGO stage II pathologically confirmed moderately differentiated adenocarcinoma in a 57-year-old female patient. A Axial PET/MR images. B Axial MR T2-weighted images. C Axial PET/CT images. D Axial CT images. E Sagittal PET/MR images. F Sagittal PET/CT images. PET/MRI and MRI show the endometrial carcinoma invading the cervical stroma. PET/CT also shows the endometrial carcinoma invading the cervical stroma, while CT is difficult to determine the extension of the tumor. The PET/MRI and PET/CT staging were consistent with the pathological stage, namely FIGO stage II. The white arrow indicates endometrial carcinoma. FIGO, International Federation of Gynecology and Obstetrics; PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography
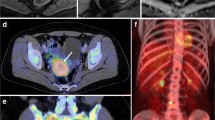
FIGO stage IIIC1, pathologically confirmed moderately differentiated adenocarcinoma in a 48-year-old female patient. A PET/MR images. B MR T2-weighted images. C PET/CT images. D CT images. Both PET/MRI and PET/CT show swelling of the left pelvic lymph node and high FDG uptake. The PET/MRI and PET/CT staging were consistent with the pathological stage, namely FIGO stage IIIC1. The white arrow indicates endometrial carcinoma, and the red arrow indicates metastatic lymph node. FIGO, International Federation of Gynecology and Obstetrics; PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography
Associations between PET/MRI quantitative parameters and clinicopathological characteristics
According to Table 5, SUVmax, ADCmean, ADCmin, SUVmax/ADCmean, and SUVmax/ADCmin showed a significant difference between stage I and stage III tumors (P < 0.001–0.021). Only SUVmax/ADCmin exhibited a significant difference between stage I and stage II tumors (P = 0.043). No parameters demonstrated a significant difference between stage II and stage III tumors.
Tumors with a myometrial invasion depth ≥ 50% had higher SUVmax/ADCmin and lower ADCmin than those with a myometrial invasion depth < 50% (P = 0.047 and 0.021, respectively). Tumors with cervical invasion had higher SUVmax/ADCmin and lower ADCmin than those without cervical invasion (P = 0.010 and 0.003, respectively). Tumors with positive pelvic lymph node metastasis had higher SUVmax, SUVmax/ADCmean, and SUVmax/ADCmin than those with negative pelvic lymph node metastasis (P = 0.022, 0.007, 0.002, respectively). Tumors with positive pelvic lymph node metastasis had lower ADCmin than those with negative pelvic lymph node metastasis (P = 0.030). ADCmean and SUVmax/ADCmin indicated a significant correlation with tumor grade (P = 0.048 and 0.036, respectively). No significant correlations were identified between PET/MRI quantitative parameters and histological type. No correlation was identified between SUVmax and ADCmean (P = 0.390) as well as between SUVmax and ADCmin (P = 0.479).
PET/MRI quantitative parameters for staging of endometrial carcinoma
ROC curve results of SUVmax, ADCmean, ADCmin, SUVmax/ADCmean, and SUVmax/ADCmin for differentiating FIGO stage I and FIGO stage II + III endometrial carcinoma are listed in Table 6. SUVmax/ADCmin had a higher area under the curve (AUC) for differentiating the FIGO stage of endometrial carcinoma than other PET/MRI parameters (AUC = 0.843, 95% CI = 0.744–0.943, P < 0.001). The cut-off value of SUVmax/ADCmin was 37.16 according to the Youden index. Tumor SUVmax/ADCmin of ≥ 37.16 had a sensitivity of 65% and specificity of 89% for differentiating the endometrial carcinoma’s FIGO stage. The ROC curves of SUVmax, ADCmean, ADCmin, SUVmax/ADCmean, and SUVmax/ADCmin are depicted in Fig. 5.
Discussion
In comparing the values of PET/MRI and PET/CT for staging in patients with endometrial carcinoma, PET/MRI had higher accuracy for FIGO stage I tumors, while the accuracy was similar in stage II and III tumors. Regarding the associations between PET/MRI quantitative parameters and tumor stage and other clinicopathological characteristics, SUVmax/ADCmin was correlated with more clinicopathological characteristics. Therefore, PET/MRI can be used as an effective diagnostic tool for preoperative staging of endometrial carcinoma.
Pathological examination is the gold standard for endometrial carcinoma staging. However, such pathological examinations can be traumatic to patients. Moreover, making an accurate diagnosis of tumor invasion depth and adjacent organ invasion can be difficult. Therefore, precise staging based on imaging has received greater research attention in recent years. Integrated 18F-FDG PET/MRI can provide the high-density resolution of soft tissues and useful functional information (e.g., metabolism and diffusion). Thus, PET/MRI has been applied in studies of clinical gynecological malignancies [11,12,13,14].
Our results revealed that PET/MRI staging, which was performed using an integrated PET/MRI scanner, had an excellent diagnostic performance for endometrial carcinoma. PET/MRI had higher diagnostic accuracy for stage I tumors than PET/CT. The diagnosis of stage I endometrial carcinoma is mainly associated with the depth of myometrial invasion. Compared with CT and PET, MRI-T2WI can display the cervix, the junctional zone, the myometrial layer, and endometrial zonal structures in all layers, which is essential for evaluating early-stage endometrial cancer. The technical principle that underlies DWI involves detecting and comparing the diffusion velocity of water molecules between cancer cells and normal cells, which can identify signs of T2WI sequence interference that is caused by irregular endometrial hyperplasia. DWI can complement routine sequences to further improve the advantages of MRI positioning [15, 16]. Building on the merits of T2WI and DWI, integrated PET/MRI is advantageous over PET/CT for diagnosing the myometrial invasion of endometrial carcinoma. Bian et al. have reported that integrated PET/MRI proved significantly more accurate for detecting myometrial invasion than PET/CT (81.8% vs. 45.9%). This is similar to our results, although the former study did not allow patients to simultaneously undergo PET/MRI and PET/CT [17]. Moreover, Tsuyoshi et al. have reported that the accuracy of PET/MRI for detecting myometrial invasion was slightly higher than that of contrast-enhanced MRI (88.9% vs. 86.1%) [18].
The PET/MRI staging accuracy for stages II and III tumors has been observed to be similar to that of PET/CT. Stages II and III are distinguished mainly according to tumor invasion location and lymph node metastasis. Our study indicated that the accuracy of PET/MRI for detecting cervical invasion was slightly higher than that of PET/CT, whereas both PET/MRI and PET/CT were equally accurate in identifying pelvic lymph node metastasis. PET can provide metabolic information and, therefore, has significant advantages in detecting metastatic lesions. Similar to our results, Kitajima et al. have revealed that the accuracy of fused PET/MRI in diagnosing lymph node metastasis was equivalent to that of PET/CT (96.7%) [4]. Moreover, Stecco et al. have reported that on a per-patient basis, fused PET/MRI had the same accuracy (85.1%) as PET/CT in detecting lymph node metastasis. Meanwhile, on a per-node basis, the accuracy of PET/MRI was greater than that of PET/CT (91.2% vs. 87%) owing to the high sensitivity of DWI [19]. Hence, DWI may increase the sensitivity of PET for diagnosing lymph node metastasis.
In addition to morphological staging, PET/MRI can also provide various functional parameters for tumor assessment. Previous studies have demonstrated the correlations between ADC values and clinicopathological characteristics of endometrial carcinoma such as tumor stage, tumor grade, tumor invasion location, and lymph node metastasis [9, 10, 20,21,22]. Recently, Gai et al. have reported that SUVmax derived from PET/CT was correlated with FIGO staging, tissue grading, depth of myometrial invasion, and lymph node metastasis [23]. However, these studies only used SUV or ADC values alone, our study used integrated PET/MRI to explore the associations between functional parameters and clinicopathological characteristics. Here, SUVmax/ADCmin correlated with clinicopathological characteristics of endometrial carcinoma more strongly than any other PET/MRI parameter. Furthermore, the ROC curve demonstrated that the SUVmax/ADCmin resulted in higher AUC for differentiating stage I tumors and other stages of endometrial carcinoma. It should be emphasized that the SUV values of the previous study [23] were obtained from PET/CT images, while the SUV values of our study were obtained from PET/MRI images. For the same patient, SUVmax measured from PET/CT and PET/MRI images are likely to have some differences, possibly due to the effects of the attenuation correction method and the PET scanner. This may lead to differences between our results and those from previous reports [24, 25]. PET and DWI can reflect the biological information of tumors from two aspects of metabolism and diffusion, respectively. Higher SUV values and lower ADC values generally reflect the more aggressive biological behavior of malignant tumors [26, 27]. Therefore, multiparametric PET/MR imaging may help to better understand tumor biology and help to personalize treatment plans and monitor treatment effects.
This study had some limitations. First, since we only performed pelvic 18F-FDG PET/MRI scanning, patients with stage IIIC2 and stage IV carcinoma were not included. Therefore, the diagnostic value of PET/MRI for distant metastasis could not be evaluated. Second, a certain interval between the PET/CT and PET/MRI examinations was present, which may have affected the results, including the SUV value and PET signal. Third, due to the limitations of the software, we could not perform ADC measurements on the entire tumor lesions, but only selected the slices containing the largest tumor diameter for measurements. This could have lead to inaccurate measurement results. Finally, the number of our patients was limited, and larger sample sizes will be required for future studies.
Conclusions
PET/MRI had higher accuracy for endometrial carcinoma staging, mainly for FIGO stage I tumors. This was mainly owing to PET/MRI having higher diagnostic accuracy for detecting the depth of myometrial invasion. Moreover, among all the measured PET/MRI quantitative parameters, SUVmax/ADCmin had the strongest association with clinicopathological characteristics. Thus, PET/MRI may be a more valuable diagnostic tool to replace traditional imaging modalities for preoperative staging in patients with endometrial carcinoma. Our findings may help physicians in selecting more appropriate individualized treatment plans for patients.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- FIGO:
-
International Federation of Gynecology and Obstetrics
- MRI:
-
Magnetic resonance imaging
- PET/CT:
-
Positron emission tomography/computed tomography
- 18F-FDG:
-
18F-fluorodeoxyglucose
- T2WI:
-
T2-weighted images
- TR:
-
Repetition time
- TE:
-
Echo time
- FOV:
-
Field of view
- DWI:
-
Diffusion-weighted imaging
- VCAR:
-
Volume computer-assisted reading
- SUVmax:
-
Maximum standardized uptake value
- ADC:
-
Apparent diffusion coefficient; ROI: region of interest
- AUC:
-
Area under the curve
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Koskas M, Amant F, Mirza MR, Creutzberg CL. Cancer of the corpus uteri: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):45–60. https://doi.org/10.1002/ijgo.13866.
Gee MS, Atri M, Bandos AI, Mannel RS, Gold MA, Lee SI. Identification of Distant Metastatic Disease in Uterine Cervical and Endometrial Cancers with FDG PET/CT: Analysis from the ACRIN 6671/GOG 0233 Multicenter Trial. Radiology. 2018;287(1):176–84. https://doi.org/10.1148/radiol.2017170963.
Kitajima K, Suenaga Y, Ueno Y, Kanda T, Maeda T, Takahashi S, et al. Value of fusion of PET and MRI for staging of endometrial cancer: comparison with 18F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur J Radiol. 2013;82(10):1672–6. https://doi.org/10.1016/j.ejrad.2013.05.005.
Musafargani S, Ghosh KK, Mishra S, Mahalakshmi P, Padmanabhan P, Gulyás B. PET/MRI: a frontier in era of complementary hybrid imaging. Eur J Hybrid Imaging. 2018;2(1):12. https://doi.org/10.1186/s41824-018-0030-6.
Zukotynski KA, Kim CK. Molecular imaging and precision medicine in uterine and ovarian cancers. PET Clin. 2017;12(4):393–405. https://doi.org/10.1016/j.cpet.2017.05.007.
Ohliger MA, Hope TA, Chapman JS, Chen LM, Behr SC, Poder L. PET/MR Imaging in gynecologic oncology. Magn Reson Imaging Clin N Am. 2017;25(3):667–84. https://doi.org/10.1016/j.mric.2017.03.012.
Umutlu L, Antoch G, Herrmann K, Grueneisen J. PET/MR imaging of the female pelvis. Semin Nucl Med. 2019;49(6):512–20. https://doi.org/10.1053/j.semnuclmed.2019.06.013.
Satta S, Dolciami M, Celli V, Di Stadio F, Perniola G, Palaia I, et al. Quantitative diffusion and perfusion MRI in the evaluation of endometrial cancer: validation with histopathological parameters. Br J Radiol. 2021;94(1125):20210054. https://doi.org/10.1259/bjr.20210054.
Jiang JX, Zhao JL, Zhang Q, Qing JF, Zhang SQ, Zhang YM, et al. Endometrial carcinoma: diffusion-weighted imaging diagnostic accuracy and correlation with Ki-67 expression. Clin Radiol. 2018;73(4):413.e1-413.e6. https://doi.org/10.1016/j.crad.2017.11.011.
Schwartz M, Gavane SC, Bou-Ayache J, Kolev V, Zakashansky K, Prasad-Hayes M, et al. Feasibility and diagnostic performance of hybrid PET/MRI compared with PET/CT for gynecological malignancies: a prospective pilot study. Abdom Radiol (NY). 2018;43(12):3462–7. https://doi.org/10.1007/s00261-018-1665-2.
Khan SR, Arshad M, Wallitt K, Stewart V, Bharwani N, Barwick TD. What’s new in imaging for gynecologic cancer? Curr Oncol Rep. 2017;19(12):85. https://doi.org/10.1007/s11912-017-0640-3.
Nakajo K, Tatsumi M, Inoue A, Isohashi K, Higuchi I, Kato H, et al. Diagnostic performance of fluorodeoxyglucose positron emission tomography/magnetic resonance imaging fusion images of gynecological malignant tumors: comparison with positron emission tomography/computed tomography. Jpn J Radiol. 2010;28(2):95–100. https://doi.org/10.1007/s11604-009-0387-3.
Brendle CB, Schmidt H, Fleischer S, Braeuning UH, Pfannenberg CA, Schwenzer NF. Simultaneously acquired MR/PET images compared with sequential MR/PET and PET/CT: alignment quality. Radiology. 2013;268(1):190–9. https://doi.org/10.1148/radiol.13121838.
Koplay M, Dogan NU, Erdogan H, Sivri M, Erol C, Nayman A, et al. Diagnostic efficacy of diffusion-weighted MRI for pre-operative assessment of myometrial and cervical invasion and pelvic lymph node metastasis in endometrial carcinoma. J Med Imaging Radiat Oncol. 2014;58(5):538–46; quiz 648. https://doi.org/10.1111/1754-9485.12209.
Beddy P, Moyle P, Kataoka M, Yamamoto AK, Joubert I, Lomas D, et al. Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2012;262(2):530–7. https://doi.org/10.1148/radiol.11110984.
Bian LH, Wang M, Gong J, Liu HH, Wang N, Wen N, et al. Comparison of integrated PET/MRI with PET/CT in evaluation of endometrial cancer: a retrospective analysis of 81 cases. PeerJ. 2019;7:e7081. https://doi.org/10.7717/peerj.7081.
Tsuyoshi H, Tsujikawa T, Yamada S, Okazawa H, Yoshida Y. Diagnostic value of 18F-FDG PET/MRI for staging in patients with endometrial cancer. Cancer Imaging. 2020;20(1):75. https://doi.org/10.1186/s40644-020-00357-4.
Stecco A, Buemi F, Cassarà A, Matheoud R, Sacchetti GM, Arnulfo A, et al. Comparison of retrospective PET and MRI-DWI (PET/MRI-DWI) image fusion with PET/CT and MRI-DWI in detection of cervical and endometrial cancer lymph node metastases. Radiol Med. 2016;121(7):537–45. https://doi.org/10.1007/s11547-016-0626-5.
Husby JA, Salvesen ØO, Magnussen IJ, Trovik J, Bjørge L, Salvesen HB, et al. Tumour apparent diffusion coefficient is associated with depth of myometrial invasion and is negatively correlated to tumour volume in endometrial carcinomas. Clin Radiol. 2015;70(5):487–94. https://doi.org/10.1016/j.crad.2014.12.016.
Ozturk M, Kalkan C, Danaci M, Kefeli M. Diffusion-weighted MRI at 3T in endometrial cancer: correlation of apparent diffusion coefficient with histopathological prognostic parameters. J Coll Physicians Surg Pak. 2021;31(12):1399–405. https://doi.org/10.29271/jcpsp.2021.12.1399.
Reyes-Pérez JA, Villaseñor-Navarro Y, Jiménez de Los Santos ME, Pacheco-Bravo I, Calle-Loja M, Sollozo-Dupont I. The apparent diffusion coefficient (ADC) on 3-T MRI differentiates myometrial invasion depth and histological grade in patients with endometrial cancer. Acta Radiol. 2020;61(9):1277–86. https://doi.org/10.1177/0284185119898658.
Gai QZ, Lv YB, Li GY, Zhang DQ, Gao Z, Fang XH. Value of metabolic parameters of primary lesions examined by 18F-FDG PET/CT for endometrial cancer in preoperative evaluation. Eur Rev Med Pharmacol Sci. 2021;25(6):2493–502. https://doi.org/10.26355/eurrev_202103_25412.
Heusch P, Buchbender C, Beiderwellen K, Nensa F, Hartung-Knemeyer V, Lauenstein TC, et al. Standardized uptake values for [18F] FDG in normal organ tissues: comparison of whole-body PET/CT and PET/MRI. Eur J Radiol. 2013;82(5):870–6. https://doi.org/10.1016/j.ejrad.2013.01.008.
Kershah S, Partovi S, Traughber BJ, Muzic RF Jr, Schluchter MD, O’Donnell JK, et al. Comparison of standardized uptake values in normal structures between PET/CT and PET/MRI in an oncology patient population. Mol Imaging Biol. 2013;15(6):776–85. https://doi.org/10.1007/s11307-013-0629-8.
Zhang L, Sun H, Du S, Xu W, Xin J, Guo Q. Evaluation of 18F-FDG PET/CT parameters for reflection of aggressiveness and prediction of prognosis in early-stage cervical cancer. Nucl Med Commun. 2018;39(11):1045–52. https://doi.org/10.1097/MNM.0000000000000909.
Inoue C, Fujii S, Kaneda S, Fukunaga T, Kaminou T, Kigawa J, et al. Correlation of apparent diffusion coefficient value with prognostic parameters of endometrioid carcinoma. J Magn Reson Imaging. 2015;41(1):213–9. https://doi.org/10.1002/jmri.24534.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (82171910); the Natural Science Foundation of Liaoning Province (2019-MS-373); the Shenyang High Level Innovative Talents Support Program (RC210138) and 345 Talent Project (M0377).
Author information
Authors and Affiliations
Contributions
Conception and design: YY, LZ and HZS. Investigation and data curation: YY and BS. Methodology: YY and LZ. Software: BW. Supervision: HZS. Writing – original draft: YY and LZ. Writing – review & editing: BS, BW and HZS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research was performed in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Shengjing Hospital of China Medical University. Written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, Y., Zhang, L., Sultana, B. et al. Diagnostic value of integrated 18F-FDG PET/MRI for staging of endometrial carcinoma: comparison with PET/CT. BMC Cancer 22, 947 (2022). https://doi.org/10.1186/s12885-022-10037-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10037-0