Abstract
Background
Therapeutic agents for dyslipidaemia, in particular statins, have been recently reported to suppress growth and metastasis of breast cancer. However, the predictive value of lipid control in breast cancer patients has not been discussed sufficiently. In addition, though immunometabolism is a relatively novel approach for tumour immunotherapy, the relationship between lipid metabolism and immune status has not been well documented. We therefore investigated the effects of lipid metabolism on antitumour immune response and cancer prognosis.
Methods
Except for patients with ductal carcinoma in situ, 938 patients treated with curative surgery were examined. The correlation between treatment for dyslipidaemia or serum lipid levels and clinicopathological features, including the prognosis, was evaluated retrospectively. Also, we stratified these results by intrinsic subtype of breast cancer, menopause, and type of therapeutic agents for dyslipidaemia. Moreover, neutrophil- to-lymphocyte ratio (NLR) and tumour-infiltrating lymphocytes (TILs) were used as indicators of systemic and local immune status, respectively.
Results
Of 194 patients treated for dyslipidaemia, recurrence-free survival (RFS) and overall survival (OS) did not differ significantly between users of drugs for dyslipidaemia and non-users (p = 0.775 and p = 0.304, log-rank, respectively). Among postmenopausal, hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer patients treated for dyslipidaemia, the good serum lipid control group had significantly better RFS (p = 0.014, log-rank), lower postoperative NLR (p = 0.012), and higher TILs in resected tissues (p = 0.024) than the poor control group. Multivariate analysis showed that postoperative serum lipid levels were a risk factor for recurrence (hazard ratio = 4.722, 95% confidence interval 1.006–22.161, p = 0.049).
Conclusions
Good control of serum lipid metabolism may improve the tumour immune microenvironment and prognosis in postmenopausal HR-positive/HER2-negative breast cancer patients.
Similar content being viewed by others
Background
Regulation and improvement of the tumour immune microenvironment are widely recognized to play an important role in cancer therapy [1, 2]. Immunometabolism is a relatively new field in tumour immunotherapy; the regulation of metabolism can affect the tumour immune microenvironment and enhance antitumour immunity [3,4,5,6]. For example, although some meta-analyses found a significant risk of breast cancer in women with type 2 diabetes mellitus [7, 8], the use of metformin in the treatment of type 2 diabetes mellitus has been reported to reduce cancer-related mortality [9]. Metformin’s antitumour activity has been associated with its modulation of components of the tumour immune microenvironment such as tumour-associated macrophages, myeloid-derived suppressor cells, and T-cells [3, 10, 11].
On the other hand, in the case of lipid metabolism, a positive association has been reported between obesity and the risk of breast cancer among postmenopausal, hormone receptor (HR)-positive women [12, 13]. Furthermore, some previous studies also have shown that the obesity prior to and after diagnosis were predictive of breast cancer recurrence and death, especially in HR positive type [14, 15]. Additionally, although epidemiologic evidence shows no association between the usage of statins, therapeutic agents for dyslipidaemia, and reduced incidence of breast cancer [16,17,18], it supports a protective effect of statins on reducing breast cancer recurrence or mortality [19]. Also, some studies suggested that lipophilic statins, but not hydrophilic statins, improved the prognosis in breast cancer patients [20, 21].
Several basic researches have shown the effects of therapeutic agents for dyslipidaemia, mainly statins, on breast cancer cells. Statins suppress cancer cell proliferation by inducing cell cycle arrest through the regulation of cyclin-dependent kinase 4/6 (CDK4/6) or cyclin D1 [22, 23]. Statins have also been shown to exert anti-angiogenic effects through a reduction in hypoxia-induced vascular endothelial growth factor secretion and expression of vascular endothelial growth factor receptor 2 and matrix metalloproteinase-9 in endothelial cells [24, 25]. Furthermore, statins have been shown to reduce the invasiveness and metastatic potential of breast cancer cells [26, 27]. However, limited information is clinically available on the systemic and local immune response to statin treatment in breast cancer patients. In this study, we evaluated the correlation between improvement in lipid metabolism along with antitumour immune response and prognosis in breast cancer patients treated with curative surgery.
Materials and methods
Patients
We analysed data from patients treated at the Osaka City General Hospital (Osaka, Japan) from the period between April 2010 and March 2017. A total of 1018 patients were diagnosed with breast cancer and underwent curative surgery during this period. We excluded 80 patients with ductal carcinoma in situ and included 938 breast cancer patients in this retrospective study. Postoperative adjuvant therapy was administered according to the intrinsic breast cancer subtype, and standard postoperative radiotherapy was administered to the remnant breast, if necessary. T and N factors as well as tumour stage were stratified based on the TNM Classification, UICC Seventh Edition [28]. Tumours were classified into intrinsic subtypes according to the immunohistochemical expression of the oestrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2). The HR-positive group comprised oestrogen receptor-positive and/or progesterone receptor-positive patients. All patients were examined for continuation of agents for dyslipidaemia before undergoing mastectomy or breast-conserving surgery.
This present study is exploratory research. First, we divided patients into two groups according to the use of agents for dyslipidaemia, and examined whether treatments for dyslipidaemia were associated with prognosis in breast cancer patients, as has been reported. Next, we evaluated serum lipid levels of patients treated with the drugs for dyslipidaemia before and after surgery. We defined patients whose postoperative serum lipid levels were in the normal range as the good control group and patients with high lipid levels as the poor control group, and examined whether lipid control by dyslipidaemia medication was associated with prognosis. We also performed subgroup analysis by menopause and intrinsic breast cancer subtypes to identify the group in which lipid control was most associated with recurrence. Finally, we examined the relationship between lipid control and clinicopathological features, including immune status, in the identified group.
We set two survival end points: (I) overall survival (OS) was defined as the time from surgery until death from any cause, and (II) recurrence-free survival (RFS) was defined as freedom from all loco-regional and distant recurrences. All patients were followed up by physical examination, blood tests, ultrasonography, computed tomography, and bone scintigraphy. The median follow-up period for the assessment of OS was 4.0 years (range, 0.1–8.9 years) and for RFS was 3.6 years (range, 0.1–8.9 years).
Blood sample analysis
Preoperative blood samples were obtained within one week before surgery, and postoperative blood samples were obtained annually. We evaluated serum lipid levels, including total cholesterol (categorized as low (< 150 mg/dL), normal (150–219 mg/dL), or high (> 219 mg/dL) and triglyceride (categorized as low (< 50 mg/dL), normal (50–149 mg/dL), or high (> 149 mg/dL)). The differential white blood cell count was analysed using a Coulter LH 750 Hematology Analyzer (Beckman Coulter, Brea, CA, USA). In this study, the neutrophil-to-lymphocyte ratio (NLR) was considered to be the systemic immune response indicator. NLR was calculated from the blood sample by dividing the absolute neutrophil count by the absolute lymphocyte count.
Histopathological evaluation
In this study, the density of tumour-infiltrating lymphocytes (TILs) was considered to be the local immune response indicator. TILs can be used as indicators of the tumour microenvironment and are important in predicting clinical outcomes in breast cancer [29, 30]. In this study, tumour specimens were used to evaluate TILs density. The assessment of haematoxylin and eosin (HE)-stained TILs was based on the criteria described by the International TILs Working Group 2014 [31]. TILs were defined as the infiltrating lymphocytes within the stromal compartment close to the tumour. To evaluate TILs, four fields of view in HE-stained areas were selected, and the percentage of TILs in each field was measured microscopically at 400× magnification. HE-stained TILs were defined to be high if TILs occupied > 10% and low when TILs occupied ≤ 10% of the HE-stained field of view (Supplementary Fig. S1).
Statistical analysis
Statistical analysis was performed using the JMP13 software program (SAS Institute, Cary, NC, USA). Associations among variables were analysed using chi-square (χ2) or Fisher’s exact test, as appropriate. OS and RFS were estimated using the Kaplan–Meier method and the log-rank test. Univariate and multivariate hazard ratios (HRs) and 95% confidence intervals (CIs) were computed using the Cox proportional hazards model. Receiver operating characteristic curve analysis was performed to select the most appropriate cut-off value for NLR. A p value < 0.05 was considered significant.
Results
The relationship between treatments for dyslipidaemia and prognosis in all breast cancer patients
Differences in clinicopathological features due to treatments for dyslipidaemia are presented in Table 1. Of 938 breast cancer patients, 194 (20.7%) received treatment for dyslipidaemia (Supplementary Fig. S2). Age (years) ranged from 20 to 94 (mean, 60.1; median, 61; standard deviation (SD), 13.4; inter quartile range (IQR), 61). Significant association was observed between patients who were administered the drug for dyslipidaemia and older age (p < 0.001), menopause (p < 0.001), and diabetes mellitus (p < 0.001). Only 89 (9.5 %) patients received neoadjuvant chemotherapy (NAC) in this study, and more patients treated for dyslipidemia did not received NAC significantly than those without treatment for dyslipidemia (p = 0.004).
In all patients, no significant differences in RFS or OS were observed due to the use of agents for dyslipidaemia (p = 0.775, p = 0.304, log-rank, respectively) (Fig. 1). Additionally, we investigated the prognostic value of administration of the drug(s) for dyslipidaemia in each breast cancer subtype; no associations between the treatment for dyslipidaemia and RFS or OS were found in each intrinsic subtype (Supplementary Figs. S3 and S4).
The relationship between lipid control and prognosis in patients treated for dyslipidaemia
In 194 patients treated for dyslipidaemia, the following anti-dyslipidaemia drugs were administered: pravastatin (29 cases), simvastatin (3 cases), fluvastatin (2 cases), atorvastatin (57 cases), pitavastatin (20 cases), rosuvastatin (51 cases), ezemitib (8 cases), bezafibrate (2 cases), fenofibrate (4 cases), ethyl icosapentate (2 cases), and elastaze (1 case). Eighty-two patients (42.3%) were treated with lipophilic statins and 80 patients (41.2%) with hydrophilic statins. The remaining 32 patients were either taking non-statin drugs or there are insufficient drug-related data for them. There was no significant difference in RFS or OS between lipophilic and hydrophilic statins (p = 0.872, p = 0.697, log-rank, respectively) (Fig. 2).
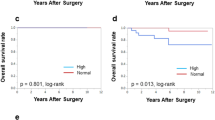
We investigated the prognostic value of serum lipid control by treatment for dyslipidaemia in each breast cancer intrinsic subtypes, and in only patients with HR-positive/HER2-negative breast cancer, the good control group had significantly better RFS than the poor control group (p = 0.045, log-rank) (Supplement Figs. S5 and S6). Because there was no death with luminal-HER2 breast cancer, statistical analysis of OS was not performed. We also classified patients with HR-positive/HER2-negative breast cancer according to menopausal status, and found that only postmenopausal patients showed significantly better RFS in the good control group compared with the poor control group (p = 0.014, log-rank) (Fig. 3).
Analyses of postmenopausal patients with HR-positive/HER2-negative breast cancer treated for dyslipidaemia
We examined the correlation between clinicopathological features and serum lipid control of 120 postmenopausal patients with HR-positive/HER2-negative breast cancer treated for dyslipidaemia (Supplement Fig. S7) (Table 2). Age (years) ranged from 47 to 90 (mean, 65.6; median, 65.5; SD, 7.0; IQR, 69). Body mass index (kg/m2) ranged from 17.4 to 36.5 (mean, 25.7; median, 25.1; standard deviation, 5.2; IQR, 25.1). Good lipid control group had significantly good serum lipid levels before surgery (p = 0.006). NLR was determined for every sample, and the NLR cut-off value for serum lipid control was 1.65 (area under the curve: 0.63; sensitivity: 76.6%; specificity: 46.4%) (Supplementary Fig. S8). Good lipid control group had significantly good serum lipid levels before surgery (p = 0.006). Patients with good lipid control also showed significantly lower postoperative NLR than poor lipid control group (p = 0.012).
Moreover, we investigated the correlation between clinicopathological features and preoperative serum lipid levels (Supplementary Table S1). Patients with good preoperative lipid levels were significantly associated with high TILs density (p = 0.024). However, preoperative serum lipid levels were not associated with long RFS (p = 0.748, log-rank) (Supplementary Fig. S9). In addition, there was no significant relationship between TILs density and preoperative NLR (p = 0.612).
Postoperative NLR (HRs = 2.573×109, 95%CI: 2.868–unparsable, p = 0.002) and serum lipid control (HRs = 5.534, 95%CI: 1.448–36.136, p = 0.010) were significantly correlated with RFS in univariate analyses. Multivariate analyses showed that good serum lipid control was an independent prognostic factor for recurrence (HRs = 4.722, 95%CI: 1.006–22.161, p = 0.049) (Table 3).
In addition, 49 patients (40.8%) were taking lipophilic statins, and 48 patients (40.0%) were taking hydrophilic statins. The remaining 23 patients were either taking non-statin drugs or drug-related data were insufficient for them. RFS tended to be better in the lipophilic statin users than in the hydrophilic statin users (p = 0.162, log-rank) (Supplementary Fig. S10).
Furthermore, in this study, we also classified postmenopausal HR-positive/HER2-negative breast cancer patients treated for dyslipidaemia by administration of Ais. Only patients treated with AIs as adjuvant endocrine therapy showed significantly better RFS in the good lipid control group (p = 0.025, log-rank) (Fig. 4).
Recurrence-free survival (RFS) using Kaplan-Meier method in postmenopausal hormone receptor (HR)-postive/human epidermal growth factor receptor 2 (HER2)-negative patients treated for dyslipidemia based on control of serum lipid levels. Patients without adjuvant endocrine therapy (a) and patients with adjuvant endocrine therapy (b)
Discussion
In this study, we verified the predictive value of anti-dyslipidaemia drug administration for the progression of breast cancer in patients treated with curative surgery. We did not observe any significant association between the use of drug(s) for dyslipidaemia and breast cancer prognosis after surgery in any breast cancer patient. Statin administration has been found to be associated with lowered risk of breast cancer-related recurrence or mortality [21, 32,33,34]. However, some studies have also reported that statin use was not significantly associated with a reduction in cancer-specific mortality [35, 36]. These inconsistent results could be attributed to age and timing of drug administration, drug types, and whether curative surgery or adjuvant therapy is being performed. Snyder et al. had indicated that statins were preferentially taken by patients who made better healthcare choices, engaged in healthier behaviours, and had better breast cancer prognoses [37]. Therefore, various subgroup analyses are very important. Some previous studies indicated that the benefits of statins for recurrence or mortality in breast cancer were most strongly seen with lipophilic statins [38, 39]. In our study, a weak non-significant improvement in RFS was observed for postmenopausal HR-positive/HER2-negative breast cancer patients treated with lipophilic statins compared with patients treated with hydrophilic statins.
We also stratified patients treated for dyslipidaemia by intrinsic subtype of breast cancer. Classification of breast cancer intrinsic subtypes is clinically useful in obtaining prognostic information [40]. In the present study, postmenopausal HR-positive/HER2-negative breast cancer patients with good serum lipid control due to the agents for dyslipidaemia showed significantly longer RFS, and these patients also had significantly lower postoperative NLR. NLR has been demonstrated to be a marker of systemic immunity and prognostic factor for breast cancer [41, 42]. Therefore, these results suggest that good control of lipid metabolism by the drugs for dyslipidaemia may improve systemic immune activity and induce suppression of recurrence in postmenopausal HR-positive/HER2-negative breast cancer patients.
Preoperative lipid metabolism was not significantly related to recurrence; thus, the data suggests that it is more important to control serum lipid levels after curative surgery. Preoperative serum lipid levels also did not have any significant relationship with preoperative NLR. However, patients whose preoperative lipid levels were low or normal were significantly associated with high TILs density in their tumour specimen. TILs can be used to monitor the tumour immune microenvironment and are important in predicting clinical outcomes in many types of cancer [29, 43, 44]. Thus, lipid metabolism may be related to the local tumour immune microenvironment.
In premenopausal HR-positive/HER2-negative breast cancer patients, there was no relationship between lipid control and recurrence. Unlike premenopausal breast cancer patients, androgens secreted from the adrenal grand in postmenopausal breast cancer patients are converted into oestrogen by aromatase mainly present in the stromal tissue. Hence, aromatase inhibitors (AIs) are recommended as adjuvant endocrine therapy for postmenopausal HR-positive breast cancer [45]. Cholesterol is the common precursor of sex hormones, including androstenedione and testosterone, the two substrates of the aromatase enzyme [46]; Thus, drugs for dyslipidaemia may act in the same way as AIs by lowering serum cholesterol levels. In this study, we found that postmenopausal HR-positive/HER2-negative breast cancer patients treated with AIs as adjuvant endocrine therapy showed significantly better RFS in the good lipid control group. Luca et al. revealed that AI treatment itself selected for acquired amplification of the CYP19A1 (aromatase) gene and promoted local autocrine oestrogen signalling in patients with AI-resistant breast cancer [47]. Aromatase is expressed from the stroma, including adipose tissue; hence, serum lipid control by treatment for dyslipidaemia may modulate components of the tumour immune microenvironment, such as cancer-associated fibroblasts, and suppress the growth of AI-resistant breast cancer cells (Fig. 5) In the future, immunohistochemical analyses of primary or recurrent tumours and investigation of immune mechanisms may facilitate both the understanding of the relationship between lipid metabolism and the tumour immune microenvironment as well as the prediction of prognosis.
Schematic illustration of effects of drugs for dyslipidaemia. Aromatase inhibitor (AI) selects for acquired amplification of the CYP19A1 (aromatase) gene and promotes local autocrine oestrogen signalling in patients with AI-resistant breast cancer. Aromatase is expressed from the stroma, including adipose tissue or cancer-associated fibroblasts; hence, serum lipid control by treatment for dyslipidaemia may modulate components of the tumour immune microenvironment and suppress the growth of AI-resistant breast cancer cells
This study has some limitations: it is a single-centre retrospective and exploratory study. The sample size was small, and some confounders such as the dose- and time-dependence of the prognostic effect among patients treated for dyslipidaemia was unclear as it was not known whether patients were adherent to their medications. Further prospective multicentre studies are therefore needed to identify the strengths and weaknesses of our findings.
Conclusions
This is the first study to demonstrate the clinical relationship between lipid metabolism and the tumour immune microenvironment in breast cancer patients. The findings of this study indicate that good control of serum lipid levels may improve the tumour immune microenvironment and predict a favourable outcome in postmenopausal HR-positive/HER2-negative breast cancer patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NLR:
-
Neutrophil-to-lymphocyte ratio
- TILs:
-
Tumor-infiltrating lymphocytes
- DFS:
-
Recurrence-free survival
- OS:
-
Overall survival
- HR:
-
Hormone receptor
- HER2:
-
Human epidermal growth factor receptor-2
- ER:
-
Estrogen receptor
- PgR:
-
Progesterone receptor
- HE:
-
Hematoxylin and eosin
- HRs:
-
Hazard ratios
- CI:
-
Confidence interval
- NAC:
-
Neoadjuvant chemotherapy
- BMI:
-
Body mass index
- AIs:
-
Aromatase inhibitors
References
Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8(3):151–60.
Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306.
Hu C, Pang B, Lin G, Zhen Y, Yi H. Energy metabolism manipulates the fate and function of tumour myeloid-derived suppressor cells. Br J Cancer. 2020;122(1):23–9.
Ho PC, Liu PS. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J Immunother Cancer. 2016;4:4.
Allison KE, Coomber BL, Bridle BW. Metabolic reprogramming in the tumour microenvironment: a hallmark shared by cancer cells and T lymphocytes. Immunology. 2017;152(2):175–84.
Li X, Wenes M, Romero P, Huang SC, Fendt SM, Ho PC. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 2019;16(7):425–41.
Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107(9):1608–17.
Sasazuki S, Charvat H, Hara A, Wakai K, Nagata C, Nakamura K, et al. Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci. 2013;104(11):1499–507.
Oh J, Chung H, Park SI, Yi SJ, Jang K, Kim AH, et al. Inhibition of the multidrug and toxin extrusion (MATE) transporter by pyrimethamine increases the plasma concentration of metformin but does not increase antihyperglycaemic activity in humans. Diabetes Obes Metab. 2016;18(1):104–8.
Ding L, Liang G, Yao Z, Zhang J, Liu R, Chen H, et al. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget. 2015;6(34):36441–55.
Verdura S, Cuyas E, Martin-Castillo B, Menendez JA. Metformin as an archetype immuno-metabolic adjuvant for cancer immunotherapy. Oncoimmunology. 2019;8(10):e1633235.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78.
Wada K, Nagata C, Tamakoshi A, Matsuo K, Oze I, Wakai K, et al. Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Ann Oncol. 2014;25(2):519–24.
Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28(21):3411–5.
Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–8.
Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23(34):8606–12.
Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120(4):833–43.
Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer. 2008;44(15):2122–32.
Ahern TP, Lash TL, Damkier P, Christiansen PM, Cronin-Fenton DP. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol. 2014;15(10):e461–8.
Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: a systematic review and meta-analysis. Int J Cancer. 2016;139(6):1281–8.
Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103(19):1461–8.
Yu X, Luo Y, Zhou Y, Zhang Q, Wang J, Wei N, et al. BRCA1 overexpression sensitizes cancer cells to lovastatin via regulation of cyclin D1-CDK4-p21WAF1/CIP1 pathway: analyses using a breast cancer cell line and tumoral xenograft model. Int J Oncol. 2008;33(3):555–63.
Wang G, Cao R, Wang Y, Qian G, Dan HC, Jiang W, et al. Simvastatin induces cell cycle arrest and inhibits proliferation of bladder cancer cells via PPARgamma signalling pathway. Sci Rep. 2016;6:35783.
Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105(6):739–45.
Massaro M, Zampolli A, Scoditti E, Carluccio MA, Storelli C, Distante A, et al. Statins inhibit cyclooxygenase-2 and matrix metalloproteinase-9 in human endothelial cells: anti-angiogenic actions possibly contributing to plaque stability. Cardiovasc Res. 2010;86(2):311–20.
Mandal CC, Ghosh-Choudhury N, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J Biol Chem. 2011;286(13):11314–27.
Beckwitt CH, Clark AM, Ma B, Whaley D, Oltvai ZN, Wells A. Statins attenuate outgrowth of breast cancer metastases. Br J Cancer. 2018;119(9):1094–105.
Greene FL, Sobin LH. A worldwide approach to the TNM staging system: collaborative efforts of the AJCC and UICC. J Surg Oncol. 2009;99(5):269–72.
Garcia-Martinez E, Gil GL, Benito AC, Gonzalez-Billalabeitia E, Conesa MA, Garcia Garcia T, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16(6):488.
Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, et al. Prediction of treatment response to neoadjuvant chemotherapy in breast cancer by subtype using tumor-infiltrating lymphocytes. Anticancer Res. 2018;38(4):2311–21.
Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res. 2016;22(6):1499–509.
Murtola TJ, Visvanathan K, Artama M, Vainio H, Pukkala E. Statin use and breast cancer survival: a nationwide cohort study from Finland. PLoS One. 2014;9(10):e110231.
Sakellakis M, Akinosoglou K, Kostaki A, Spyropoulou D, Koutras A. Statins and risk of breast cancer recurrence. Breast Cancer. 2016;8:199–205.
Borgquist S, Broberg P, Tojjar J, Olsson H. Statin use and breast cancer survival - a Swedish nationwide study. BMC Cancer. 2019;19(1):54.
Mc Menamin UC, Murray LJ, Hughes CM, Cardwell CR. Statin use and breast cancer survival: a nationwide cohort study in Scotland. BMC Cancer. 2016;16:600.
Berwick MR, Slope LN, Smith CF, King SM, Newton SL, Gillis RB, et al. Location dependent coordination chemistry and MRI relaxivity, in de novo designed lanthanide coiled coils. Chem Sci. 2016;7(3):2207–16.
Snyder CF, Frick KD, Peairs KS, Kantsiper ME, Herbert RJ, Blackford AL, et al. Comparing care for breast cancer survivors to non-cancer controls: a five-year longitudinal study. J Gen Intern Med. 2009;24(4):469–74.
Liu B, Yi Z, Guan X, Zeng YX, Ma F. The relationship between statins and breast cancer prognosis varies by statin type and exposure time: a meta-analysis. Breast Cancer Res Treat. 2017;164(1):1–11.
Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after diagnosis of breast cancer and survival: a population-based cohort study. Epidemiology. 2015;26(1):68–78.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74.
Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16(1):55–9.
Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2.
Liu H, Zhang T, Ye J, Li H, Huang J, Li X, et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother. 2012;61(10):1849–56.
Lee WS, Kang M, Baek JH, Lee JI, Ha SY. Clinical impact of tumor-infiltrating lymphocytes for survival in curatively resected stage IV colon cancer with isolated liver or lung metastasis. Ann Surg Oncol. 2013;20(2):697–702.
Early Breast Cancer Trialists’ Collaborative G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–52.
Ali S, Buluwela L, Coombes RC. Antiestrogens and their therapeutic applications in breast cancer and other diseases. Annu Rev Med. 2011;62:217–32.
Magnani L, Frige G, Gadaleta RM, Corleone G, Fabris S, Kempe MH, et al. Acquired CYP19A1 amplification is an early specific mechanism of aromatase inhibitor resistance in ERalpha metastatic breast cancer. Nat Genet. 2017;49(3):444–50.
Acknowledgements
We thank Yayoi Matsukiyo and Tomomi Okawa (Department of Breast and Endocrine Surgery, Osaka City University Graduate School of Medicine) for helpful advice regarding data management.
Funding
This study was supported by JSPS KAKENHI Grant Number JP 19K18067, 17K10559, and 20K08938. The funding body had no role in the design of the study, collection, analysis, and interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparation of this manuscript. WG collected the data and wrote the manuscript. SK, KT, SI, YA and TM performed the operation and designed the study. YA and SK summarized the data and revised the manuscript. MS, HT, KH, and MO provided a substantial contribution to the study design, performed the operation, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
A written informed consent to participate in the study was obtained from each subject in accordance with the declaration of Helsinki principles. Each patient or the patient’s family was fully informed of the investigational nature of this study and provided their written, informed consent. The study protocol was approved by the Ethics Committee of Osaka City University (approve number #926).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure S1.
Region of histopathological TILs evaluation. TILs were measured by examining the occupation ratio of immune cells present in the tumour stroma of hematoxylin and eosin stained specimens at 400x magnification. We determined that the proportion of TILs in the tumor stroma was> 10% as High (a) and ≤10% as Low (b)
Additional file 2: Supplementary Figure S2.
Consort diagram. A total of 1018 patients were diagnosed with breast cancer and underwent curative surgery. We excluded 80 patients with ductal carcinoma in situ, and this retrospective study comprised 938 breast cancer patients. Of the 938 breast cancer patients, 194 were receiving treatment for dyslipidaemia.
Additional file 3: Supplementary Figure S3.
Recurrence-free survival (RFS) using Kaplan-Meier method in patients based on users or non-users of drugs for dyslipidaemia with different intrinsic breast cancer subtype. Luminal (a), Luminal-human epidermal growth factor receptor 2 (HER2) (b), HER2-enrich (c) and triple-negative breast cancer (TNBC) (d).
Additional file 4: Supplementary Figure S4.
Overall survival (OS) using Kaplan-Meier method in patients based on users or non-users of drugs for dyslipidemia with different intrinsic breast cancer subtype. Luminal (a), Luminal-human epidermal growth factor receptor 2 (HER2) (b), HER2-enrich (c) and triple-negative breast cancer (TNBC) (d).
Additional file 5: Supplementary Figure S5.
Recurrence-free survival (RFS) using Kaplan-Meier method in patients treated for dyslipidemia based on control of serum lipid levels with different intrinsic breast cancer subtype. Luminal (a), Luminal-human epidermal growth factor receptor 2 (HER2) (b), HER2-enrich (c) and triple-negative breast cancer (TNBC) (d).
Additional file 6: Supplementary Figure S6.
Overall survival (OS) using Kaplan-Meier method in patients treated for dyslipidemia based on control of serum lipid levels with different intrinsic breast cancer subtype. Luminal (a), HER2-enrich (b) and triple-negative breast cancer (TNBC) (c).
Additional file 7: Supplementary Figure S7.
Consort diagram. Of the 120 postmenopausal patients with hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer treated for dyslipidaemia, 56 were in good lipid control group.
Additional file 8: Supplementary Figure S8.
Receiver operating characteristic curve analyses of the NLR in postmenopausal hormone receptor (HR)-postive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer patients
Additional file 9: Supplementary Figure S9.
Recurrence-free survival (RFS) and overall survival (OS) in postmenopausal hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer patients treated for dyslipidaemia based on preoperative serum lipid levels. Estimated Kaplan-Meier curves of RFS (a) and OS (b).
Additional file 10: Supplementary Figure S10.
Recurrence-free survival (RFS) using Kaplan-Meier method in postmenopausal hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer patients treated for dyslipidaemia based on statin type.
Additional file 11: Supplementary Table S1.
Differences in clinicopathological features due to preoperative serum lipid levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Goto, W., Kashiwagi, S., Kamei, Y. et al. Relationship between serum lipid levels and the immune microenvironment in breast cancer patients: a retrospective study. BMC Cancer 22, 167 (2022). https://doi.org/10.1186/s12885-022-09234-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09234-8