Abstract
Background
A primary pulmonary invasive mucinous adenocarcinoma (IMA) is a rare subtype of invasive adenocarcinoma of the lung. The prognosis of advanced IMA depending on chemotherapy regimen has not been fully investigated. Here, we compared the clinical outcomes of patients with advanced IMA treated with different palliative chemotherapies that included novel therapeutics.
Methods
This single-center retrospective study included a total of 79 patients diagnosed with IMA and treated with palliative chemotherapy. The primary outcome was the comparison of overall survival according to palliative chemotherapy type. Risk factors associated with death were evaluated as a secondary outcome.
Results
The study cohort of 79 patients comprised 27 progressive or recurrent cases and 52 initial metastatic patients. Thirteen patients (16.5%) received targeted therapy and 18 cases (22.8%) received immunotherapy. When we compared the survival outcomes of the different treatment regimens, patients with IMA treated by immunotherapy (undefined vs. non-immunotherapy 17.0 months, p < 0.001) had better overall survival rates. However, there was no difference in the prognosis between the cases treated with a targeted therapy (35.6 vs. non-targeted therapy 17.0 months, p = 0.211). None of the conventional regimens produced a better outcome. By multivariable analysis, immunotherapy (HR 0.28; 95% CI 0.11–0.74; P = 0.008) was found to be an independent prognostic factor for death.
Conclusions
This study suggests that immunotherapy for patients with advanced IMA may provide favorable outcomes than other chemotherapy options.
Similar content being viewed by others
Background
A primary pulmonary invasive mucinous adenocarcinoma (IMA) is an adenocarcinoma variant composed of histologically goblet and/or columnar cells with abundant intracytoplasmic mucin in the lung tumor [1]. In the 2015 World Health Organization (WHO) classification, IMA was reclassified into one of the various subtypes of invasive adenocarcinoma because its clinical, radiologic, and genetic characteristics were found to be distinct from non-mucinous adenocarcinoma [2, 3]. IMA comprises approximately 5% of all non-small cell lung cancers (NSCLCs) [1]. Numerous novel therapies that target oncogene mutations have improved the prognosis for NSCLC patients [4]. However, most advanced IMAs are usually ineligible for clinical trials of tyrosine kinase inhibitors (TKIs) that target NSCLCs because they principally harbor KRAS mutations and rarely contain targetable mutations in IMA such as those in the epidermal growth factor receptor (EGFR) [5, 6]. Almost all patients with advanced IMA to date have received conventional chemotherapy (CTx) [7].
There have been few studies of the clinical outcomes in patients with IMA after the 2015 WHO classification was adopted. Survival outcomes for IMAs are similar to those for non-mucinous adenocarcinomas [8]. Patients with IMA lesions showing acinar-predominant features on computed tomography (CT) exhibit a poorer prognosis [9]. Some studies have suggested that patients with a surgically resected IMA have similar overall survival (OS) outcomes to non-mucinous adenocarcinoma cases [10, 11]. In patients with metastatic IMA, there was no difference found in a previous report between IMA and non-mucinous adenocarcinoma patients [7]. However, the prognosis of IMA in accordance with the treatment used is not as well characterized as that of non-mucinous adenocarcinoma.
Novel therapies have improved the survival results for metastatic lung cancer patients [12]. One of these new treatments involves the use of immune checkpoint inhibitors and several different studies have demonstrated their benefits in metastatic NSCLCs [13,14,15]. In addition, the use of TKIs for newly characterized oncogenic mutations such as ROS1, anaplastic lymphoma receptor tyrosine kinase (ALK), and BRAF has led to prolonged survival in NSCLC patients [16,17,18]. However, the response of novel treatments to patients with IMA remains unclear. Our present study thus aimed to further investigate the prognosis of patients with advanced IMA treated with different palliative chemotherapies that included novel drugs.
Materials and methods
Study population
This retrospective study included patients with IMA who were treated with palliative chemotherapy between January 2010 and April 2020 at Asan Medical Center. The diagnosis of IMA was based on the histopathologic report from biopsy specimens and in accordance with the WHO classification of lung tumors [19]. The inclusion criteria for the patient subjects were as follows; 1) a pathological diagnosis of advanced IMA which included local recurrence or progressive lesion after surgical resection and initially metastatic disease; 2) the receipt of palliative chemotherapy. A histopathologic finding of mucinous adenocarcinoma for the patients diagnosed prior to the introduction of the 2015 WHO classification was regarded as IMA. Because the aim of the study was to evaluate patients with advanced IMA treated with palliative chemotherapy, patients who received adjuvant chemotherapy after surgical resection at the time of initial diagnosis were also included. Patients were excluded if they had a colloid adenocarcinoma described in their histopathologic report, failed to receive palliative chemotherapy after the diagnosis of advanced IMA.
Clinicopathological features of the study patients
Patient data including clinical characteristics, tumor mutation status, and treatment options were collected from their medical records. The following clinical variables collected for analysis: age, sex, eastern cooperative oncology group performance status (ECOG PS), initial clinical stage by TNM 8th edition [20], smoking history (never smokers, former smokers, or current smokers), initial chest CT findings (solid mass or consolidation to the dominant presentation), recurred or metastatic sites, mutational status (EGFR mutation, ALK rearrangement, ROS1 rearrangement, or KRAS mutation), and Programmed death (PD)-1 expression. EGFR and KRAS mutations were detected by PCR from paraffin-embedded tumor samples. Fluorescent in situ hybridization was used to identify ALK and ROS rearrangements [21]. Positive for PD-L1 expression was defined by ≥1% of tumor cells.
Terms of chemotherapy
We defined the term of a chemotherapy line as a period of the same chemotherapy repeated periodically and the term of a chemotherapy cycle as a single dose of chemotherapy treatment on a regular schedule. The type of chemotherapy was classified into three types; conventional chemotherapy as a cytotoxic chemotherapy that is widely used to lung cancer; targeted therapy as a drug to target specific genes and proteins that are involved in the survival of cancer cells; immunotherapy as a substance to stimulate or suppress the immune system to help the body fight cancer. Treatment methods including chemotherapy lines, cycles, conventional chemotherapy regimen, targeted therapy, and immunotherapy were assessed from the start of palliative chemotherapy to the inclusion period.
Statistical analysis
The data collected for the study patients were compared using the χ2 test or Fisher’s exact test for categorical variables, and independent two sample t-test for continuous variables. Progression-free survival (PFS) was defined as the duration from the date of IMA diagnosis until the date of local progression or the development of a new lesion or death or last follow-up. The OS was calculated from the date of IMA diagnosis until the date of death due to any cause. The primary outcome of this study was the comparison of the PFS and OS outcomes in the patients in accordance with the palliative chemotherapy they received. The secondary outcome included the risk factors associated with death. Kaplan–Meier analysis was used to estimate the PFS and OS, whereas a log-rank test was used to test the significance of the differences in these variables. Univariable and multivariable Cox proportional hazards regression models were used to identify risk factors associated with death. A final model was constructed using a stepwise method with backward selection. P values < 0.15 in the univariate analysis were set for the entry of variables. Two-sided p values < 0.05 were considered to indicate significance. All analyses were performed using SPSS ver. 24.0 (IBM Corporation, Armonk, NY) software.
Results
Patient characteristics
A total 79 patients with IMA who satisfied the inclusion criteria comprised 27 cases that underwent palliative chemotherapy for recurrent or progressive IMA lesions that arose after surgery and 52 patients who were diagnosed with an initially metastatic IMA. The characteristics of patients with IMA included in this study are listed in Table 1.
The median age of this cohort was 62.0 years (interquartile range [IQR], 54.0–72.0 years) and there were about an equal number of men and women. All of ECOG PS were either 0 (3.3%) or 1 (96.7%) in patients with records. Most patients were former (45.6%) or never (51.9%) smokers. There was no difference between the two initial chest CT findings. Almost all of the patients (97.5%) included in this study had a distant metastasis; the two patients who did not had received palliative chemotherapy for a local recurrence. The most common M stage was M1a (75.3%), followed by M1c (14.3%). 10.1% (8/79) of the patients were positive for EGFR mutations, and 5.8% (4/69) and 91.7% (11/12) had an ALK rearrangement and KRAS mutation, respectively. None of the study patients was positive for a ROS1 rearrangement. PD-1 was expressed in 42.4% (14/33) of the cohort.
Palliative chemotherapy
The mean of chemotherapy line was 2.4 (SD, ± 1.1). Of the 79 patients in the study cohort, 19 (24.1%) underwent only first line chemotherapy whereas 36 received 3rd or 4th line chemotherapy (45.6%; Table 2). The median number of chemotherapy cycles was 4.0 (IQR, 2.0–10.0) at the 1st line, 4.0 (IQR, 2.0–8.0) at the 2nd line, and 4.0 (IQR, 2.0–7.5) at the 3rd line of treatment.
The most common agent used for the conventional chemotherapy regimens among the study patients was platinum with pemetrexed (50.6%), followed by platinum with gemcitabine (38.0%). Eight patients (10.1%) received an EGFR TKI and 4 cases (5.1%) were administered an ALK-TKI. One patient (1.3%) received the entrectinib TKI for an NRTK1 fusion. Immunotherapy was administrated to 18 patients (22.8%), of which 4 cases (5.1%) received a combination of immunotherapy and conventional therapy, such as pembrolizumab, pemetrexed, and carboplatin, as a first line. None of the study patients was treated using both immunotherapy and targeted therapy during their course of palliative chemotherapy. Nivolumab (8.9%) was used most commonly, followed by atezolimumab (5.1%). According to the line of chemotherapy, conventional chemotherapy was most common treatment option (Fig. 1A). Targeted therapy was used in the first line in almost all cases (92.3%) whereas only two patients received targeted therapy as a 2nd line regimen, of which 1 received crizotinib after conventional chemotherapy and the other was treated with gefitinib followed by osimertinib. Among the regimens involving conventional agents, platinum with pemetrexed (40/79) was most commonly administrated 1st line therapy, followed by platinum with gemcitabine (16/79) (Fig. 1B). Taxane agents such as docetaxel were usually used as a 2nd line (10/60) whilst navelbine was commonly used in 4th line regimens (7/15). The highest proportion (40.0%) of patients treated by immunotherapy was in the 2nd line, comprising 4 cases receiving nivolumab and 4 receiving atezolizumab (Fig. 1C).
Palliative chemotherapy in accordance with the chemotherapy treatment line. CTx = chemotherapy. PP = platinum with pemetrexed, GP = platinum with gemcitabine, TP = platinum with taxane, P = pemetrexed, G = gemcitabine, T = taxane, N = navelbine. Shown are the numbers of patients stratified by their treatments, i.e. conventional chemotherapy, targeted therapy, and immunotherapy (Panel A), conventional regimen (Panel B), and the type of immunotherapy (Panel C)
Progression-free and overall survival
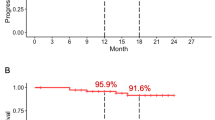
The PFS rates were analyzed in our study cohort in accordance with the chemotherapy line (Fig. 2). In the 1st line regimens, patients receiving targeted therapies (median 15.4 months; 95% CI 11.9–19.0) showed a longer PFS (median 5.0 months; 95% CI 4.2–5.9, P = 0.045). On the other hand, immunotherapy (median 5.4 months; 95% CI 2.2–8.6) was found to be associated with a trend toward a longer PFS (i.e. not statistically significant (P = 0.136) in the 2nd line. The PFS outcomes from targeted therapies in the 2nd line were not analyzed because only two patients were involved. The median survival of the total cohort of 79 patients with IMA was 20.1 months (95% confidence interval (CI), 14.7–25.6). We next compared the OS outcomes in accordance with the therapeutic regimen (Fig. 3). The IMA study patients that received a targeted therapy (median 35.6 months; 95% CI 14.8–56.4) showed a clear non-survival benefit than the patients that underwent non-targeted treatments (median 17.0 months; 95% CI 11.3–23.4, P = 0.211). Notably however, patients with IMA treated using immunotherapy (undefined) showed a longer OS than the cases that received non-immunotherapy interventions (median 17.0 months; 95% CI 12.0–22.1, P < 0.001). The OS outcomes did not significantly differ among the conventional regimens.
Progression-free survival (PFS) outcomes in the IMA study cohort determined using Kaplan-Meier estimates. The PFS following first line chemotherapy was compared accreting to targeted therapy (Panel A), or immunotherapy (Panel B). PFS outcome following 2nd line chemotherapy was also analyzed based on immunotherapy (Panel C)
Prognostic factors
By univariate analysis, M1c (Hazard ratio (HR) 2.37; 95% CI 1.14–4.91; P = 0.021) and immunotherapy (HR 0.28; 95% CI 0.11–0.69; P = 0.006) were identified as significant variables. Multivariate analysis that controlled for covariables such as the initial metastatic IMA, M stage, pemetrexed and immunotherapy revealed immunotherapy (HR 0.28; 95% CI 0.11–0.74; P = 0.008) as an independent prognostic factor for death (Table 3).
Discussion
In our current study, we found that the prognosis of patients with IMA who received palliative chemotherapy was variable in accordance with the type of chemotherapeutic intervention. Patients with IMA treated by immunotherapy appeared to have a better outcome than those who received chemotherapy. Of note in particular, immunotherapy was found to be associated with improved survival in multivariable analysis. On the other hand, targeted therapy did not improve the survival outcomes of the advanced IMA cases in our present series, although the PFS was better in these patients compared with those without targeted therapy. In addition, no OS differences were evident based on conventional agents. We thus contend from our current data that immunotherapy should be considered, if possible, as a principal treatment option in patients with advanced IMA.
In our present analyses, the median survival of patients with IMA was 20.1 months. When compared with the findings of previous reports, our measured OS is similar to that reported by Cha et al. of 17.9–20.9 months from patients with metastatic IMA, with no OS differences found between IMA and non-IMA adenocarcinoma cases in that prior study [7]. Several previous clinical trials that compared treatment efficacies reported a median survival of 14.2–21.5 months from the use of conventional chemotherapy [22,23,24]. On assessing the conventional chemotherapy regimens in our present IMA population, we noted that the combination of pemetrexed or gemcitabine with platinum agents was the most common first line protocol for these treatments. Real-world practices reported in other studies have also shown a similar pattern. In prior reports on non-squamous NSCLC, the most common regimen was carboplatin plus pemetrexed (25.7%) in the US, and pemetrexed-based therapy (68%) in China [25, 26]. Notably, the proportion (about 42%) of patients who received second-line therapies in the American study [25] and the Chinese study [26] was lower than that (75.9%) in our present study. In our current cohort, only 13 patients (16.5%) were found to have oncogenic mutations whereas 11 KRAS mutations were detected in a setting involving 12 patients with IMA in previous reports [27, 28]. Considering that the patients in our current cohort had similar clinicopathologic features and treatment environments to those previously reported for IMA, the clinical outcomes of IMA seems to be comparable to those for non-squamous NSCLC.
It has been well established that KRAS mutations are the most frequent genetic alternations seen in IMA [8]. On the other hand, IMAs usually lack other oncogenic mutations such as EGFR variants, or translocations of ALK or ROS1 [29]. KRAS mutations are mutually exclusive from EGFR mutations or ALK/ROS1 rearrangements based on oncogene addiction theory [30]. The molecular mechanisms driving IMA remain unclear, but some reports have suggested possible mechanisms for identifying novel therapeutic targets. A previous animal study has suggested that the induction of the signature genes FOXA3 or SPDEF, which are enriched in mucin-producing cancers, along with a KRAS mutation in the lung epithelium, is sufficient to develop mucinous lung tumors in transgenic mice [28]. Other studies have revealed that a loss of TTF-1 expression owing to an NKX2–1 mutation, which occurs in approximately 19% of IMA cases, would de-suppress the expression of the mucin-related genes MUC5Ac, MUC5B, and MUC3 [31]. In addition, novel driver mutations, NRG1 fusions, have been recurrently identified in IMA, i.e. as a regulator of goblet-cell formation in human bronchial epithelial cells [32]. The NRG1 protein mediates juxtacrine signals through the HER2:HER3 receptors that may play a role in the transformation and acquisition of goblet-cell morphology in IMA [33]. Recently, several clinical trials have been performed on drugs that target KRAS-mutated IMAs. A phase 1 trial of sotorasib, a small molecule that selectively targets the KRAS G12C subtype, revealed an 88.1% disease control response in NSCLC [34]. Driver mutations such as KRAS thus represent promising future therapeutic targets for the treatment of IMA.
The OS outcomes were found in our present series to be significantly improved in patients treated with immunotherapy. Numerous prior studies have reported that immunotherapy produces favorable outcomes in NSCLCs and that the clinical response predictions in these patients depend on the expression of biomarkers such as PD-L1 or on the tumor mutation burden [35,36,37]. Previous analysis of the expression of PD-L1 in IMA has indicated that PD-L1 positive tumors are infrequent [38]. However, other findings suggest the possibility of a good response to immunotherapy by IMA as KRAS mutations show an association with this response [30]. Although most prior randomized trials have not been designed to examine treatment differences between molecular subgroups, immunotherapy showed a significantly greater benefit for KRAS mutant tumors in one previous meta-analysis [39]. However, another study using real-world data demonstrated that a KRAS mutation did not confer any significant OS difference [40]. An attractive biological explanation for these discrepant findings is the molecular and environmental diversity of KRAS mutation subgroups [30]. Three of these subgroups have been defined in accordance with the presence of co-mutations, and have differences in terms of the immune environment and the responses to immunotherapy. In addition, IMAs have distinct expression profiles of immune checkpoint regulators such as VTCN1, which represents a potential immunotherapy target [28]. Although the biology to support the benefits of immunotherapy in IMA remains unclear, further studies are warranted to address whether key mutations such as KRAS can offer predictive insights into the immunotherapy responses of IMA tumors.
Our present study had several limitations of note. First, it was a retrospective study with a relatively small sample size from a single institution. However, we tried to include patients with IMA having similar characteristics to minimize possible bias and investigated all of the regimens which patients received as palliative chemotherapy. This is also, to our knowledge, the first study to identify the clinical outcomes of patients with IMA who had received immunotherapy. Prospective, randomized studies will be needed to clarify the clinical outcomes of patients with IMA in accordance with their palliative treatments. A second limitation was that we included patients who had been diagnosed prior to the WHO reclassification with mucinous adenocarcinoma, not IMA. Patients with non-IMA could therefore have been included in our cohort. We did however exclude patients with other variants of invasive adenocarcinoma such as colloids who has been diagnosed before 2015, which would have helped to reduce the heterogeneity of our cohort. A third limitation was that targeted therapies did not show survival benefits in our present series, which contrasts with the findings of previous studies [41]. Although we did observe an improved PFS from 1st line targeted therapies among our current patients, these treatments were not found to be associated with the IMA prognosis in our multivariate analysis. The distinct characteristics of EGFR mutations that have been found to have no female predominance and operate in a different tumorigenic pathway in IMA could have impacted on these targeted treatment responses [42]. Finally, PD L-1 expression was not examined in all the patients in our cohort. This marker is used as a predictor of the response to immunotherapy and a future examination of its status in IMA could provide insights into the causality behind the good response of these cancers to immunotherapy.
Conclusions
This study suggested that the type of palliative chemotherapy is associated with the prognosis of patients with advanced IMA. Patients with IMA treated by immunotherapy may have better outcomes than those treated using other chemotherapies. Further studies are needed to demonstrate the efficacy of these treatments as a palliative intervention for patients with IMA.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IMA:
-
Invasive mucinous adenocarcinoma
- WHO:
-
World Health Organization
- NSCLC:
-
Non-small cell lung cancer
- TKI:
-
Tyrosine kinase inhibitor
- EGFR:
-
Epidermal growth factor receptor
- CTx:
-
Chemotherapy
- CT:
-
Computed tomography
- OS:
-
Overall survival
- ALK:
-
Anaplastic lymphoma receptor tyrosine kinase
- PD:
-
Programmed death
- PFS:
-
Progression-free survival
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
References
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–85. https://doi.org/10.1097/JTO.0b013e318206a221.
Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. 2015;10(10):1383–95. https://doi.org/10.1097/JTO.0000000000000654.
Luo J, Wang R, Han B, Zhang J, Zhao H, Fang W, et al. Analysis of the clinicopathologic characteristics and prognostic of stage I invasive mucinous adenocarcinoma. J Cancer Res Clin Oncol. 2016;142(8):1837–45. https://doi.org/10.1007/s00432-016-2201-9.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54. https://doi.org/10.1038/nature25183.
Shim HS, Kenudson M, Zheng Z, Liebers M, Cha YJ, Hoang Ho Q, et al. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol. 2015;10(8):1156–62. https://doi.org/10.1097/JTO.0000000000000579.
Ichinokawa H, Ishii G, Nagai K, Kawase A, Yoshida J, Nishimura M, et al. Distinct clinicopathologic characteristics of lung mucinous adenocarcinoma with KRAS mutation. Hum Pathol. 2013;44(12):2636–42. https://doi.org/10.1016/j.humpath.2013.05.026.
Cha YJ, Kim HR, Lee HJ, Cho BC, Shim HS. Clinical course of stage IV invasive mucinous adenocarcinoma of the lung. Lung Cancer. 2016;102:82–8. https://doi.org/10.1016/j.lungcan.2016.11.004.
Boland JM, Maleszewski JJ, Wampfler JA, Voss JS, Kipp BR, Yang P, et al. Pulmonary invasive mucinous adenocarcinoma and mixed invasive mucinous/nonmucinous adenocarcinoma-a clinicopathological and molecular genetic study with survival analysis. Hum Pathol. 2018;71:8–19. https://doi.org/10.1016/j.humpath.2017.08.002.
Lin G, Li H, Kuang J, Tang K, Guo Y, Han A, et al. Acinar-predominant pattern correlates with poorer prognosis in invasive mucinous adenocarcinoma of the lung. Am J Clin Pathol. 2018;149(5):373–8. https://doi.org/10.1093/ajcp/aqx170.
Lee HY, Cha MJ, Lee KS, Lee HY, Kwon OJ, Choi JY, et al. Prognosis in resected invasive mucinous adenocarcinomas of the lung: related factors and comparison with resected nonmucinous adenocarcinomas. J Thorac Oncol. 2016;11(7):1064–73. https://doi.org/10.1016/j.jtho.2016.03.011.
Moon SW, Choi SY, Moon MH. Effect of invasive mucinous adenocarcinoma on lung cancer-specific survival after surgical resection: a population-based study. J Thorac Dis. 2018;10(6):3595–608. https://doi.org/10.21037/jtd.2018.06.09.
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. https://doi.org/10.1016/S0140-6736(16)30958-8.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. https://doi.org/10.1056/NEJMoa1810865.
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. https://doi.org/10.1056/NEJMoa1910231.
Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–6. https://doi.org/10.1093/annonc/mdz131.
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38. https://doi.org/10.1056/NEJMoa1704795.
Leonetti A, Facchinetti F, Rossi G, Minari R, Conti A, Friboulet L, et al. BRAF in non-small cell lung cancer (NSCLC): pickaxing another brick in the wall. Cancer Treat Rev. 2018;66:82–94. https://doi.org/10.1016/j.ctrv.2018.04.006.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60. https://doi.org/10.1097/JTO.0000000000000630.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung Cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. https://doi.org/10.1016/j.jtho.2015.09.009.
Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–70. https://doi.org/10.1200/JCO.2011.35.6345.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. https://doi.org/10.1056/NEJMoa0909530.
Winfree KB, Torres AZ, Zhu YE, Muehlenbein C, Aggarwal H, Woods S, et al. Treatment patterns, duration and outcomes of pemetrexed maintenance therapy in patients with advanced NSCLC in a real-world setting. Curr Med Res Opin. 2019;35(5):817–27. https://doi.org/10.1080/03007995.2018.1547273.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–46. https://doi.org/10.1200/JCO.18.00149.
Abernethy AP, Arunachalam A, Burke T, McKay C, Cao X, Sorg R, et al. Real-world first-line treatment and overall survival in non-small cell lung cancer without known EGFR mutations or ALK rearrangements in US community oncology setting. PLoS One. 2017;12(6):e0178420. https://doi.org/10.1371/journal.pone.0178420.
Lei L, Wang WX, Yu ZY, Liang XB, Pan WW, Chen HF, et al. A real-world study in advanced non-small cell lung cancer with KRAS mutations. Transl Oncol. 2020;13(2):329–35. https://doi.org/10.1016/j.tranon.2019.12.004.
Nakaoku T, Tsuta K, Ichikawa H, Shiraishi K, Sakamoto H, Enari M, et al. Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin Cancer Res. 2014;20(12):3087–93. https://doi.org/10.1158/1078-0432.CCR-14-0107.
Guo M, Tomoshige K, Meister M, Muley T, Fukazawa T, Tsuchiya T, et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med. 2017;9(4):462–81. https://doi.org/10.15252/emmm.201606711.
Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res. 2017;6(5):508–12. https://doi.org/10.21037/tlcr.2017.06.10.
Adderley H, Blackhall FH, Lindsay CR. KRAS-mutant non-small cell lung cancer: converging small molecules and immune checkpoint inhibition. EBioMedicine. 2019;41:711–6. https://doi.org/10.1016/j.ebiom.2019.02.049.
Snyder EL, Watanabe H, Magendantz M, Hoersch S, Chen TA, Wang DG, et al. Nkx2-1 represses a latent gastric differentiation program in lung adenocarcinoma. Mol Cell. 2013;50(2):185–99. https://doi.org/10.1016/j.molcel.2013.02.018.
Kettle R, Simmons J, Schindler F, Jones P, Dicker T, Dubois G, et al. Regulation of neuregulin 1β1-induced MUC5AC and MUC5B expression in human airway epithelium. Am J Respir Cell Mol Biol. 2010;42(4):472–81. https://doi.org/10.1165/rcmb.2009-0018OC.
Fernandez-Cuesta L, Plenker D, Osada H, Sun R, Menon R, Leenders F, et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014;4(4):415–22. https://doi.org/10.1158/2159-8290.CD-13-0633.
Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS G12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–17. https://doi.org/10.1056/NEJMoa1917239.
Incorvaia L, Fanale D, Badalamenti G, Barraco N, Bono M, Corsini LR, et al. Programmed death ligand 1 (PD-L1) as a predictive biomarker for pembrolizumab therapy in patients with advanced non-small-cell lung cancer (NSCLC). Adv Ther. 2019;36(10):2600–17. https://doi.org/10.1007/s12325-019-01057-7.
Chen R, Tao Y, Xu X, Shan L, Jiang H, Yin Q, et al. The efficacy and safety of nivolumab, pembrolizumab, and atezolizumab in treatment of advanced non-small cell lung cancer. Discov Med. 2018;26(143):155–66.
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–104. https://doi.org/10.1056/NEJMoa1801946.
Miyazawa T, Marushima H, Saji H, Kojima K, Hoshikawa M, Takagi M, et al. PD-L1 expression in non-small-cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surg. 2019;25(1):1–9. https://doi.org/10.5761/atcs.oa.18-00163.
Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–6. https://doi.org/10.1001/jamaoncol.2017.4427.
Passiglia F, Cappuzzo F, Alabiso O, Bettini AC, Bidoli P, Chiari R, et al. Efficacy of nivolumab in pre-treated non-small-cell lung cancer patients harbouring KRAS mutations. Br J Cancer. 2019;120(1):57–62. https://doi.org/10.1038/s41416-018-0234-3.
Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016;5:CD010383. https://doi.org/10.1002/14651858.CD010383.pub2.
Wakejima R, Inamura K, Ninomiya H, Nagano H, Mun M, Okumura S, et al. Mucinous lung adenocarcinoma, particularly referring to EGFR-mutated mucinous adenocarcinoma. Pathol Int. 2020;70(2):72–83. https://doi.org/10.1111/pin.12879.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YJJ, DGH and JCL contributed to the conceptualization of the manuscript. YJJ, DGH and WJ contributed to the collection of the data. CMC, DHL and SWK contributed to the analysis of the data. SY, WSK, WJ and JCL contributed to the methodology. YJJ, DGH, WSK, and JCL contributed to the writing of the manuscript. CMC, DHL, SWK and SY contributed to the editing of the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Asan Medical Center Institutional Review Board, Seoul, Korea (IRB No.2020–0645). The same ethics committee (the Asan Medical Center Institutional Review Board, Seoul, Korea) confirmed the requirement for the patients’ informed consent was waived because of the retrospective nature and minimal risk to the subjects. Administrative permission to access the clinical data used in this study has been granted by the Asan Medical Center Institutional Review Board, Seoul, Korea. It was conducted in full accordance with the Guidelines for Good Clinical Practice and the 1964 Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jang, Y.J., Hyun, Dg., Choi, CM. et al. Optimizing palliative chemotherapy for advanced invasive mucinous adenocarcinoma of the lung. BMC Cancer 21, 731 (2021). https://doi.org/10.1186/s12885-021-08472-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-08472-6