Abstract
Background
Cervical cancer, one of the most common cancers affecting females in South Africa, commonly requires a cisplatin-based-treatment regimen, which has been associated with ototoxic side effects. However, cisplatin-associated ototoxicity is largely under-reported in South Africa, despite its impact of hearing loss having serious overt ramifications on the quality of life of these patients. Hence, a prospective cohort study was undertaken to assess the audiological changes in female cervical cancer patients receiving cisplatin therapy.
Objective
To present details of the feasibility study and initial results on hearing patterns in cervical cancer patients receiving cisplatin chemotherapy. .
Methods
Fifty cervical cancer patients commencing with cisplatin chemotherapy underwent audiological assessments at a hospital in South Africa at various time intervals. Assessments included case history, otoscopic examination, immittance audiometry, pure tone audiometry (including high-frequency audiometry), speech audiometry, and distortion product otoacoustic emission testing. Data analysis involved the use of descriptive statistics and the Cochran-Armitage trend test for a linear trend in proportions.
Results
Fifty participants, aged between 32 and 79 years (Mean: 53 years; SD = 11.00), were recruited. Clinical findings revealed an incidence of 100% ototoxic hearing loss at the one-month post-treatment, i.e., 98% after three cycles of cisplatin and 2% at one-month post-chemotherapy. Sensorineural hearing loss and high-frequency tinnitus were most common. Deterioration in hearing thresholds was more evident in the extended high-frequency range, with the number of “no-responses,” from 11,200 Hz to 20,000 Hz, increasing with each successive audiological evaluation. This study further indicated that recruitment and follow-up of study participants within a limited resource setting are possible. However, cognizance must be given to a multidisciplinary approach and constant engagement with participants through regular contact either telephonically or via a short-message-system.
Conclusion
Exposure to cisplatin treatment contributed to hearing loss in females with cervical cancer, highlighting the need for ototoxicity monitoring during chemotherapy treatments. Furthermore, the results indicate that it is possible to conduct prospective cohort studies, using a multidisciplinary approach in limited-resource environments with appropriate planning and training strategies, as this study was able to achieve its aim successfully.
Similar content being viewed by others
Background
Cervical cancer is the second most prevalent cancer, accounting for over 20% of all female cancer types in Africa [1]. In South Africa, it is the most common cancer amongst the black female population, with an age-standardized incidence rate of 28.25 per 100,000 and a lifetime risk of 1 in 33 [2]. Treatment of cervical cancer may include surgery, radiotherapy, chemotherapy, or a combination of modalities based on International Federation of Gynecology and Obstetrics (FIGO) stage [3]. Concurrent chemoradiotherapy using weekly cisplatin as the chemotherapeutic agent is the standard of care as an outpatient therapy for locally advanced cervical cancer (LACC), commonly diagnosed in our setting [4].
Cisplatin, however, possesses ototoxic properties [5], where patients exposed to this drug experience loss of hearing and/or vestibular function resulting from the functional and cellular damage of the inner ear [6], for which no medical treatment or prevention currently exists [7]. As cervical cancer is an acquired immunodeficiency syndrome (AIDS)-defining illness, this condition adds further distress for women who are on anti-retroviral therapy (ART), which also has been reported to have ototoxic effects [8]. Affected women generally present with a host of chronic conditions for which they are then prescribed other classes of ototoxic drugs, which can include but not limited to aminoglycosides, loop diuretics, quinine, and non-steroidal anti-inflammatory drugs [9].
The impact of hearing loss on communication has serious overt ramifications on an individual’s quality of life [10]. Consequently, effective communication is often hindered, where the hearing-impaired individual may experience social, emotional, and vocational difficulties [11]. This may be the case for patients with cervical cancer who already present with various symptoms, who now also experience a reduced hearing sensitivity to the point that they miss relevant information regarding treatment regimens. Therefore, it is crucial that the incidence and severity of cisplatin-associated ototoxicity are known, so that the extent of this additional comorbidity may be acknowledged, and appropriate interventions set in place.
The incidence of cisplatin-associated ototoxicity ranges from 13 to 96% [12] and varies due to several factors. For example, differences in treatment dosages, both within a cycle and the total amount administered over multiple cycles (accumulative dose), the time interval between treatment courses, method of administration, treatment duration, as well as differences in the patient population, such as patient demographics, physiology, and clinical factors, all of which accounts for these discrepancies. A review revealed that despite many international studies focusing on cisplatin-associated ototoxicity, only one study (retrospective cross-sectional) reported on its incidence in SA [13]. This study revealed that 55% of the patients developed ototoxicity while receiving high-dose (≥60 mg/m2) cisplatin treatment; however, it is unclear to what extent previous noise exposure, pre-existing hearing loss or the use of previous ototoxic medication may have impacted this condition.
The longitudinal nature and degree of variability in hearing loss, after initiation of chemotherapy, required that a “fit-for-purpose” prospective study be conducted. This would allow scrutiny of audiological status prior to, during and at intervals after treatment, in the presence of confounders to exposures but also among other variables of interest. This approach also sought to reduce measurement error and avoid bias resulting from missing data associated with retrospective chart audits.
The utilization of this type of study design is relatively uncommon in the audiology domain in SA, hence we embarked on a planning and feasibility phase. Here, we present the details of the feasibility study and initial results on the hearing patterns of cervical cancer patients during the course of cisplatin chemotherapy.
Methods
Aim
To present details of the feasibility study and initial results on hearing patterns in cervical cancer patients receiving cisplatin chemotherapy.
Study design
A prospective cohort study design was reported on.
Setting
The study was conducted at a referral hospital offering tertiary services in KwaZulu-Natal (KZN), South Africa (SA), as defined in the regulations relating to categories of hospitals in SA. This hospital is also one of the main referral centers for cancer patients and houses an audiology department.
Population
The study population comprised of female patients diagnosed with cervical cancer receiving a cisplatin-based chemotherapeutic treatment regimen.
Sampling
Females who were 18 years or older, with an incident diagnosis of cervical cancer, commencing with the first cycle of chemotherapy were invited to participate in the study. Patients presenting with profound hearing loss at baseline assessment, or those who had previously received cisplatin chemotherapy, or had a history of medical conditions such as tuberculosis and malaria were excluded, as the treatment for such conditions often require the use of ototoxic drugs. However, since cervical cancer is considered an AIDs-defining illness, all participants were tested for their HIV status, and HIV positive women who were on ART were documented.
Data collection procedures
Data was collected over a 10-month period during which 50 participants underwent audiological evaluations prior to chemotherapy initiation, at the beginning of the fourth cycle and at one-month post-treatment.
Staff at the oncology department (doctors, nurses, and radiotherapists) willingly assisted with the recruitment of participants. In addition, the primary researcher checked the statistics report of the oncology department weekly to ensure that no patients were missed. Individual data collection commenced following informed consent.
A detailed case history interview was conducted at each assessment to obtain information on participant’s audiological and otological status, medical conditions, medication, noise exposure, and communicative abilities related to audition. Information on the risk factors and symptoms of ototoxic hearing loss was also documented during the case history interview, in addition to patient contact details (mobile phone numbers of participants and close relatives) for the purpose of follow-up. Participants were reminded of their appointments, either telephonically or via a short message system (SMS). The monitoring of audiometry was conducted before the fourth cycle of chemotherapy, as this is generally the mid-point of the treatment regimen for patients with cervical cancer.
Appropriate instructions were provided to the participants before the commencement of each audiological procedure. The audiological test, suggested for ototoxicity monitoring by the American Speech And Hearing Association (ASHA, 1994) [14], was utilized and included the following: review of medical file, case history interview, otoscopic examination, immittance audiometry (tympanometry and acoustic reflex threshold testing), pure tone audiometry (air conduction up to 20 kHz, and bone conduction), speech reception threshold testing, speech discrimination testing and distortion product otoacoustic emission testing [14, 15]. A qualified technician calibrated all equipment, while the researcher conducted daily biological calibrations before data collection.
To accommodate local prerequisites, all correspondence with participants was translated into isiZulu, which is the local language spoken by the majority of the population in KwaZulu-Natal. Therefore, information and informed consent documents, case history questionnaires, and instructions for audiological procedures were available in both English and isiZulu. An isiZulu linguist verified translations. The primary investigator was also able to converse in isiZulu, having completed a course at university. The entire audiological test took a maximum of 45 min to complete.
All audiometric test results were interpreted according to the norms prescribed in the literature [16]. On identification of a significant ototoxic hearing loss, an audiological retest was conducted within 24 h to verify the change [14]. The presence of a significant ototoxic change was determined using the ASHA Association [14] criteria, defined as follows:
(a) ≥ 20 dB decrease at any one test frequency,
(b) ≥10 dB decrease at any two adjacent frequencies, or.
(c) loss of response at three consecutive frequencies where responses were previously obtained.
On identification of a reduction in hearing ability, participants were counseled regarding treatment options such as compensatory communication strategies, as well as rehabilitation technology options, and referred to the necessary medical personnel, i.e., either the oncologist, ear-nose-throat specialist, or the psychologist. Participants were also encouraged to refrain from noisy environments, as this would exacerbate the experienced hearing loss.
Data analysis
Feasibility issues were evaluated by considering the eligibility and accrual of participants, the capacity of the study site; follow up rates, and the results obtained in the audiological evaluation of the participants. Descriptive statistics were used to summarize demographic and clinical characteristics of the study participants, where mean and standard deviation (SD) was used for normally distributed variables and median and interquartile range (IQR) for non-normal variables. Shapiro Wilk test was used to assess normality. Categorical variables were presented using frequency and percentages and comparisons were performed using the Fisher’s exact test. The Cochran-Armitage trend test for trend in proportions was used to examine changes in case history findings over time. Ototoxic hearing loss was determined using the ASHA (1994) [14] guidelines. Statistical significance was accepted at p < 0.05 for all statistical tests. All data were analyzed using the STATA 15 software (StataCorp. 2017, Texas, USA).
Results
Participant characteristics
Approximately five of the 30 patients diagnosed with cervical cancer at the study site each month, commenced with chemotherapy. As four participants did not meet the selection criteria, the study sample comprised 50 females. As indicated in Table 1, the study population comprised of three ethnic groups (as categorized by the South African National Cancer Registry) between 32 and 79 years of age, with the mean age being 53 years (SD = 11).
The majority of participants in this cohort were black African women (n = 44) (88%), with the remaining six participants (12%) comprising of an equal distribution of the Indian/Asian and Coloured women, respectively. Fifty percent (n = 25) of the participants were diagnosed with Stage IIB cervical cancer, with stage IA2 being the least common, as diagnosed in one patient (2%). Twenty-three (46%) females were HIV positive. All participants received concomitant chemoradiation therapy. Chemotherapy consisted of cisplatin (50 mg/m2 body surface weekly for six cycles) in combination with dexamethasone (48 mg) and ondansetron (24 mg). All participants received 3 cycles of cisplatin chemotherapy (cumulative dosage of 150 mg/m2) at mid- cycle. Eight patients (16%) were found to have discontinued treatment after the third cycle, having received a cumulative cisplatin dosage of 150 mg/m2, while the remaining participants completed their cycles to varying degrees as indicated in Table 1. All participants presented with normal renal function during the course of treatment.
Increasing complaints of reduced hearing sensitivity and tinnitus
A summary of the case history enquiry at each audiological evaluation is presented in Table 2.
At baseline, seven (14%) participants reported reduced hearing sensitivity, with the number of complaints increasing at each follow-up, although the increase was not significant (p = 0.36) (Table 2). Bilateral hearing difficulties were more frequently reported than unilateral. Tinnitus was found to be the most common otologic symptom experienced by participants, i.e., 21 (42%) at baseline, 26 (52%) after three cycles of chemotherapy, and 17 (34%) at the one-month follow-up (Table 2). Reports of tinnitus increased after the three cycles of chemotherapy and decreased at the one-month post-chemotherapy audiological evaluation. Results show no evidence of significant changes over time for reported tinnitus (p = 0.42) (Table 2). A similar pattern is seen with the reports of otalgia. High-frequency tinnitus was the most commonly reported at all audiological evaluations, as reflected in Fig. 1. Only two participants reported previous repeated ear infections on all successive evaluation times, i.e., one on the right ear and the other on the left ear.
Deterioration in hearing thresholds more evident in the extended high-frequency range
Otoscopic examination findings were normal in 48 (96%) participants bilaterally, with a tympanic membrane perforation visualized in the left ear of one participant and the right ear of another. Tympanometry was consistent with Type A tympanograms being obtained in the right ear of 49 participants (98%) and the left ear of 49 participants (98%).
Clinical findings, as reflected in Table 3, showed an increase, although not statistically significant (p= > 0.1), in the number of participants presenting with hearing loss from baseline to mid-cycle chemotherapy.
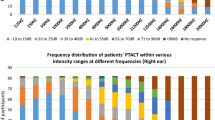
A steady increase in the number of participants with hearing loss, while not significant, is evident at each successive audiological evaluation. Sensorineural hearing loss was the most common type of hearing loss diagnosed at each audiological evaluation, with only one participant (2%) presenting with a mixed hearing loss (Table 3). Ototoxic hearing loss was evident in 49 (98%) participants after three cycles of chemotherapy and 1 ( 2%) participant at the one-month post chemotherapy evaluation (Table 3). An increase in the number of ‘no-responses’ at each subsequent audiological evaluation was evident from 11,200 Hz to 20,000 Hz, as reflected in Fig. 2; thus, revealing the extended high-frequency range to be most affected.
There is a progression of hearing loss over time, as reflected by the increase in median pure tone thresholds from the baseline assessment to each of the follow-up evaluations, especially evident from 8000 Hz to 14,000 Hz bilaterally (Fig. 3).
Distribution of pure-tone hearing thresholds in right and left ears prior to chemotherapy initiation (visit 0), mid-cycle (visit 1) and post-treatment (visit 2) measured at frequencies ranging from 125 Hz to 14,000 Hz. The bottom and top of the box are the 25th and 75th centiles and the line in the box is the median; the lower and upper lines represent the minimum and maximum values. The distribution, depicted by the median, displays an increasing trend in hearing threshold bilaterally between 8000 Hz – 14,000 Hz.Footnote: The medians of 16,000, 18,000 and 20,000 Hz are not presented on the table due to the large number of ‘No Responses’ (responses beyond the limits of the audiometer) at these frequencies
At one month post treatment follow-up, 11/23 (47.8%) HIV positive participants progressed to Grade 1 (n = 8), Grade 2(n = 3) stages of hearing loss respectively, based on the NCI-CTCAE Grading Scale [17]. In comparison, 8/27 (29.6%) HIV-negative participants progressed to Grade 1 (n = 7), Grade 2 (n = 1) stages of hearing loss respectively. The Fisher’s exact test revealed no significant difference (p = 0.25), possibly attributable to the small sample size and limited follow-up.
Figure 4 and Fig. 5 revealed a decrease in the difference between the DPOAE and the noise floor for all frequencies tested in the right ear of 49 participants and the left ear of 49 participants, respectively. However, this difference was not found to be statistically significant (p > 0.05).
Discussion
Our pilot data has demonstrated the feasibility of undertaking such a study in a resource-constrained environment, provided that the basic principles of epidemiology is adhered to and that the project is underpinned by strong intersectoral collaboration and multidisciplinary team effort. It was possible to accrue and follow up patients through study sensitization and regular contact, respectively. We observed that employing a multidisciplinary approach to the recruitment of participants was beneficial and therefore required ‘buy-in’ from the relevant healthcare personnel. This would only be possible if the healthcare personnel has been informed of the purpose of the study and the benefit to the patient population. The study site, being the main referral center for oncology services, was deemed suitable due to the high incidence of cervical cancer within a large catchment area, allowing for the generalizability of data. The hospital also has an oncology department, as well as an audiology department for robust and scientific assessment, hence site capacity is an essential evaluation parameter for such studies, given the larger scale investments that are placed into the conduct of prospective cohort studies. An audiology department away from the hospital can result in higher attrition, as patients may be too ill or may not have the funds to travel for follow up visits to additional sites. Therefore, an assessment of the environment is essential to ensure proper planning concerning patient recruitment and their time and travel management.
The selection of patients with locally advanced cervical cancer was appropriate to study the impact of cisplatin on hearing loss, in this setting, given the high incidence of cervical cancer and the synchronicity of the treatment protocol. These assessment parameters allow for a higher patient population and greater probability of the inclusion criteria being met within the study period.
Patient follow-up messages and appointment reminders were communicated through mobile phones via voice contact short message system (SMS). This has highlighted the growing influence of mobile phones as a tool for research and public health intervention and adds to the growing body of evidence on the value of mobile devices within health platforms [18, 19, 20].
Our study also showed an increasing number of women with complaints of reduced hearing sensitivity and clinical hearing loss at each successive audiological evaluation. All participants in the study presented with significant ototoxic change by the one-month post-treatment evaluation, similar to studies conducted in India [5, 21]. The incidence of ototoxicity in the current study is higher as compared to other studies [22, 23, 24, 25]. These differences can be ascribed to methodology differences as the current study utilized high-frequency audiometry during the audiological evaluations, whereas Nitz et al. (2013) [24], and Nagy et al. (1999) [25], utilized pure tone audiometry in the conventional frequency range. Furthermore, diseases such as diabetes [26], hypertension [27] and HIV as well as treatment for HIV [28] may also negatively influence hearing, as seen in the current study with 15 participants presenting with hearing loss at baseline. Complaints of reduced hearing sensitivity were much lower than that revealed by the baseline audiological assessment, indicating that the participants may have gradually adjusted to the reduced hearing sensitivity due to the loss being gradual. This is generally seen in presbycusis and is consistent with the age characteristics of our study population.
As is seen in ototoxic hearing loss, the findings of our feasibility study revealed sensorineural hearing loss to be most common, due to the structures of the inner ear being most susceptible to damage by cisplatin chemotherapy; with apoptotic degeneration of the hair cell in the organ of Corti being most prominent [29]. In addition, the extended high-frequency range appeared to be more affected. This is consistent with literature stating that the outer hair cells in the basal turn of the cochlea are most affected, resulting in an initial elevation of high-frequency audiometric thresholds, followed by a progressive loss in the lower frequencies with continued therapy [6, 30]. This, therefore, highlights the need for the inclusion of high-frequency audiometry in the ototoxicity-monitoring programme.
The most common otologic symptom experienced by the study participants was tinnitus. With no national averages available in the country and 42% of participants reporting tinnitus at baseline, the current authors postulate that an increase in the stress and anxiety associated with receiving cisplatin chemotherapy, as well as thoughts about their prognosis, may have resulted in tinnitus. Hasson et al. [31] reported a linear association between tinnitus and the magnitude and duration of stress, which is consistent with the pattern observed in the current study, whereby the number of participants reporting tinnitus increased during treatment but decreased post-treatment. The resolution of tinnitus is in keeping with Bokemeyer et al. [32], who reported that tinnitus resolved or decreased in some patients receiving chemotherapy after a median duration of 6 months (range 1–18 months). Melamed et al. [33] and Reddel et al. [34] also indicated that while the reversibility of tinnitus was common, threshold abnormalities persisted.
Furthermore, 23 (46%) of the participants were HIV positive and receiving ART thus, indicating that it is not feasible to exclude patients with HIV in this cervical cancer population, as it may substantially increase the duration of recruitment. In light of this, it is essential to include information on HIV status, additional comorbidities and other potential ototoxic drugs as confounders when assessing cisplatin-associated ototoxicity. Studies indicate that some diseases such as hypoalbuminemia, anemia [35], renal insufficiency [32], and diabetes [36] are considered to place patients at a higher risk for ototoxicity. Additional factors that may increase the risk for ototoxicity include cumulative dosage, the number of cycles administered, method of administration, exposure to high levels of concomitant noise, chemicals and other ototoxic medication [36], genetic risk factors (megalin and glutathione S-transferases gene polymorphism) [37], pre-exposure hearing ability and age [38, 39].
Conclusion
Our study has demonstrated that a prospective cohort study within the audiological domain is feasible in our setting, allowing for the collection of relevant medical and audiological information to investigate the hearing patterns of cervical cancer patients. This experience communicated the need for this study and may bear relevance to other resource-limited settings with a high cervical cancer burden where audiologists may wish to study hearing loss in similar cohorts, so long as the relevant expertise and support is available.
Availability of data and materials
The data that supports the findings of this study are available on request from the corresponding author.
References
Denny L, Kuhn L, editors. Cervical cancer prevention and early detection from a south African perspective. Durban: Health Systems Trust; 2017. http://www.hst.org.za/publications/south-african-health-review-2017
South African National Cancer Registry. Cancer in South Africa; 2017. https://www.nicd.ac.za/wp-content/uploads/2020/12/NCR_2017_Final_02dec2020.pdf
Burt LM, McCormak M, Lecuru F, Kanyike DM, Bvochora-Nsingo M, Ndlovu N, et al. Cervix Cancer in sub-Saharan Africa: an assessment of cervical Cancer management. JCO Global Oncology. 2021;7:173–82. https://doi.org/10.1200/GO.20.00079.
Jemu M, van Wijk L, Parker M, Jones G. Tumour and treatment factors influencing the outcome of chemo-radiation in stage IIB cervical cancer: a single institution experience. Southern African Journal of Gynaecological Oncology. 2018;10(1):5–10. https://doi.org/10.1080/20742835.2018.1441694.
Arora R, Thakur JS, Azad RK, Mohindroo NK, Sharma DR, Seam RK. Cisplatin-based chemotherapy: add high-frequency audiometry in the regimen. Indian J Cancer. 2009;46(4):7. https://doi.org/10.4103/0019-509X.55551.
Rybak L, Ramkumar V. Ototoxicity. Kidney Int. 2007;72(8):931–5. https://doi.org/10.1038/sj.ki.5002434.
Malhotra H. Cisplatin ototoxicity. Indian J Cancer. 2009;46(4):262–3. https://doi.org/10.4103/0019-509X.55545.
Bisht M, Bist SS. Ototoxicity: the hidden menace. Indian J Otolaryngol Head Neck Surg. 2011;63(3):255–9. https://doi.org/10.1007/s12070-011-0151-8.
Schellack N, Naude A. An overview of pharmacotherapy-induced ototoxicity : review article. S Afr Fam Pract. 2013;55(4):357–65. https://doi.org/10.1080/20786204.2013.10874377.
Tambs K. Moderate effects of hearing loss on mental health and subjective well-being. Psychosom Med. 2014;66:776–82. https://doi.org/10.1097/01.psy.0000133328.03596.fb.
Tye-Murray N. Foundations of aural rehabilitation: children, adults, and their family members: Cengage learning; 2014.
Olgun Y. Cisplatin ototoxicity: where we are? J Int Adv Otol. 2013;9(3):403–16 http://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=94058566&site=ehost-live.
Whitehorn H, Sibanda M, Lacerda M, Spracklen T, Ramma L, Dalvie S, Ramesar R. High prevalence of cisplatin-induced ototoxicity in Cape Town, South Africa. South Afr Med J. 2014;104(4):288–91. https://doi.org/10.7196/samj.7389.
American Speech-Language and Hearing Association. Audiologic management of individuals receiving cochleotoxic drug therapy. ASHA. 1994; https://inte.asha.org/policy/g11994-00003/.
Fausti SA, Helt WJ, Gordon JS. Audiologic monitoring for ototoxicity and patients management. In: Pharmacology and ototoxicity for audiologists [internet]. United States: Thomson Delmar Learning; 2007. [230-51].
Paken J, Govender CD, Sewram V. Research protocol: cisplatin-associated ototoxicity amongst patients receiving cancer chemotherapy and the feasibility of an audiological monitoring program. BMC Womens Health. 2017;17(1):129. https://doi.org/10.1186/s12905-017-0486-8.
National Cancer Institute. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 5.0, 2017. [Available from https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf]
Dalal S, Holmes MD, Laurence C, Bajunirwe F, Guwatudde D, Njelekela M, et al. Feasibility of a large cohort study in sub-Saharan Africa assessed through a four-country study. Glob Health Action. 2015;8 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4444761/.
Koshy E, Car J, Majeed A. Effectiveness of mobile-phone short message service (SMS) reminders for ophthalmology outpatient appointments: observational study. BMC Ophthalmol 2008;8. https://doi.org/10.1186/1471-2415-8-9.
Anhøj J, Møldrup C. Feasibility of collecting diary data from asthma patients through mobile phones and SMS (short message service): response rate analysis and focus group evaluation from a pilot study. J Med Internet Res. 2004;6. https://pubmed.ncbi.nlm.nih.gov/15631966/.
Malgonde MS, Nagpure P, Kumar M. Audiometric patterns in ototoxicity after radiotherapy and chemotherapy in patients of head and neck cancers. Indian J Palliat Care. 2015;21(2):164.
Strumberg D, Brügge S, Korn M, Koeppen S, Ranft J, Scheiber G, et al. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. 2002;13(2):229–36. https://doi.org/10.1093/annonc/mdf058.
Schultz C, Goffi-Gomez MVS, Liberman PHP, Carvalho AL. Report on hearing loss in oncology. Braz J Otorhinolaryngol. 2009;75(5):634–41. https://doi.org/10.1016/s1808-8694(15)30510-3.
Nitz A, Kontopantelis E, Bielack S, Koscielniak E, Klingebiel T, Langer T, et al. Prospective evaluation of cisplatin-and carboplatin-mediated ototoxicity in paediatric and adult soft tissue and osteosarcoma patients. Oncol Lett. 2013;5(1):311–5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3525486/. https://doi.org/10.3892/ol.2012.997.
Nagy JL, Adelstein DJ, Newman CW, Rybicki LA, Rice TW, Lavertu P. Cisplatin ototoxicity: the importance of baseline audiometry. Am J Clin Oncol. 1999;22(3):305–8. https://doi.org/10.1097/00000421-199906000-00020.
Malucelli DA, Malucelli FJ, Fonseca VR, Zeigeboim B, Ribas A, de Trotta F, et al. Hearing loss prevalence in patients with diabetes mellitus type 1. Braz J Otorhinolaryngol. 2012;78(3):105–15.
Agarwal S, Mishra A, Jagade M, Kasbekar V, Nagle SK. Effects of hypertension on hearing. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 3):S614–S8.
Luque AE, Orlando MS, Leong UC, Allen PD, Guido JJ, Yang H, et al. Hearing function in patients living with HIV/AIDS. Ear Hear. 2014;35(6):e282–e90. https://doi.org/10.1097/AUD.0000000000000064.
Callejo A, Sedó-Cabezón L, Juan ID, Llorens J. Cisplatin-induced ototoxicity: effects, mechanisms and protection strategies. Toxics. 2015;3(3):268–93. https://doi.org/10.3390/toxics3030268.
Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec Adv Integr Anat Evol Biol. 2012;295(11):1837–50. https://doi.org/10.1002/ar.22578.
Hasson D, Theorell T, Wallén MB, Leineweber C, Canlon B. Stress and prevalence of hearing problems in the Swedish working population. BMC Public Health. 2011;11(1):130. https://doi.org/10.1186/1471-2458-11-130.
Bokemeyer C, Berger C, Hartmann J, Kollmannsberger C, Schmoll H, Kuczyk M, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77(8):1355. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2150148/pdf/brjcancer00084-0161.pdf.
Melamed LB, Selim MA, Facs F, Schuchman D. Cisplatin ototoxicity in gynecologic cancer patients. Cancer Treat Rev. 1985;55:41–3.
Reddel RR, Kefford RF, Grant JM, Coates AS, Fox RM, and , Tattersall MHN. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat Rev 1982;66:19–23.
Blakley BW, Gupta AK, Myers SF, Schwan S. Risk factors for ototoxicity due to cisplatin. Arch Otolaryngol Head Neck Surg. 1994;120(5):541–6. https://doi.org/10.1001/archotol.1994.01880290051009.
Reavis KM, McMillan G, Austin D, Gallun F, Fausti SA, Gordon JS, et al. Distortion-product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear. 2011;32(1):61–74. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5588870/. https://doi.org/10.1097/AUD.0b013e3181e8b6a7.
Kirkim G, Olgun Y, Aktas S, Kiray M, Kolatan E, Altun Z, Erçetin P, Bagriyanik A, Yilmaz O. Ellidokuz H Is there a gender-related susceptibility for cisplatin ototoxicity? Eur Arch Otorhinolaryngol. 2015;272(10):2755–63. https://doi.org/10.1007/s00405-014-3283-0.
Coradini PP, Cigana L, Selistre SG, Rosito LS, Brunetto AL. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007;29(6):355–60. https://journals.lww.com/jpho-online/fulltext/2007/06000/ototoxicity_from_cisplatin_therapy_in_childhood.2.aspx. https://doi.org/10.1097/MPH.0b013e318059c220.
Helson L, Okonkwo E, Anton L, Cvitkovic E. Cis-platinum ototoxicity. Clin Toxicol. 1978;13(4):469–78. https://doi.org/10.3109/15563657808988252.
Acknowledgements
We are grateful to the participants of this research project for giving their time in the study and the hospital management for allowing this study to be undertaken at the institution.
Disclaimer
The views expressed in this article are the views of the authors and not an official position of the institution or funder.
Funding
This work was supported by the Oticon Research Foundation under Grant number 15–3622, Medical Research Council of South Africa under the National Health Scholarship Programme, and the University of KwaZulu-Natal under Competitive Research Grant.
Author information
Authors and Affiliations
Contributions
JP contributed to the conception and design of the study, collected and analyzed data, and drafted the initial manuscript. VS contributed to the conceptualization of the study, analyzed data, and critically revised the manuscript. CDG contributed to the conceptualization of the study and reviewed the manuscript providing valuable intellectual content. MP reviewed the manuscript providing relevant intellectual content. BTA performed statistical analysis of data and reviewed the results aspect of the manuscript. All authors approved the final manuscript as submitted.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study has been conducted in accordance with the Declaration of Helsinki and has been approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee (BE 064/13) and the study site before data collection commenced. Acknowledging the demographic and language profile of the country, communication with patients was undertaken in either English or isiZulu, based on their preference. All participants gave written informed consent, which were available in both languages. Participants were informed as to the nature, and the purpose of the study, any potential risks that may be involved, participation is voluntary, the nature of their participation, their right to withdraw at any time if they so desired, and that refusal to participate did not affect their clinical care. Furthermore, patients were assured of strict confidentiality and anonymity, as all participants were allocated numbers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Paken, J., Govender, C.D., Pillay, M. et al. Feasibility and first results of a prospective cohort study to investigate cisplatin-associated ototoxicity amongst cancer patients in South Africa. BMC Cancer 21, 822 (2021). https://doi.org/10.1186/s12885-021-08567-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08567-0