Abstract
Background
Routine measurement of tumor markers is not recommended in daily clinical practice for patients with cancer of unknown primary (CUP). We evaluated the diagnostic value of tumor markers in identifying favorable or unfavorable subsets in patients with CUP.
Methods
We retrospectively reviewed the medical records of patients who were diagnosed with CUP between October 2010 and July 2015 at the National Cancer Center Hospital. The tumor markers of the patients were examined, including squamous cell carcinoma antigen, cytokeratin fraction, carcinoembryonic antigen, sialyl Lewis X, neuron-specific enolase, pro-gastrin-releasing peptide, α-fetoprotein, protein induced by vitamin K absence or antagonist II, prostate-specific antigen, soluble interleukin-2 receptor, carbohydrate antigen 19–9, cancer antigen 125, cancer antigen 15–3, NCC-ST-439 (ST439), elastase-1, human chorionic gonadotropin, and sialyl-Tn (STN).
Results
Among 199 patients with suspected CUP, 90 were diagnosed with confirmed CUP (12 in the favorable subset and 78 in the unfavorable subset). No tumor markers showed 100% sensitivity for unfavorable subsets. ST439 (p = 0.03) and STN (p = 0.049) showed 100% specificity for unfavorable subsets.
Conclusions
For patients with suspected CUP who show elevated ST439 or STN levels, the treatment strategy should be based on the premise that the patient is likely to be placed in the unfavorable subset.
Similar content being viewed by others
Background
Cancer of unknown primary (CUP) is defined as a cancer lacking any detectable primary site after full evaluation. Only metastatic sites are histologically confirmed. CUP is a rare malignancy, accounting for approximately 3%–5% of all newly diagnosed patients with malignancies [1]. In addition, some are found to be non-cancerous during a thorough examination [2]. Approximately 20% of patients with CUP have a favorable prognosis [1]. This patient group includes men with adenocarcinoma of bone metastases and elevated prostate-specific antigen (PSA), women with papillary adenocarcinoma of the peritoneal cavity, women with adenocarcinoma involving the axillary lymph nodes, patients with poorly differentiated carcinoma with midline distribution, patients with well-differentiated neuroendocrine tumors or poorly differentiated neuroendocrine carcinomas, patients with squamous cell carcinoma involving cervical lymph nodes, patients with adenocarcinoma with a colon cancer profile, and patients with squamous cell carcinoma of isolated inguinal adenopathy [1]. These patients should be identified at initial evaluation and receive specific therapy to extend the prognosis.
Approximately 80% of patients with CUP do not have any favorable subsets, and the prognosis of these patients is worse [1]. The median survival time of these patients is only 6–7 months [1], and a standard of care for this patient group is absent [3]. Although most patients with unfavorable subsets are treated based on suspected tissue-of-origin, there is no survival advantage compared with empiric platinum-based combination chemotherapy [4]. A previous report analyzed 93 patients who received platinum-based combination chemotherapy, and the response rate was 39.8% [5]. A meta-analysis has shown that no type of chemotherapy has been proven to lengthen survival time [6].
Evaluation of tumor markers is useful for diagnosis and the reduction of inappropriate diagnostic tests for patients with suspected malignancy [7]. Although patients with CUP commonly overexpress several tumor markers, the diagnostic, predictive, and prognostic utilities are unexplained. Routine measurement of tumor markers for patients with CUP is not recommended in daily clinical practice [8].
Tumor markers are not recommended for finding the primary site of CUP, except in limited situations. The guidelines published by the European Society for Medical Oncology mention that useful tumor markers for diagnosing the primary tumor site include human chorionic gonadotropin (hCG) and α-fetoprotein (AFP) in patients with poorly differentiated carcinoma of midline distribution for germ-cell tumors, PSA in men with bone metastases for prostate cancer, cancer antigen 125 (CA125) in women with primary peritoneal serous adenocarcinoma for ovarian, fallopian tube, and peritoneal cancers, and thyroglobulin for differentiated thyroid cancer [1, 8,9,10,11,12,13].
Squamous cell carcinoma antigen is a marker that is elevated in squamous cell carcinomas, such as head and neck, esophageal, and uterine cervical cancers [14]. Cytokeratin fraction (cytokeratin 19 fragment) is elevated in non-small cell lung cancers [15]. Carcinoembryonic antigen, present in the fetal digestive cells, is elevated in gastric, colorectal, and other cancers of the digestive system [16]. Sialyl Lewis X is a polymeric glycoprotein elevated in lung, ovarian, and pancreatic cancers [17]. Neuron-specific enolase increases with the tumorigenesis in neuroendocrine cells, such as in small-cell lung cancer and neuroblastoma [18]. Pro-gastrin-releasing peptide is a gastrointestinal hormone; it is a marker for small-cell lung cancer [19]. Protein induced by vitamin K absence or antagonist II is precursor of the coagulation factor prothrombin; it is a marker for hepatocellular carcinoma [20]. Soluble interleukin-2 receptor is the alpha chain of interleukin 2 receptor, which exists in the free-form in blood; it is elevated in lymphoid malignancies such as non-Hodgkin's lymphoma, adult T-cell lymphoma/leukemia, and acute lymphocytic leukemia [21]. Carbohydrate antigen 19–9 is a cell surface glycoprotein complex; it is elevated in gastrointestinal cancers, such as pancreatic, gallbladder, and bile duct cancers [22]. Cancer antigen 15–3 is a mucin-type glycoprotein, which is elevated in breast cancers [23]. NCC-ST 439 is a mucin-type glycoprotein that is elevated in breast and gastrointestinal cancers [24]. Elastase-1 is a proteolytic enzyme; it is a marker for pancreatic cancer [25]. Sialyl-Tn is a sugar chain antigen; it is elevated in ovarian and gastrointestinal cancers [26]. However, these tumor markers are not recommended for identifying the primary site of CUP.
The diagnostic evaluation of patients with CUP takes time, sometimes up to several months. In addition, deciding whether a subset is favorable or not must be carefully considered because it has a great impact on the treatment selection and prognosis. Only a few studies have examined whether tumor markers can be used to classify subsets. If tumor markers could be used to classify favorable and unfavorable subsets, then the best treatment option could be more quickly recommended to patients with CUP. Identifying favorable subsets during the initial evaluation can lead to appropriate treatment and prolonged survival. We evaluated the diagnostic value of tumor markers that are routinely used in our hospital for identifying favorable or unfavorable subsets in patients with CUP.
Methods
Study cohort
We retrospectively reviewed the medical records of patients who were diagnosed with CUP at National Cancer Center Hospital (NCCH) (Tokyo, Japan) between October 2010 and July 2015. This single-institution medical record-based retrospective observational study was approved by the Institutional Review Board of NCCH (NCCH 2012–335), which waived the requirement for informed consent. Patient registration was based on an opt-out model. The study was conducted according to the principles of the Declaration of Helsinki.
Diagnosis of CUP
Since our facility is a cancer-specialized hospital, most patients with suspected cancer were referred to us before they had undergone adequate examination. Patients then underwent a fundamental workup and additional focused imaging based on their cancer distribution and histopathology. Examinations were performed according to the guidelines of the European Society for Medical Oncology and the Japanese Society of Medical Oncology [12].
Patients were evaluated through an initial workup, including physical examination, laboratory studies (a complete blood count, urinalysis, basic serum chemistries, and tumor marker analysis), and imaging procedures (computed tomography scan or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis). The selected women were evaluated with a pelvic examination by a gynecologist or mammography and breast MRIs to look for breast lesions. For selected men or women, an examination of the prostate or urinary tract by a urologist was completed to look for urinary tract lesions.
The diagnosis of CUP was confirmed when the primary site of cancer was unknown after these initial workups, based on the consensus of medical oncology specialists.
Tumor markers
The patients were evaluated for the following tumor markers: squamous cell carcinoma antigen (cut-off: 1.5 ng/ml), cytokeratin 19 fragment (cut-off: 2.2 ng/ml), carcinoembryonic antigen (cut-off: 5.0 ng/ml), sialyl Lewis X (cut-off: 38.0 U/ml), neuron-specific enolase (cut-off: 15.0 ng/ml), pro-gastrin-releasing peptide (cut-off: 81.0 pg/ml), AFP (cut-off: 10.0 ng/ml), protein induced by vitamin K absence or antagonist II (PIVKA-II) (cut-off: 40 mAU/ml), PSA (cut-off: 2.7 ng/ml), soluble interleukin-2 receptor (sIL-2R) (cut-off: 587 U/ml), carbohydrate antigen 19–9 (cut-off: 37 U/ml), CA125 (cut-off: 35 U/ml), cancer antigen 15–3 (cut-off: 28 U/ml), NCC-ST 439 (ST439) (cut-off: 4.5 U/ml), elastase-1 (cut-off: 300 ng/dl), hCG (cut-off: 3.0 mIU/ml), and sialyl-Tn (STN) (cut-off: 45.0 U/ml). These cut-off values were based on the facility standard.
Definition of favorable and unfavorable subsets
The following patient populations were placed in the favorable subset: men with adenocarcinoma who have bone metastases and elevated PSA; women with adenocarcinoma who have peritoneal carcinomatosis; women with adenocarcinoma who have axillary lymph node metastases; patients with poorly differentiated carcinoma with midline distribution; patients with well-differentiated neuroendocrine tumors or poorly differentiated neuroendocrine carcinomas; patients with squamous cell carcinoma involving the cervical nodes; patients with a colon cancer profile; and patients with squamous cell carcinoma of isolated inguinal adenopathy [12, 13]. Patients without any of these factors were placed in the unfavorable subset.
Statistical analyses
Univariate analyses were performed to evaluate the correlation between tumor markers and favorable or unfavorable subsets. All statistical analyses were performed using JMP software (version 14.3.0 for Windows; SAS Institute Japan Inc., Cary, NC, USA), and results were considered significant with a two-sided p-value of < 0.05.
Results
Patient characteristics
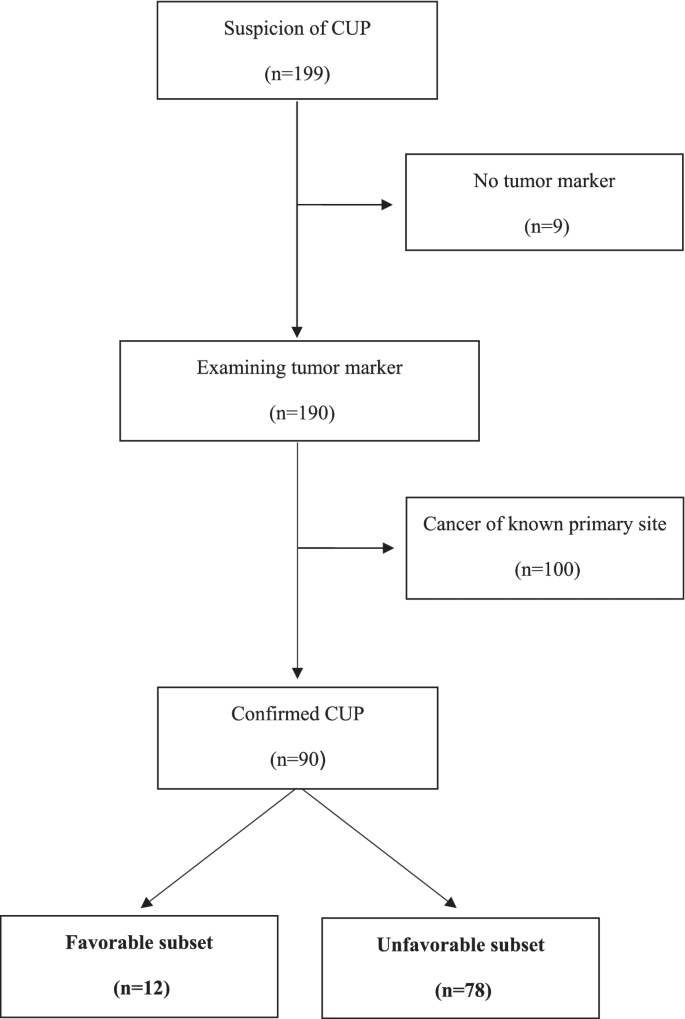
Between October 2010 and July 2015, 199 patients with suspected CUP were referred to the NCCH. Among them, 190 patients were examined via tumor markers, 100 were diagnosed with cancer of known primary site, and 90 were diagnosed with confirmed CUP (12 in the favorable subset and 78 in the unfavorable subset) (Fig. 1).
Median age was 68 years (range: 47–78) in the favorable subset and 66 years (range: 34–83) in the unfavorable subset. Females accounted for 83% of the favorable subset and 55% of the unfavorable subset. The Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of most patients was 0 or 1. The estimated primary organs of 12 patients in the favorable subset were breast (5 patients), ovary/peritoneum (5 patients), and skin (2 patients). The characteristics of the patients are shown in Table 1.
Sensitivity and specificity of tumor markers
No tumor markers showed 100% sensitivity for unfavorable subsets. However, PIVKA-II, PSA, ST439, elastase-1, and STN showed 100% specificity for unfavorable subsets. Among them, ST439 (p = 0.03) and STN (p = 0.049) showed a significant correlation between favorable and unfavorable subsets (Table 2).
Treatment regimen
Among the patients placed in the unfavorable subset, many were treated with drug therapy based on suspected tissue-of-origin, such as lung, based on the consensus of medical oncologists (Table 3 and 4). Patients within the normal range of ST439 were significantly more likely to receive drug therapy for ovarian cancer (p = 0.004). Patients with elevated ST439 levels were significantly more likely to receive drug therapy for colorectal (p = 0.036) or salivary gland cancer (p = 0.031).
Discussion
Despite tumor markers being easily accessible, their diagnostic ability for patients in unfavorable subsets had previously been unknown. Thus, we have evaluated tumor markers to identify patients in unfavorable subsets. ST439 and STN showed 100% specificity for patients in the unfavorable subset. No patients with elevated ST439 or STN above the reference value in the favorable subset were detected. In about 30% of the patients in the unfavorable subset, ST439 or STN was above the reference range. These results demonstrate that when ST439 or STN is elevated at the initial workup, a patient could be included in the unfavorable subset. In CUP treatment, the final diagnosis is not based soley on pathology, but on a combination of clinical factors. In addition, the standard of care for patients in unfavorable subsets is absent [3] and their prognosis is worse [1]. Therefore, it is necessary to differentiate between the favorable and unfavorable subsets within a limited time frame, such as one month [12]. Based on the findings from this study, routine evaluation of ST439 and STN could enable screening for treatment-ineffective subsets and prognostic estimation. This would enable identifying unfavorable subsets during the initial assessment for CUP. Refraining from aggressive treatments for patients in unfavorable subsets, who have a poor ECOG-PS, and early preparation for palliative care could improve the patients' quality of life. Evaluation of ST439 and STN at a patient’s first visit may help in the initial diagnosis of CUP in daily practice.
On the contrary, markers such as CA125, hCG, and PSA did not show a significant correlation between favorable and unfavorable subsets. This suggests that these markers are helpful when confirming favorable subsets with other clinical findings but are difficult to use alone for distinguishing between favorable and unfavorable subsets. The favorable subset included only five patients whose estimated primary organ was ovarian/peritoneal. There were no patients whose estimated primary organ was prostate or germ cell. A larger sample size is needed for further assessment. In addition, recent advances in histopathological examination of germ-cell tumors and malignant lymphomas suggest that anaplastic carcinoma of median development may not remain in a favorable subset as previously thought [27]. Therefore, the diagnostic abilities of tumor markers associated with malignant lymphomas (sIL-2R) and germ-cell tumors (hCG and AFP) may be limited given the current state of medicine.
An analysis comparing tumor markers and survival outcomes could not be carried out in this study because anticancer therapy was selected based on CUP histology or metastatic distribution and varied from patient to patient. We selected a primary site-directed treatment based on the suspected primary organ evaluated by a panel of oncologists. A previous report showed that patients with unfavorable subset CUP whose suspected primary organ was breast or ovary had higher response rates and a better prognosis compared with other unfavorable subsets [28].
This study has several limitations. It had a retrospective design and a relatively small sample size, with all data obtained from a single institution. In addition, the cut-off values were selected based on the facility standard. Whether these values are appropriate for distinguishing between favorable and unfavorable subsets of patients with CUP is unknown. Moreover, it is unclear why ST439 and STN can identify favorable or unfavorable subsets.
Additional research is needed regarding tumor markers that can identify favorable or unfavorable subsets in patients with CUP. Tumor markers can be utilized for the diagnosis of CUP in daily clinical practice.
Conclusions
We evaluated diagnostic value of tumor markers in identifying favorable or unfavorable subsets in patients with CUP. ST439 and STN showed 100% specificity for the unfavorable subset. If ST439 or STN is elevated in patients with CUP, they could be included in the unfavorable subset.
Availability of data and materials
The datasets generated during and analyzed during the current study are not publicly available due to protect patient privacy but are available from the corresponding author on reasonable request.
Abbreviations
- AFP:
-
α-Fetoprotein
- CA125:
-
Cancer antigen 125
- CUP:
-
Cancer of unknown primary
- ECOG-PS:
-
Eastern cooperative oncology group-performance status
- hCG:
-
Human chorionic gonadotropin
- MRI:
-
Magnetic resonance imaging
- NA:
-
Not applicable
- NCCH:
-
National Cancer Center Hospital
- PIVKA-II:
-
Protein induced by vitamin K absence or antagonist II
- PSA:
-
prostate-specific antigen
- sIL-2R:
-
Soluble interleukin-2 receptor
- SLX:
-
Sialyl Lewis X
- ST439:
-
NCC-ST 439
- STN:
-
Sialyl-Tn
References
Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428–35.
Sato J, Shimoi T, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, et al. The incidence of nonmalignant diseases among patients with suspected carcinoma of unknown primary site. Intern Med. 2019;58:1423–8.
Tomuleasa C, Zaharie F, Muresan MS, Pop L, Fekete Z, Dima D, et al. How to diagnose and treat a cancer of unknown primary site. J Gastrointestin Liver Dis. 2017;26:69–79.
Kato S, Alsafar A, Walavalkar V, Hainsworth J, Kurzrock R. Cancer of unknown primary in the molecular era. Trends Cancer. 2021;7:465–77.
Yonemori K, Ando M, Shibata T, Katsumata N, Matsumoto K, Yamanaka Y, et al. Tumor-marker analysis and verification of prognostic models in patients with cancer of unknown primary, receiving platinum-based combination chemotherapy. J Cancer Res Clin Oncol. 2006;132:635–42.
Golfinopoulos V, Pentheroudakis G, Salanti G, Nearchou AD, Ioannidis JP, Pavlidis N. Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat Rev. 2009;35:570–3.
Molina R, Bosch X, Auge JM, Filella X, Escudero JM, Molina V, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463–74.
Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39:1990–2005.
Bugat R, Bataillard A, Lesimple T, Voigt JJ, Culine S, Lortholary A, et al. Summary of the Standards, Options and Recommendations for the management of patients with carcinoma of unknown primary site (2002). Br J Cancer. 2003;89(Suppl 1):S59-66.
Collado Martín R, GarcíaPalomo A, de la Cruz Merino L, BorregaGarcía P, Barón Duarte FJ. Spanish Society for Medical Oncology. Clinical guideline SEOM: cancer of unknown primary site. Clin Transl Oncol. 2014;16:1091–7.
Ettinger DS, Handorf CR, Agulnik M, Bowles DW, Cates JM, Cristea M, et al. Occult primary, version 3.2014. J Natl Compr Canc Netw. 2014;12:969–74 version 3.2014.
Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v133–8.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Occult primary (cancer of unknown primary [CUP]). Version 1. 2022. National Comprehensive Cancer Network. 2021. https://www.nccn.org/professionals/physician_gls/pdf/occult.pdf. Accessed 12 Apr 2022.
Derakhshan S, Poosti A, Razavi AE, Moosavi MA, Mahdavi N, Naieni FB, et al. Evaluation of squamous cell carcinoma antigen 1 expression in oral squamous cell carcinoma (tumor cells and peritumoral T-lymphocytes) and verrucous carcinoma and comparison with normal oral mucosa. J Appl Oral Sci. 2021;29:e20210374.
Dall’Olio FG, Abbati F, Facchinetti F, Massucci M, Melotti B, Squadrilli A, et al. CEA and CYFRA 21–1 as prognostic biomarker and as a tool for treatment monitoring in advanced NSCLC treated with immune checkpoint inhibitors. Ther Adv Med Oncol. 2020;12:1758835920952994.
Campos-da-Paz M, Dórea JG, Galdino AS, Lacava ZGM, de Fatima Menezes Almeida Santos M. Carcinoembryonic antigen (CEA) and hepatic metastasis in colorectal cancer: update on biomarker for clinical and biotechnological approaches. Recent Pat Biotechnol. 2018;12:269–79.
Fujita T, Murayama K, Hanamura T, Okada T, Ito T, Harada M, et al. CSLEX (Sialyl Lewis X) is a useful tumor marker for monitoring of breast cancer patients. Jpn J Clin Oncol. 2011;41:394–9.
Kečkéš Š, Palaj J, Waczulíková I, Dyttert D, Mojtová E, Kováč G, et al. Pretreatment levels of chromogranin A and neuron-specific enolase in patients with gastroenteropancreatic neuroendocrine neoplasia. In Vivo. 2021;35:2863–8.
Fang L, Huang Z, Lin Y, Fu J, Liang X, Liu F. Clinical application of pro-gastrin-releasing peptide. Clin Lab. 2018;64:1259–68.
Tarao K, Nozaki A, Komatsu H, Komatsu T, Taguri M, Tanaka K, et al. Real impact of tumor marker AFP and PIVKA-II in detecting very small hepatocellular carcinoma (≤ 2 cm, Barcelona stage 0) - assessment with large number of cases. World J Hepatol. 2020;12:1046–54.
Umino K, Fujiwara SI, Ikeda T, Kawaguchi SI, Toda Y, Ito S, et al. Predictive value of soluble interlukin-2 receptor level at diagnosis on the outcome for patients with classical Hodgkin lymphoma treated with ABVD with or without radiotherapy. Ann Hematol. 2019;98:2121–9.
Lee T, Teng TZJ, Shelat VG. Carbohydrate antigen 19–9 - tumor marker: Past, present, and future. World J Gastrointest Surg. 2020;12:468–90.
Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, et al. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–5.
Sakurai Y, Kodaira S, Teramoto T, Sugano K, Abe O, Ohtake H, et al. Clinical evaluation of serum level of NCC-ST-439 as a new tumor marker for colorectal diseases–fluctuation of serum NCC-ST-439 in patients with colorectal cancers before and after surgery. Gan To Kagaku Ryoho. 1989;16:3205–12.
Lim JH, Park JS, Yoon DS. Preoperative fecal elastase-1 is a useful prognostic marker following curative resection of pancreatic cancer. HPB (Oxford). 2017;19:388–95.
Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26–33.
Pentheroudakis G, Stoyianni A, Pavlidis N. Cancer of unknown primary patients with midline nodal distribution: midway between poor and favourable prognosis? Cancer Treat Rev. 2011;37:120–6.
Kodaira M, Yonemori K, Shimoi T, Yoshida A, Yoshida M, Kitano A, et al. Prognostic impact of presumed breast or ovarian cancer among patients with unfavorable-subset cancer of unknown primary site. BMC Cancer. 2018;18:176.
Acknowledgements
We thank all the patients whose data were used for the study. We thank Kyoko Onozawa for the secretarial assistance she provided. Editage (Cactus Communications) provided editorial support in the form of medical writing, table assembly, collating author comments, copyediting, fact-checking, referencing, and high-resolution image creation based on the authors’ detailed directions.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by ST. The first draft of the manuscript was written by ST and TS. All authors commented on previous versions of the manuscript. ST, TS, MY, MT, SY, CM, AS, SK, KY, YK, HO, TN, EN, KS, and KY read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The retrospective study protocol was approved by the National Cancer Center Research Ethics Review Committee (Tokyo, Japan; NCCH 2014–092), which waived the requirement for informed consent. All research was conducted in accordance with the Declaration of Helsinki. Patients are able to opt-out of the use of their data for research on the hospital’s website.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Takamizawa, S., Shimoi, T., Yoshida, M. et al. Diagnostic value of tumor markers in identifying favorable or unfavorable subsets in patients with cancer of unknown primary: a retrospective study. BMC Cancer 22, 412 (2022). https://doi.org/10.1186/s12885-022-09514-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09514-3