Abstract
Introduction
Although myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN) – including chronic myeloid leukemia (CML) – and myelodysplastic/myeloproliferative neoplasms (MDS/MPN) are largely clinically distinct myeloid malignancies, epidemiological studies rarely examine them separately and often combine them with lymphoid malignancies, limiting possible etiological interpretations for specific myeloid malignancies.
Methods
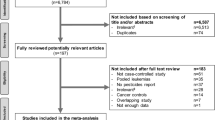
We systematically evaluated the epidemiological literature on the four chemical agents (1,3-butadiene, formaldehyde, benzene, and tobacco smoking, excluding pharmaceutical, microbial and radioactive agents, and pesticides) classified by the International Agency for Research on Cancer as having sufficient epidemiological evidence to conclude that each causes “myeloid malignancies.” Literature searches of IARC Monographs and PubMed identified 85 studies that we critically assessed, and for appropriate subsets, summarized results using meta-analysis.
Results
Only two epidemiological studies on 1,3-butadiene were identified, but reported findings were inadequate to evaluate specific myeloid malignancies. Studies on formaldehyde reported results for AML and CML – and not for MDS or MPN – but reported no increased risks. For benzene, several specific myeloid malignancies were evaluated, with consistent associations reported with AML and MDS and mixed results for CML. Studies of tobacco smoking examined all major myeloid malignancies, demonstrating consistent relationships with AML, MDS and MPN, but not with CML.
Conclusions
Surprisingly few epidemiological studies present results for specific myeloid malignancies, and those identified were inconsistent across studies of the same exposure, as well as across chemical agents. This exercise illustrates that even for agents classified as having sufficient evidence of causing “myeloid malignancies,” the epidemiological evidence for specific myeloid malignancies is generally limited and inconsistent. Future epidemiological studies should report findings for the specific myeloid malignancies, as combining them post hoc – where appropriate – always remains possible, whereas disaggregation may not. Furthermore, combining results across possibly discrete diseases reduces the chances of identifying important malignancy-specific causal associations.
Similar content being viewed by others
Introduction
Hematopoietic and lymphoid malignancies (also known as lymphohematopoietic malignancies, or LHM) arise from stem and progenitor cells derived from hematopoietic stem cells. These diseases, though, represent several heterogeneous groups of neoplasms that are biologically, etiologically or clinically distinct [1]. LHM are classified based on the progenitor cells from which they arise, the vast majority being of lymphoid (i.e., derived from the lymph and lymphatic system) or myeloid (deriving from the bone marrow) origin, although much rarer malignancies may arise from dendritic or histiocytic cells.
Lymphoid malignancies generally are associated with lymphoid progenitor cells that mature into cells of the immune system, including B lymphocytes [B-cells], T lymphocytes [T-cells], and Natural Killer [NK] cells), but are categorized by the stage of differentiation of the tumor cells rather than the cell in which the initial transforming event occurred [2]. Lymphoid malignancies include various lymphomas, as well as acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL). Myeloid malignancies arise from myeloid progenitor cells and include all granulocytic (e.g., erythrocytes, or red blood cells) and mast cell lineages [3]. Myeloid malignancies include myelodysplastic syndrome (MDS), acute myeloid leukemia (AML, which has replaced the term acute nonlymphocytic leukemkia, ANLL), myeloproliferative neoplasms (MPN), chronic myeloid (or “myelogenous”) leukemia (CML) – and myelodysplastic/myeloproliferative neoplasms (MDS/MPN) [2]. Multiple myeloma is a malignant disorder involving plasma cells which originate from B-cells. Most of these sub-groups of LHM contain multiple entities with diverse etiologies and possible underlying risk factors.
The 2008 revision of the World Health Organization (WHO) classification of LHMs led to changes in the classification of leukemias and especially myeloid leukemias for epidemiological research based on improved understanding of the lineage of the cells, as well as the molecular genetics and pathologic characteristics of the different malignancies. The WHO classification was further updated in 2016 for lymphoid [4] and for myeloid malignancies [5].
The primary objective of this paper is to evaluate the published epidemiological evidence on the myeloid malignancies for chemical agents classified by the International Agency for Research on Cancer (IARC) as Group 1 carcinogens (that is, “carcinogenic to humans,” commonly referred to as “known human carcinogens”) and for which the epidemiological evidence of a causal association was considered sufficient. The epidemiological and toxicological evidence for associations with exposure to certain chemicals (e.g., benzene) appears to be stronger for specific myeloid malignancies – especially AML and MDS – than for leukemias as a group or lymphoid malignancies, which are generally more closely related to infections and immunological functions [6].
PART I: overview of the myeloid malignancies
Since 2001, the WHO has included genetic information relevant to the diagnosis and classification of LHMs, and the 2008 WHO classification of myeloid neoplasms built on the 2001 classification. The underlying pathology in myeloid malignancies is based on clonal proliferations arising in hematopoietic stem or progenitor cells, and specific diseases are often associated with genetic or epigenetic changes in genes involved in regulation of cell growth. The 2016 update to the 4th Edition of the WHO Classification of Tumors of the Hematopoietic and Lymphoid Tissues additionally incorporated clinical features, morphology, immuno-phenotyping, cytogenetics, and molecular genetics to classify both acute and chronic myeloid leukemias into subtypes and discrete disease entities of clinical significance [7]. A brief review of the current pathology and classification of the myeloid malignancies illustrates several ways in which specific myeloid malignancies differ and a basis for epidemiologically examining them separately (Part II).
Myelodysplastic syndromes (MDS)
MDS refers to a heterogeneous collection of clonal disorders of pluripotent hematopoietic progenitor cells (HPC) that demonstrate lower than normal blood cell counts (cytopenias), an increased percentage of blasts in bone marrow, and dysplasia in erythroid cells, granulocytes, or megakaryocytes [5]. MDS generally has an insidious onset, often diagnosed due to vague symptoms arising as a manifestation of cytopenias, and a variable prognosis, depending upon the molecular genetic profile of the subtype and individual response to therapy. Approximately 20–30% of MDS patients over the age of 65 go on to develop AML, suggesting that at least some proportion of these cases may represent the same underlying disease processes or share causal factors [8]. Some acquired mutations seen in the development of MDS include those in genes involved in RNA splicing (SRSF2), DNA methylation (DNMT3a, TET2, IDH 1/2), chromatin modification (ASXL1) or the cohesion complex (STAG2) [9].
MDS is more prevalent in older adults, with the majority of cases diagnosed in individuals over the age of 60 [10]. Rates of MDS appear to be increasing, which may be due to improvements over time in diagnostic specificity combined with clearer diagnostic criteria for MDS [11].
Acute myeloid leukemia (AML)
The classification of AML includes 20 definitive and 2 provisional subtypes [5]. AML generally has a rapid onset, often diagnosed due to the development of infections, bleeding, or fatigue that result from pancytopenia, and a variable prognosis, depending upon the molecular genetic profile of the subtype and individual response to therapy.
AML is a genetically diverse disease, with 40–55% of patients having chromosome abnormalities that can be identified using conventional analysis techniques [12,13,14]. The most common genetic change is the loss of genetic material in chromosome 5 or chromosome 7 [13]. Others include deletions in parts of chromosomes (e.g., the long arms of chromosomes 5, 7, and 9), insertion of genetic material, inversions of genetic material (e.g., involving chromosome 16), duplications, and translocations (e.g., t[8;21], t[15;17], and 11q23 translocation]) [13]. Approximately 40–50% of AML patients have a normal karyotype and harbour mutations within specific genes including IDH1, IDH2, FLT3, and NPM1.
Some AMLs develop secondary to MDS, and these occur in patients with acquired mutations in genes encoding for myeloid transcription factors (RUNX1, CEBPA) or signal transduction proteins (FLT3) [9]. However, de novo AMLs are also diagnosed in patients with mutations in RUNX1, CEBPA, FLT3 or MLL, but these patients do not have mutations in the genes associated with prior MDS (described above) [9]. Estey (2018) estimated that one-third of patients clinically diagnosed with de novo AML will exhibit genetic mutations specific for secondary AML [9].
AML is more common in the elderly, with more than 58% of cases diagnosed among those 65 years of age or older [10].
Myeloproliferative neoplasms (MPN)
MPNs (previously known as myeloproliferative disorders, or MPD) are a group of clonal hematopoeitic neoplasms, including polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF). These conditions are associated with the proliferation of one or more of the myeloid lineages (i.e., increased blood cell counts), without dysplasia. CML shares several features with these disorders, e.g., dysregulated production of a particular lineage of mature myeloid cells, a tendency to progress to acute leukemia, and abnormalities in thrombosis and hemostasis. Many diagnoses of MPNs occur in patients that have acquired mutations in the Janus kinase 2 (JAK2) gene, seen in 95% of patients diagnosed with PV and over 50% of patients diagnosed with MF and ET) [15]. Other mutations seen in patients with MPN include calreticulin (CALR), myeloproliferative leukemia virus oncogene (MPL) [16].
SEER data are limited for MPN, however, ET represented 45.5% of the cases and PV accounted for 41.5% of the cases. The incidence rate was slightly higher in males compared to females, 3.3 vs. 3.0 per 100,000, respectively. Incidence rates increased with age from 0.5 per 100,000 for under age 40 to 18.6 per 100,000 for ages 80 and over [10].
Chronic myeloid leukemia (CML)
In CML, the proliferating cells are mature cells of the myeloid lineage, which have differentiated into functional formed elements of the blood. The development of CML involves an acquired cytogenetic abnormality in the pluripotent hematopoietic stem cells (HSCs) or myeloid progenitor cells located in the bone marrow. Ninety-five percent of CML cases involve the reciprocal translocation of genetic material between chromosome 22 and chromosome 9 [t(9;22)(q34;q11)]. This translocation results in an abnormally shortened version of chromosome 22, known as the “Philadelphia (Ph) chromosome” [17, 18].
In the United States, the median age at diagnosis of CML was 65 years, while the median age at death was 77 years. The incidence rate among males, for all races and ethnicities and all age-groups, was 2.4 per 100,000 population, while among females the rate was 1.4 per 100,000. Incidence among white males was 2.5 per 100,000 while the incidence was 2.2 per 100,000 among black males. Incidence among those under 65 years of age was 1.1 per 100,000 population, but nearly seven times higher (i.e., 7.6 per 100,000) among those 65 and over. Incidence among the population aged 65 and over was highest among white males (11.1 per 100,000), followed by black males (8.2 per 100,000), white females (5.7 per 100,000) and black females (4.9 per 100,000) [10].
Myelodysplastic syndrome/Myeloproliferative Neoplams (MDS/MPN)
The 2016 Classification of LHM includes a category for MDS/MPN. These neoplasms are characterized by both dysplastic and proliferative features. Examples include chronic myelomonocytic leukemia (CMML), atypical chronic myeloid leukemia (aCML) and juvenile myelomonocytic leukemia (JMML) [7]. However, these specific myeloid neoplasms are very rare and infrequently considered in epidemiological studies; therefore, they are not discussed further.
PART II: epidemiological evaluation of four environmental agents and specific myeloid malignancies
Methods
We reviewed the list of carcinogenicity classifications by cancer site published on the IARC Monographs website [19, 20]. The IARC has identified 28 agents as having sufficient evidence of carcinogenicity in humans for neoplasms the IARC grouped as “leukemia and/or lymphoma”. We assessed the human evidence summaries in the “Evaluation” sections of the relevant IARC monographs for each agent.
We excluded from our review 10 pharmaceutical agents (azathioprine, busulfan, chlorambucil, cyclophosphamide, etoposide with cisplatin and bleomycin, melphalan, MOPP [vincristine-prednisone-nitrogen mustard-procarbazine], semustine [methyl-CCNU], thiotepa, and treosulfan) because most of these are chemotherapy agents in which exposure is voluntary and the expected benefit likely offsets the possible leukemogenic effect. We also eliminated radioactive (e.g., X- and gamma radiation, fission-products radionuclides [including strontium-90], thorium-232 and its decay products) and microbiological (Epstein Barr virus, helicobacter pylori, hepatitis C virus, Human immunodeficiency virus type 1, Human T-cell lymphotropic virus type 1, Kaposi sarcoma herpes virus) agents. We excluded two pesticides - pentachlorophenol and lindane - because IARC identified the human evidence as sufficient for causing NHL (lymphomas). We also excluded IARC’s evaluation “occupational exposures in the rubber-manufacturing industry” because workers in the industry are exposed to multiple chemicals and it cannot be determined which specific agents may be causally related to leukemia.
After these exclusions, four leukemogenic chemical agents remained: 1,3-butadiene, formaldehyde, benzene and tobacco smoking. For each of these, we conducted a focused systematic review of the literature using searches of the relevant IARC Monographs and key word searches of PubMed to identify epidemiological studies that reported results separately for specific subtypes of myeloid malignancies. Keywords included “benzene”, “1,3-butadiene,” “formaldehyde,” “cigarette,” “smoking,” “leukemia,” “myeloid,” “AML,” “CML,” “MDS,” and “MDN.” Where results of independent studies of acceptable quality were available, we conducted meta-analyses using random-effects models [21]. For each study, the following characteristics were extracted consistent with PRISMA guidelines [22]: study design, study population, geographic location, study period, exposure categories, number of deaths observed or number of cases in exposed and unexposed groups, relative risk measures (SMRs, HRs, RRs, and ORs) 95% confidence intervals (CI) and covariates adjusted for in models. Using meta-analysis, summary relative risk estimates were calculated by specific categories of myeloid malignancy including AML, CML and MDS. Cohort studies and case-control studies were analysed separately as well as overall and where possible for the highest exposure categories. When multiple results were published on the same study population, we preferentially selected for meta-analysis those based on incidence data, those representing the most complete results, or results reported for higher exposure categories. Publication bias was assessed using a visual inspection of the funnel plots as well as Egger’s test (see supplemental file). Heterogeneity was evaluated using the I2 statistic, which provides a measure for quantifying inconsistency of effects across studies. All meta-analyses were conducted using R version 3.6.1 (2019-07-05).
Results
1,3-butadiene (butadiene)
The IARC last reviewed the carcinogenicity of butadiene in 2009 [23]. The epidemiological evidence for exposure to butadiene and risk of leukemia is based primarily on studies conducted among workers in the butadiene monomer industry and workers in the styrene–butadiene rubber (SBR) manufacturing industry. However, results on specific types of leukemia are available only from studies conducted in the SBR manufacturing industry.
A study of approximately 17,000 workers from eight SBR facilities across the United States and Canada reported an increased risk of leukemia among 16,610 workers (12,412 exposed to butadiene), based on 58 leukemia deaths [24]. Because standardized mortality ratio analyses were not conducted, it is not clear whether excess mortality from leukemias occurred. A positive dose-response was reported between cumulative exposure to butadiene and risk of leukemia. Despite the individual exposure estimates and the relatively large number of leukemia deaths, results by leukemia subtype were not reported.
The mortality follow-up was extended through 1998 for 15,649 men employed since 1943, 75% of whom were exposed to butadiene [25]. A total of 71 deaths from leukemia was observed (SMR 1.16, 95% CI, 0.91–1.47). No consistent patterns were observed by categories of years since hire or by years worked. The excess leukemia mortality was concentrated among men hired in the 1950s (31 deaths; SMR, 1.50; 95% CI, 1.01–2.11). In the analysis by leukemia subtype the SMR was 1.02 (95% CI 0.56–1.71, 14 deaths) for AML and 1.67 (95% CI 0.83–2.99, 11 deaths) for CML. Mortality from AML was elevated in maintenance laborers and from CML in laboratory workers; however these were based on only five and three deaths, respectively.
Time-dependent exposure-response relationships between several butadiene exposure indices and leukemia (81 decedents) as well as all myeloid neoplasms (56 decedents from myeloid and monocytic leukemia, myelofibrosis, myelodysplasia, myeloproliferative disorders and polycythemia vera) were evaluated [26]. The butadiene exposure indices included cumulative exposure in ppm–years, total number of exposures to peaks (> 100 ppm) and average intensities of exposure in parts per million. All three exposure indices were associated positively with the risk for leukemia whereas the myeloid neoplasms were more clearly associated with peak exposures. This highlights the potential additional role choice of exposure metric may play in evaluating risk [27].
Only two studies evaluated the risk of myeloid malignancies [25, 26], based on the same study cohort. Risks of AML were not increased, and risk of CML was increased but not statistically significantly. For myeloid leukemias (including CML), a relationship was reported for peak exposure, but not cumulative exposure.
Formaldehyde
The IARC last reviewed the carcinogenicity of formaldehyde in 2009 (23). The epidemiological literature on exposure to formaldehyde and risk of leukemia published since the IARC meeting was reviewed in detail [28]. Twenty studies that reported results for leukemia overall were included, three of which also reported results for myeloid leukemia. Since then, some of the occupational epidemiological studies have been updated or re-analyzed, and new studies have been published that examine myeloid leukemias in relation to formaldehyde exposure (Table 1).
Peak exposure in a cohort of workers employed in six plants producing formaldehyde in the United States was re-defined and analysed with respect to specific leukemia types. Absolute peak exposure, duration of time worked at the highest peak or time since highest peak exposure generated no clear associations with myeloid leukemia or AML. Cumulative exposure also was unrelated to risk of leukemia, myeloid leukemia, AML, or CML. The authors concluded, “Findings from this re-analysis do not support the hypothesis that formaldehyde is a cause of AML” [31]. The use of peak exposure in this and other epidemiological studies presents specific challenges that have been explored separately [27].
The other occupational cohort study of formaldehyde producers also reported no clear associations between different metrics of formaldehyde exposure and myeloid leukemia [30]. In a study of garment workers in the United States [29, 35], moderately elevated relative risks for myeloid leukemia were associated with duration of employment, a surrogate for cumulative exposure, and duration of follow-up, a surrogate for latency. A large cancer registry study in the Nordic countries “did not provide clear evidence for an association between occupational solvent exposure and AML” [34].
There were no deaths from myeloid leukemias among a cohort of laminated plastic workers from Italy [36]. A European community-based cohort study [33] found no increased risks of AML or CML among study subjects with low-level occupational exposure to formaldehyde (no study subjects were reported to have high occupational exposure to formaldehyde).
SMR results for myeloid leukemia, AML and CML, including those for the highest categories of exposure from the most recent updates of the industrial cohorts, are summarized in Table 2. Overall, the updated cohort study analyses demonstrate no clear or consistent excess risk of myeloid leukemia or AML or CML. None of the formaldehyde studies evaluated MDS or MPN. Table 3 presents meta-analysis results by myeloid malignancy, specifically ML, AML and CML. No statistically significant increased meta-relative risk estimates were seen. Based on the I2 test, heterogeneity was low, and based on Egger’s test, publication bias appears unlikely.
Benzene
The IARC last reviewed the carcinogenicity of benzene in 2018 [6]. Risk estimates for one or more myeloid malignancies were reported in 31 independent studies. Characteristics of the design of these studies are summarized in Table 4, and results are summarized in Table 5.
A cohort exposed to benzene in a variety of manufacturing and user industries, including paints and painting, printing, footwear, paints, chemicals in 12 cities in China was followed for mortality. In the most recent update of this cohort, 73 leukemia deaths were observed, including 60 among benzene-exposed workers [51]. Similar risks were reported for AML and CML, while the risk of MDS was inestimable due to zero cases of MDS among the unexposed group (Table 5). A case-cohort analysis of combined AML/MDS (44 cases) and CML (18 cases) from the 12-city China cohort examined the timing of exposure [66]. The investigators found that high cumulative exposure or high intensity exposure experienced 2 to 10 years before diagnosis increased the risk of MDS/AML among workers who were first exposed under 30 years of age, but not for workers first exposed 30 years of age or older [66].
Pooled results for AML, CML, MDS and MPN were reported using data from three separate nested case-control studies of petroleum workers from Canada, the UK, and Australia. No significantly elevated risks of AML by cumulative exposure, average exposure intensity, maximum exposure intensity, duration of employment, and peak exposure were reported [55]. Increased relative risk of MDS for cumulative exposure greater than 2.93 ppm-years and peak exposure less than 3 ppm were reported, but not for CML or MPN [58]. The most recent follow-up of incidence in the UK petroleum distribution and oil refinery workers reported deficits of MDS [67]. Similarly, the most recent mortality follow-up of the Canadian Petroleum Workers cohort reported no excess of MDS [68].
An increased mortality risk of MDS associated with 25 ppm-years or more of benzene exposure was reported, however, this finding was based on only one death [42]. A much larger registry-based study of occupational exposure to benzene and incidence of AML indicated no increased risk [34]. Age-stratified analyses indicated a possible increased risk of AML in workers under age 50 and in the highest benzene exposure group [34].
Since studies did not report results for the same subtypes of leukemia, it is problematic to combine all results using meta-analysis. We, therefore, conducted meta-analyses of results reported for myeloid leukemias combined or specifically for AML, MDS and CML (Table 6). The meta-analysis of results for AML was based on 27 estimates from 26 publications and generated a summary RR of 1.30 (95% CI 1.09–1.55; I2 = 48.91%) with similar increases, but some variation in the summary RR across exposure categories (i.e., high, low, any exposure). The results for low and any exposure to benzene were not statistically significantly elevated. Egger’s test was significant for the overall result and for cohort studies, but not for the other meta-analyses (Table 6). Visual inspection of funnel plots indicated possible publication bias favoring negative results (see supplemental file).
For CML, the meta-analysis of overall results was based on 18 estimates from 17 studies resulting in a summary RR of 1.25 (95% CI 1.00–1.55; I2 = 0%) with large variation in the summary RR across exposure categories. Publication bias appears unlikely. The meta-RR for myeloid leukemias combined, based on seven studies, was 1.56 (95% CI 1.10–2.20; I2 = 45.06%) with wide variability by exposure category (high, low, any exposure). Evidence of publication bias was present for the overall and cohort meta-analyses, but small numbers of studies hindered results for the exposure categories (Table 6). Visual inspection of funnel plots indicated that publication bias favored positive results (see supplemental file).
The meta-analysis for MDS was based on nine studies and generated a summary RR of 1.87 (95% CI 1.39–2.52; I2 = 40.73%) with similar risks for the low exposure category (m-RR = 2.29, 95% CI 1.51–3.48, I2 = 0%) and the high exposure category (m-RR = 1.80, 95% CI 1.18–2.75, I2 = 51.97%). Publication bias appears unlikely. The meta-analyses for the overall category for each outcome were also calculated by study type (Table 6). The meta-analyses for CML by study type revealed a large difference between the case-control (m-RR = 1.93; 95% CI 1.05–3.56, I2 = 25.76%) and cohort studies (m-RR = 1.13; 95% CI 0.89–1.45, I2 = 0%), possibly reflecting reporting bias, as many of the case-control studies were population-based and dependent on self-reported exposure. This difference by study design was not observed for AML, MDS or the category of all myeloid leukemias combined.
The interpretation of results on risk of specific leukemia types from exposure to benzene is complicated by the heterogeneity in exposure circumstances. However, the evidence indicates a similar association between occupational exposure to benzene and specific myeloid neoplasms, but the association appears strongest for MDS, especially among more recent studies. This raises the question of whether earlier studies identifying associations between generally very high benzene exposure and AML might have reflected the occurrence of secondary AML following unrecognized (or misdiagnosed) primary cases of MDS. It is noteworthy that a very large record linkage study from the Nordic countries reported no association between benzene exposure and AML incidence [34].
Tobacco smoking
The IARC last evaluated the carcinogenicity of tobacco smoking in 2009 [69]. From the IARC review and the PubMed search, we identified 42 studies on tobacco smoking and risk of myeloid malignancies, 27 of which reported results for current or ever smokers (current and former smokers combined) as summarized in Table 7. The remaining studies reported results for different groups of smokers, defined according to dose (cigarettes per day), duration (years of smoking) or cumulative consumption (pack-years). Whenever possible, we selected results by duration of smoking, since this is the exposure metric most strongly associated with lung cancer risk (Table 8).
One of the largest studies to examine leukemia risks among smokers was a prospective cohort of 1.3 million middle-aged women recruited for breast cancer screening during 1996–2001 and followed for mortality through 2009 [84]. The investigators identified death due to myeloid neoplasm in 831 cohort members and reported a statistically significantly increased risk (RR = 1.33, 95% CI 1.24–1.42). The increase was driven by a significant RR for MPN/MDS (RR = 1.42, 95% CI 1.31–1.55), whereas the RR for AML was not elevated (RR = 1.10, 95% CI 0.96–1.26). Relative risks also increased with increased intensity of smoking for myeloproliferative/myelodysplastic disease, but not for AML [84].
A cohort study of over 330,000 Swedish construction workers with follow-up for mortality through 2004 reported a statistically significant association between “current” smoking and AML risk (RR = 1.50, 95%CI: 1.06, 2.11), but no association with CML (RR = 0.69, 95% CI 0.42–1.14). For AML, relative risks did not increase with increasing intensity of smoking [78].
Table 9 presents summary risk estimates for myeloid malignancies by various smoking exposure metrics and study type. Several meta-analyses demonstrated significant heterogeneity, as indicated by the high I2 statistic and associated low p-values. However, publication bias generally was not indicated.
The meta-analysis for smoking and AML was based on 28 studies and resulted in a summary RR of 1.43 (95% CI 1.25–1.62; I2 = 56.25%) with slightly higher meta-RR for current smokers and a lower meta-RR for ever smokers. Meta-analysis of results for CML was based on 14 studies, generating a summary RR of 0.93 (95% CI 0.74–1.16; I2 = 40.44%) with similar results for current and ever smokers. The meta-RR for myeloid leukemias combined (i.e., based on eight studies not differentiating by leukemia type) was 1.54 (95% CI 0.79–3.01; I2 = 81.80%) with similar results for current and ever smokers. The meta-analysis of overall results on MDS was based on 22 studies and resulted in a summary RR of 1.66 (95% CI 1.38–2.00; I2 = 61.89%) with similar results for current and ever smokers. Only three studies were identified for MPN resulting in a summary RR of 1.70 (95% CI 1.23–2.34; I2 = 0%) for current smokers. Publication bias appears unlikely for the meta-analyses on smoking except for the Egger’s test results for the category of current smoker and CML. Visual inspection of the funnel plot, however, did not suggest any publication bias (see supplemental file).
In contrast to benzene, the evidence on the risk of specific leukemia subtypes from tobacco smoking indicates an association with AML, but not with CML. Similar to benzene, there is evidence of an increased risk of MDS. Although only three studies were identified, risk of myeloproliferative/myelodysplastic neoplasm (MPN) appears to be increased among smokers.
Discussion
The myeloid malignancies clearly have different clinical features and characteristic genetic aberrations and therefore, they should be evaluated separately in epidemiological studies intended to identify risk factors and potential causes.
We found little consistency in the way leukemias were evaluated, and they often were analyzed in aggregate, mixing myeloid and lymphocytic leukemias. The more recent benzene cohort studies were the exception, as they specifically evaluated AML, CML and MDS separately. Some analyses evaluated myeloid malignancies separately from the lymphocytic neoplasms, but still combined AML and CML, despite evidence of different mutations in genes and other risk factors that indicate different etiologies. Despite the determination that the epidemiological evidence was sufficient for purposes of establishing causation for leukemia, our review identified only small numbers of studies that actually reported results for specific types of myeloid neoplasms. Furthermore, where specific diseases were considered, small numbers of observed events often limited the precision of risk estimates.
For example, for butadiene, only one study analyzed risks by specific leukemia type, and findings were mixed: statistically significant associations were reported for CML among laboratory workers (based on only three deaths) but not for AML [25]. That results on butadiene exposure and myeloid malignancies are based on a single study and do not allow any causal conclusions does not necessarily mean that the IARC conclusion of “sufficient” human evidence is incorrect. Rather, it indicates that the relationship, if any, with one or more specific type of leukemia cannot be discerned based on available epidemiological evidence.
The updated meta-RRs for formaldehyde showed no consistent relationship with AML, CML or myeloid leukemia overall, confirming an earlier meta-analysis [28]. A further meta-analysis of the highest exposure groups was not conducted due to a lack of a common exposure metric. A very large registry-based linkage study demonstrated no increased risk and, in fact, reported a statistically significant deficit of incident AML cases among groups potentially occupationally exposed to formaldehyde [34]. Our findings suggest that the updated human evidence for formaldehyde and leukemia (of any type) may not be sufficient as determined by IARC and should be revisited. Combined with the lack of support from animal and mechanistic studies, it is unlikely that formaldehyde causes leukemia in humans [112].
A causal relationship between benzene exposure and AML has been recognized for decades and our meta-analyses indicate a significant increased risk overall and at high levels of exposure, yet the largest study, the Nordic registry study, demonstrated no association [34]. Other recent high-quality studies also indicate no clear association between benzene and AML risk [33, 42, 48, 55], possibly due to generally low exposure concentrations that do not exceed a possible exposure threshold for risk. The most comprehensive study on benzene and incident leukemia and myeloid neoplasm risks demonstrated a stronger association between benzene and risk of MDS than for AML, especially among workers with high peak exposures [58]. However, subsequent analyses of the Canadian sub-cohort were not consistent with these findings [68]. An association was reported between benzene and CML, but this was limited to case-control studies and may reflect potential reporting bias. Nevertheless, these findings epidemiologically underscore the importance of examining and contrasting results for specific malignancies (at least initially) and that exposure metric may play an important role in identifying causal associations [27].
Findings for tobacco smoking and myeloid leukemia were consistently positive for AML and negative for CML. The meta-RR for AML demonstrated a 50% statistically significantly increased risk, whether based on seven studies reporting individual leukemia types or the six in which AML and CML were reported separately. These results, and specifically the statistically significant positive meta-RR for AML and null findings for CML, further underscore the importance of examining epidemiologically narrowly defined or disease-specific relationships.
An ancillary finding of this evaluation is the surprisingly limited body of epidemiological studies aimed at addressing and differentiating risks by specific types of myeloid malignancy. While observing small numbers of any specific leukemia will plague all but the largest studies (or studies in which a strong association is indicated), we would argue that arbitrarily combining possibly discrete disease entities to improve “statistical power” will not help elucidate their specific causes; rather, this technique likely will dilute any true malignancy-specific associations and may lead to erroneous conclusions. One exception might be the subset of MDS cases that progresses to AML: these may reflect different clinical stages in the progression of the same disease. Nevertheless, publishing results based even on small numbers will facilitate combining results across studies using meta-analysis. It is conceivable that apparently negative findings based on small numbers may not be published, leading to potential “small numbers” or “negative study” publication bias, of which we found some evidence. While concerns of uncontrolled confounding arise in many occupational epidemiological settings, it is unlikely to be problematic in this context, primarily because there are no common exposures or risk factors that are strongly associated with all (or even multiple) types of leukemia. Progress in understanding the genetic factors underlying each of the myeloid neoplasms likely will guide future epidemiological studies to improve their ability to define appropriate combinations of myeloid malignancies and to isolate environmental risk factors that may be among their causes.
Nevertheless, our detailed evaluation of the four environmental chemical agents summarized here highlights important differences in risks by myeloid malignancies and provides support for reporting disease-specific findings from studies of environmental agents and risk of specific myeloid leukemias or other LHM. They also build on clinical observations that treatments with chemotherapy drugs lead to high incidence of AML and MDS (and possibly ALL) and that the genetic changes in therapy-related myeloid neoplasm reflect some specificity for the type of chemotherapy administered, but that chemotherapy does not lead to appreciable increases in CML, MPN, or lymphoid malignancies [2]. Meanwhile, epidemiological findings based on small numbers of specific LHM should be reported but appropriately caveated and not over-interpreted, as these results statistically will be unstable with likely false-positive and perhaps more likely false-negative relative risk estimates. Similarly, findings based on analyses of multiple types of leukemias and other LHM should be examined further and if possible, groups of LHM deconstructed, to identify the specific neoplasms that may be driving an observed association or situations where true associations may be diluted or masked.
Availability of data and materials
All data generated or analysed during this study are derived from publicly available peer-reviewed scientific publications and are presented in this article and its supplemental file.
Abbreviations
- aCML:
-
Atypical chronic myeloid leukemia
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- ANLL:
-
Acute nonlymphocytic leukemkia
- B-cells:
-
B lymphocytes
- CI:
-
Confidence interval
- CLL:
-
Chronic lymphocytic leukemia
- CML:
-
Chronic myeloid leukemia
- CMML:
-
Chronic myelomonocytic leukemia
- cpd:
-
Cigarettes per day
- HPC:
-
Hematopoietic progenitor cells
- IARC:
-
International Agency for Research on Cancer
- JMML:
-
Juvenile myelomonocytic leukemia
- LHM:
-
Lymphohematopoietic malignancies
- m-RR:
-
Meta-relative risk
- MDS:
-
Myelodysplastic syndrome (MDS)
- MPN:
-
Myeloproliferative neoplasms
- MDS/MPN:
-
Myelodysplastic/myeloproliferative neoplasms
- methyl-CCNU:
-
semustine
- MOPP:
-
Vincristine-prednisone-nitrogen mustard-procarbazine
- NHL:
-
Non-Hodgkin lymphoma
- NK cells:
-
Natural killer cells
- PMR:
-
Proportionate mortality ratio
- PPM:
-
parts per million
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- pyrs:
-
Pack-years
- RR:
-
Relative risk
- SBR:
-
Styrene–butadiene rubber
- SMR:
-
Standardized mortality ratio
- T-cells:
-
T lymphocyte
- WHO:
-
World Health Organization.
References
Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112(12):4384–99. https://doi.org/10.1182/blood-2008-07-077982.
Linet MS, Morton LM, Devesa SS, Dores GM. Leukemias. In: Thun MJ, et al., editors. Schottenfeld and Fraumeni Cancer epidemiology and prevention. 4th ed. New York: Oxford University Press; 2018. p. 715–44.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. https://doi.org/10.1182/blood-2009-03-209262.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. https://doi.org/10.1182/blood-2016-01-643569.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. https://doi.org/10.1182/blood-2016-03-643544.
International Agency for Research on Cancer (IARC) (2018) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Benzene. Volume 120. Lyon, France.
World Health Organization (2016) WHO classification of Tumours of Haematopoietic and lymphoid tissues, WHO classification of Tumours, revised 4th edition, volume 2. Geneva: World Health Organization.
Koeffler HP, Leong G. Preleukemia: one name, many meanings. Leukemia. 2017;31(3):534–42. https://doi.org/10.1038/leu.2016.364.
Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93(10):1267–91. https://doi.org/10.1002/ajh.25214.
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
Hofmann WK, Koeffler HP. Myelodysplastic syndrome. Annu Rev Med. 2005;56:1–16.
Natelson EA. Benzene-induced acute myeloid leukemia: a clinician's perspective. Am J Hematol. 2007;82(9):826–30.
Mrózek K, Bloomfield CD. Clinical significance of the most common chromosome translocations in adult acute myeloid leukemia. J Natl Cancer Inst Monogr. 2008;(39):52–7. https://doi.org/10.1093/jncimonographs/lgn003.
Blau O. Gene mutations in acute myeloid leukemia – incidence, prognostic influence, and association with other molecular markers. In: Guenova M, Balatzenko G, editors. Leukemias – updates and new insights. IntechOpen; 2015. https://doi.org/10.5772/60928.
Schieber M, Crispino JD, Stein B. Myelofibrosis in 2019: moving beyond JAK2 inhibition. Blood Cancer J. 2019;9(9):74. https://doi.org/10.1038/s41408-019-0236-2.
Helbig G. Classical Philadelphia-negative myeloproliferative neoplasms: focus on mutations and JAK2 inhibitors. Med Oncol. 2018;35(9):119. https://doi.org/10.1007/s12032-018-1187-3.
Zhou H, Xu R. Leukemia stem cells: the root of chronic myeloid leukemia. Protein Cell. 2015;6(6):403–12. https://doi.org/10.1007/s13238-015-0143-7.
NIH (2019) Chromosome 22. https://ghrnlmnihgov/chromosome/22 Reviewed: September 2016; Published: December 10, 2019. Accessed: 10 Dec 2019.
Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Wild CP. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103(24):1827–39. https://doi.org/10.1093/jnci/djr483.
International Agency for Research on Cancer (IARC) (2019) List of classifications by cancer site with sufficient or limited evidence in humans, Volumes 1to 125. https://monographs.iarc.fr/wp-content/uploads/2019/07/Classifications_by_cancer_site.pdf. Accessed 10 Dec 2019.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.100009.
International Agency for Research on Cancer (IARC) (2012) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Chemical Agents and Related Occupations. Volume 100F. Lyon, France.
Macaluso M, Larson R, Delzell E, Sathiakumar N, Hovinga M, Julian J, Muir D, Cole P. Leukemia and cumulative exposure to butadiene, styrene and benzene among workers in the synthetic rubber industry. Toxicology. 1996;113(1–3):190–202.
Sathiakumar N, Graff J, Macaluso M, Maldonado G, Matthews R, Delzell E. An updated study of mortality among north American synthetic rubber industry workers. Occup Environ Med. 2005;62(12):822–9.
Cheng H, Sathiakumar N, Graff J, Matthews R, Delzell E. 1,3-butadiene and leukemia among synthetic rubber industry workers: exposure-response relationships. Chem Biol Interact. 2007;166(1–3):15–24.
Checkoway H, Lees PSJ, Dell LD, Gentry PR, Mundt KA. Peak exposures in epidemiologic studies and Cancer risks: considerations for regulatory risk assessment. Risk Anal. 2019;39(7):1441–64. https://doi.org/10.1111/risa.13294.
Checkoway H, Boffetta P, Mundt DJ, Mundt KA. Critical review and synthesis of the epidemiologic evidence on formaldehyde exposure and risk of leukemia and other lymphohematopoietic malignancies. Cancer Causes Control. 2012;23(11):1747–66. https://doi.org/10.1007/s10552-012-0055-2.
Meyers AR, Pinkerton LE, Hein MJ. Cohort mortality study of garment industry workers exposed to formaldehyde: update and internal comparisons. Am J Ind Med. 2013;56(9):1027–39. https://doi.org/10.1002/ajim.22199.
Coggon D, Ntani G, Harris EC, Palmer KT. Upper airway cancer, myeloid leukemia, and other cancers in a cohort of British chemical workers exposed to formaldehyde. Am J Epidemiol. 2014;179(11):1301–11. https://doi.org/10.1093/aje/kwu049.
Checkoway H, Dell LD, Boffetta P, Gallagher AE, Crawford L, Lees PS, Mundt KA. Formaldehyde exposure and mortality risks from acute myeloid leukemia and other Lymphohematopoietic malignancies in the US National Cancer Institute cohort study of Workers in Formaldehyde Industries. J Occup Environ Med. 2015;57(7):785–94. https://doi.org/10.1097/JOM.0000000000000466.
Beane Freeman LE, Blair A, Lubin JH, Stewart PA, Hayes RB, Hoover RN, Hauptmann M. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: the National Cancer Institute cohort. J Natl Cancer Inst. 2009;101(10):751–61. https://doi.org/10.1093/jnci/djp096.
Saberi Hosnijeh F, Christopher Y, Peeters P, Romieu I, Xun W, Riboli E, Raaschou-Nielsen O, Tjønneland A, Becker N, Nieters A, Trichopoulou A, Bamia C, Orfanos P, Oddone E, Luján-Barroso L, Dorronsoro M, Navarro C, Barricarte A, Molina-Montes E, Wareham N, Vineis P, Vermeulen R. Occupation and risk of lymphoid and myeloid leukaemia in the European prospective investigation into Cancer and nutrition (EPIC). Occup Environ Med. 2013;70(7):464–70.
Talibov M, Lehtinen-Jacks S, Martinsen JI, Kjærheim K, Lynge E, Sparén P, Ryggvadottir L, Weiderpass E, Kauppinen T, Kyyrönen P, Pukkala E. Occupational exposure to solvents and acute myeloid leukemia: a population-based, case-control study in four Nordic countries. Scand J Work Environ Health. 2014;40(5):511–7. https://doi.org/10.5271/sjweh.3436.
Pinkerton LE, Hein MJ, Stayner LT. Mortality among a cohort of garment workers exposed to formaldehyde: an update. Occup Environ Med. 2004;61(3):193–200.
Pira E, Romano C, Verga F, La Vecchia C. Mortality from lymphohematopoietic neoplasms and other causes in a cohort of laminated plastic workers exposed to formaldehyde. Cancer Causes Control. 2014;25(10):1343–9. https://doi.org/10.1007/s10552-014-0440-0.
Adegoke OJ, Blair A, Shu XO, Sanderson M, Jin F, Dosemeci M, Addy CL. Zheng W (2003) occupational history and exposure and the risk of adult leukemia in Shanghai. Ann Epidemiol. 2003;13(7):485–94.
Albin M, Björk J, Welinder H, Tinnerberg H, Mauritzson N, Billström R, Strömberg U, Mikoczy Z, Johansson B, Ahlgren T, Nilsson PG, Mitelman F, Hagmar L. Cytogenetic and morphologic subgroups of myelodysplastic syndromes in relation to occupational and hobby exposures. Scand J Work Environ Health. 2003;29(5):378–87.
Blair A, Zheng T, Linos A, Stewart PA, Zhang YW, Cantor KP. Occupation and leukemia: a population-based case-control study in Iowa and Minnesota. Am J Ind Med. 2001;40(1):3–14.
Björk J, Albin M, Welinder H, Tinnerberg H, Mauritzson N, Kauppinen T, Strömberg U, Johansson B, Billström R, Mikoczy Z, Ahlgren T, Nilsson PG, Mitelman F, Hagmar L. Are occupational, hobby, or lifestyle exposures associated with Philadelphia chromosome positive chronic myeloid leukaemia? Occup Environ Med. 2001a;58(11):722–7.
Bonzini M, Grillo P, Consonni D, Cacace R, Ancona C, Forastiere F, Cocco PL, Satta G, Boldori L, Carugno M, Pesatori CA. Cancer risk in oil refinery workers: a pooled mortality study in Italy. Med Lav. 2019 Feb 22. 2019;110(1):3–10. https://doi.org/10.23749/mdl.v110i1.7842.
Collins JJ, Anteau SE, Swaen GM, Bodner KM, Bodnar CM. Lymphatic and hematopoietic cancers among benzene-exposed workers. J Occup Environ Med. 2015;57(2):159–63. https://doi.org/10.1097/JOM.0000000000000324.
Copley GB, Schnatter AR, Armstrong TW, Irons RD, Chen M, Wang XQ, Kerzic P. Hospital-based case-control study of MDS subtypes and benzene exposure in Shanghai. J Occup Environ Med. 2017;59(4):349–55. https://doi.org/10.1097/JOM.0000000000000952.
Costantini AS, Benvenuti A, Vineis P, Kriebel D, Tumino R, Ramazzotti V, Rodella S, Stagnaro E, Crosignani P, Amadori D, Mirabelli D, Sommani L, Belletti I, Troschel L, Romeo L, Miceli G, Tozzi GA, Mendico I, Maltoni SA, Miligi L. Risk of leukemia and multiple myeloma associated with exposure to benzene and other organic solvents: evidence from the Italian multicenter case-control study. Am J Ind Med. 2008;51(11):803–11. https://doi.org/10.1002/ajim.20592.
Divine BJ, Hartman CM, Wendt JK. Update of the Texaco mortality study 1947-93: part II. Analyses of specific causes of death for white men employed in refining, research, and petrochemicals. Occup Environ Med. 1999;56(3):174–80.
Divine BJ, Hartman CM. Update of a study of crude oil production workers 1946-94. Occup Environ Med. 2000;57(6):411–7.
Guénel P, Imbernon E, Chevalier A, Crinquand-Calastreng A, Goldberg M. Leukemia in relation to occupational exposures to benzene and other agents: a case-control study nested in a cohort of gas and electric utility workers. Am J Ind Med. 2002;42(2):87–97.
Huebner WW, Wojcik NC, Jorgensen G, Marcella SP, Nicolich MJ. Mortality patterns and trends among 127,266 U.S.-based men in a petroleum company: update 1979-2000. J Occup Environ Med. 2009;51(11):1333–48. https://doi.org/10.1097/JOM.0b013e3181be6c18.
Ireland B, Collins JJ, Buckley CF, Riordan SG. Cancer mortality among workers with benzene exposure. Epidemiology. 1997;8(3):318–20.
Kirkeleit J, Riise T, Bråtveit M, Moen BE. Increased risk of acute myelogenous leukemia and multiple myeloma in a historical cohort of upstream petroleum workers exposed to crude oil. Cancer Causes Control. 2008;19(1):13–23.
Linet MS, Yin SN, Gilbert ES, Dores GM, Hayes RB, Vermeulen R, Tian HY, Lan Q, Portengen L, Ji BT, Li GL, Rothman N, Chinese Center for Disease Control and Prevention-U.S. National Cancer Institute Benzene Study Group. A retrospective cohort study of cause-specific mortality and incidence of hematopoietic malignancies in Chinese benzene-exposed workers. Int J Cancer. 2015.
McCraw DS, Joyner RE, Cole P. Excess leukemia in a refinery population. J Occup Med. 1985;27(3):220–2.
Poynter JN, Richardson M, Roesler M, Blair CK, Hirsch B, Nguyen P, Cioc A, Cerhan JR, Warlick E. Chemical exposures and risk of acute myeloid leukemia and myelodysplastic syndromes in a population-based study. Int J Cancer. 2017;140(1):23–33. https://doi.org/10.1002/ijc.30420.
Rhomberg L, Goodman J, Tao G, Zu K, Chandalia J, Williams PR, Allen B. Evaluation of acute nonlymphocytic leukemia and its subtypes with updated benzene exposure and mortality estimates: a Lifetable analysis of the Pliofilm cohort. J Occup Environ Med. 2016;58(4):414–20. https://doi.org/10.1097/JOM.0000000000000689.
Rushton L, Schnatter AR, Tang G, Glass DC. Acute myeloid and chronic lymphoid leukaemias and exposure to low-level benzene among petroleum workers. Br J Cancer. 2014;110(3):783–7. https://doi.org/10.1038/bjc.2013.780.
Sathiakumar N, Delzell E, Cole P, Brill I, Frisch J, Spivey G. A case-control study of leukemia among petroleum workers. J Occup Environ Med. 1995;37(11):1269–77.
Satin KP, Wong O, Yuan LA, Bailey WJ, Newton KL, Wen CP, Swencicki RE. A 50-year mortality follow-up of a large cohort of oil refinery workers in Texas. J Occup Environ Med. 1996;38(5):492–506.
Schnatter AR, Glass DC, Tang G, Irons RD, Rushton L. Myelodysplastic syndrome and benzene exposure among petroleum workers: an international pooled analysis. J Natl Cancer Inst. 2012;104(22):1724–37. https://doi.org/10.1093/jnci/djs411.
Stenehjem JS, Kjærheim K, Bråtveit M, Samuelsen SO, Barone-Adesi F, Rothman N, Lan Q, Grimsrud TK. Benzene exposure and risk of lymphohaematopoietic cancers in 25,000 offshore oil industry workers. Br J Cancer. 2015;112(9):1603–12. https://doi.org/10.1038/bjc.2015.108.
Strom SS, Gu Y, Gruschkus SK, Pierce SA, Estey EH. Risk factors of myelodysplastic syndromes: a case-control study. Leukemia. 2005;19(11):1912–8.
Teras LR, Diver WR, Deubler EL, Krewski D, Flowers CR, Switchenko JM, Gapstur SM. Residential ambient benzene exposure in the United States and subsequent risk of hematologic malignancies. Int J Cancer. 2019;145(10):2647–60. https://doi.org/10.1002/ijc.32202.
Wong O, Harris F, Smith TJ. Health effects of gasoline . II Mortality patterns of distribution workers in the United States exposure. Environ Health Perspect. 1993;101(Suppl 6):63–76.
Wong O, Harris F, Rosamilia K, Raabe GK. An updated mortality study of workers at a petroleum refinery in Beaumont, Texas, 1945 to 1996. J Occup Environ Med. 2001a;43(4):384–401.
Wong O, Harris F, Rosamilia K, Raabe GK. Updated mortality study of workers at a petroleum refinery in Torrance, California, 1959 to 1997. J Occup Environ Med. 2001b;43(12):1089–102.
Wong O, Harris F, Armstrong TW, Hua F. A hospital-based case-control study of acute myeloid leukemia in Shanghai: analysis of environmental and occupational risk factors by subtypes of the WHO classification. Chem Biol Interact. 2010;184(1–2):112–28. https://doi.org/10.1016/j.cbi.2009.10.017.
Linet MS, Gilbert ES, Vermeulen R, Dores GM, Yin SN, Portengen L, Hayes RB, Ji BT, Lan Q, Li GL, Rothman N, Chinese Center for Disease Control and Prevention–US National Cancer Institute Benzene Study Group. Benzene exposure response and risk of myeloid neoplasms in Chinese workers: a multicenter case-cohort study. J Natl Cancer Inst. 2019;111(5):465–74. https://doi.org/10.1093/jnci/djy143.
Sorahan T, Mohammed N 2016) Incidence of Myelodysplastic syndrome in UK petroleum distribution and oil refinery workers, 1995-2011. Int J Environ Res Public Health. 13(5). Pii: E474. doi: https://doi.org/10.3390/ijerph13050474.
Schnatter AR, Wojcik NC, Jorgensen G. Mortality update of a cohort of Canadian petroleum workers. J Occup Environ Med. 2019;61(3):225–38. https://doi.org/10.1097/JOM.0000000000001523.
International Agency for Research on Cancer (IARC) (2012) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Personal Habits and Indoor Combustions. Volume 100E. Lyon, France.
Avgerinou C, Giannezi I, Theodoropoulou S, Lazaris V, Kolliopoulou G, Zikos P, Alamanos Y, Leotsinidis M, Symeonidis A. Occupational, dietary, and other risk factors for myelodysplastic syndromes in Western Greece. Hematology. 2017;22(7):419–29. https://doi.org/10.1080/10245332.2016.1277006.
Batty GD, Kivimaki M, Gray L, Smith GD, Marmot MG, Shipley MJ. Cigarette smoking and site-specific cancer mortality: testing uncertain associations using extended follow-up of the original Whitehall study. Ann Oncol. 2008;19(5):996–1002. https://doi.org/10.1093/annonc/mdm578.
Björk J, Albin M, Mauritzson N, Strömberg U, Johansson B, Hagmar L. Smoking and myelodysplastic syndromes. Epidemiology. 2000;11(3):285–91.
Björk J, Albin M, Mauritzson N, Strömberg U, Johansson B, Hagmar L. Smoking and acute myeloid leukemia: associations with morphology and karyotypic patterns and evaluation of dose-response relations. Leuk Res. 2001b;25(10):865–72.
Björk J, Johansson B, Broberg K, Albin M. Smoking as a risk factor for myelodysplastic syndromes and acute myeloid leukemia and its relation to cytogenetic findings: a case-control study. Leuk Res. 2009;33(6):788–91. https://doi.org/10.1016/j.leukres.2008.10.009.
Brown LM, Gibson R, Blair A, Burmeister LF, Schuman LM, Cantor KP, Fraumeni JF Jr. Smoking and risk of leukemia. Am J Epidemiol. 1992;135(7):763–8.
Brownson RC, Chang JC, Davis JR. Cigarette smoking and risk of adult leukemia. Am J Epidemiol. 1991;134(9):938–41.
Dalamaga M, Petridou E, Cook FE, Trichopoulos D. Risk factors for myelodysplastic syndromes: a case-control study in Greece. Cancer Causes Control. 2002;13(7):603–8.
Fernberg P, Odenbro A, Bellocco R, Boffetta P, Pawitan Y, Zendehdel K, Adami J. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: a nationwide cohort study in Sweden. Cancer Res. 2007;67(12):5983–6.
Ido M, Nagata C, Kawakami N, Shimizu H, Yoshida Y, Nomura T, Mizoguchi H. A case-control study of myelodysplastic syndromes among Japanese men and women. Leuk Res. 1996;20(9):727–31.
Kabat GC, Augustine A, Hebert JR. Smoking and adult leukemia: a case-control study. J Clin Epidemiol. 1988;41(9):907–14.
Kabat GC, Wu JW, Moore SC, Morton LM, Park Y, Hollenbeck AR, Rohan TE. Lifestyle and dietary factors in relation to risk of chronic myeloid leukemia in the NIH-AARP diet and health study. Cancer Epidemiol Biomark Prev. 2013;22(5):848–54. https://doi.org/10.1158/1055-9965.EPI-13-0093.
Kane EV, Roman E, Cartwright R, Parker J. Morgan G (1999) tobacco and the risk of acute leukaemia in adults. Br J Cancer. 1999 Dec;81(7):1228–33.
Kasim K, Levallois P, Abdous B, Auger P, Johnson KC. Lifestyle factors and the risk of adult leukemia in Canada. Cancer Causes Control. 2005;16(5):489–500.
Kroll ME, Murphy F, Pirie K, Reeves GK, Green J, Beral V; Million Women Study Collaborators. Alcohol drinking, tobacco smoking and subtypes of haematological malignancy in the UK million women study. Br J Cancer. 2012;107(5):879–87. https://doi.org/10.1038/bjc.2012.333.
Leal AD, Thompson CA, Wang AH, Vierkant RA, Habermann TM, Ross JA, Mesa RA, Virnig BA, Cerhan JR. Anthropometric, medical history and lifestyle risk factors for myeloproliferative neoplasms in the Iowa Women's health study cohort. Int J Cancer. 2014;134(7):1741–50. https://doi.org/10.1002/ijc.28492.
Linet MS, McLaughlin JK, Hsing AW, Wacholder S, Co-Chien HT, Schuman LM, Bjelke E, Blot WJ. Cigarette smoking and leukemia: results from the Lutheran brotherhood cohort study. Cancer Causes Control. 1991;2(6):413–7.
Lv L, Lin G, Gao X, Wu C, Dai J, Yang Y, Zou H, Sun H, Gu M, Chen X, Fu H, Bao L. Case-control study of risk factors of myelodysplastic syndromes according to World Health Organization classification in a Chinese population. Am J Hematol. 2011;86(2):163–9. https://doi.org/10.1002/ajh.21941.
Ma X, Lim U, Park Y, Mayne ST, Wang R, Hartge P, Hollenbeck AR, Schatzkin A. Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol. 2009;169(12):1492–9. https://doi.org/10.1093/aje/kwp074.
Ma X, Park Y, Mayne ST, Wang R, Sinha R, Hollenbeck AR, Schatzkin A, Cross AJ. Diet, lifestyle, and acute myeloid leukemia in the NIH-AARP cohort. Am J Epidemiol. 2010;171(3):312–22. https://doi.org/10.1093/aje/kwp371.
Mele A, Szklo M, Visani G, Stazi MA, Castelli G, Pasquini P, Mandelli F. Hair dye use and other risk factors for leukemia and pre-leukemia: a case-control study. Italian leukemia study group. Am J Epidemiol. 1994;139(6):609–19.
Mills PK, Newell GR, Beeson WL, Fraser GE, Phillips RL. History of cigarette smoking and risk of leukemia and myeloma: results from the Adventist health study. J Natl Cancer Inst. 1990;82(23):1832–6.
Musselman JR, Blair CK, Cerhan JR, Nguyen P, Hirsch B, Ross JA. Risk of adult acute and chronic myeloid leukemia with cigarette smoking and cessation. Cancer Epidemiol. 2013;37(4):410–6. https://doi.org/10.1016/j.canep.2013.03.012.
Nagata C, Shimizu H, Hirashima K, Kakishita E, Fujimura K, Niho Y, Karasawa M, Oguma S, Yoshida Y, Mizoguchi H. Hair dye use and occupational exposure to organic solvents as risk factors for myelodysplastic syndrome. Leuk Res. 1999;23(1):57–62.
Nisse C, Haguenoer JM, Grandbastien B, Preudhomme C, Fontaine B, Brillet JM, Lejeune R, Fenaux P. Occupational and environmental risk factors of the myelodysplastic syndromes in the north of France. Br J Haematol. 2001;112(4):927–35.
Parodi S, Merlo DF, Stagnaro E, Working Group for the Epidemiology ofHematolymphopoietic Malignancies in Italy. Coffee and tea consumption and risk of leukaemia in an adult population: a reanalysis of the Italian multicentre case-control study. Cancer Epidemiol. 2017;47:81–7. https://doi.org/10.1016/j.canep.2017.01.005.
Pasqualetti P, Festuccia V, Acitelli P, Collacciani A, Giusti A, Casale R. Tobacco smoking and risk of haematological malignancies in adults: a case-control study. Br J Haematol. 1997;97(3):659–62.
Pedersen KM, Bak M, Sørensen AL, Zwisler AD, Ellervik C, Larsen MK, Hasselbalch HC, Tolstrup JS. Smoking is associated with increased risk of myeloproliferative neoplasms: a general population-based cohort study. Cancer Med. 2018;7(11):5796–802. https://doi.org/10.1002/cam4.1815.
Pekmezovic T, Suvajdzic Vukovic N, Kisic D, Grgurevic A, Bogdanovic A, Gotic M, Bakrac M, Brkic N. A case-control study of myelodysplastic syndromes in Belgrade (Serbia Montenegro). Ann Hematol. 2006;85(8):514–9.
Pogoda JM, Preston-Martin S, Nichols PW, Ross RK. Smoking and risk of acute myeloid leukemia: results from a Los Angeles County case-control study. Am J Epidemiol. 2002;155(6):546–53.
Richardson DB, Terschüren C, Pohlabeln H, Jöckel KH, Hoffmann W. Temporal patterns of association between cigarette smoking and leukemia risk. Cancer Causes Control. 2008;19(1):43–50.
Sandler DP, Shore DL, Anderson JR, Davey FR, Arthur D, Mayer RJ, Silver RT, Weiss RB, Moore JO, Schiffer CA, et al. Cigarette smoking and risk of acute leukemia: associations with morphology and cytogenetic abnormalities in bone marrow. J Natl Cancer Inst. 1993;85(24):1994–2003.
Severson RK, Davis S, Heuser L, Daling JR, Thomas DB. Cigarette smoking and acute nonlymphocytic leukemia. Am J Epidemiol. 1990;132(3):418–22.
Speer SA, Semenza JC, Kurosaki T, Anton-Culver H. Risk factors for acute myeloid leukemia and multiple myeloma: a combination of GIS and case-control studies. J Environ Health. 2002;64(7):9–16.
Stagnaro E, Ramazzotti V, Crosignani P, Fontana A, Masala G, Miligi L, Nanni O, Neri M, Rodella S, Costantini AS, Tumino R, Viganò C, Vindigni C, Vineis P. Smoking and hematolymphopoietic malignancies. Cancer Causes Control. 2001;12(4):325–34.
Strom SS, Oum R, Elhor Gbito KY, Garcia-Manero G, Yamamura Y. De novo acute myeloid leukemia risk factors: a Texas case-control study. Cancer. 2012;118(18):4589–96. https://doi.org/10.1002/cncr.27442.
Ugai T, Matsuo K, Sawada N, Iwasaki M, Yamaji T, Shimazu T, Sasazuki S, Inoue M, Tsugane S, Japan Public Health Center-based Prospective Study Group. Smoking and subsequent risk of leukemia in Japan: the Japan public health center-based prospective study. J Epidemiol. 2017a;27(7):305–10. https://doi.org/10.1016/j.je.2016.07.005.
Ugai T, Matsuo K, Sawada N, Iwasaki M, Yamaji T, Shimazu T, Sasazuki S, Inoue M, Kanda Y, Tsugane S, Japan Public Health Centre-based Prospective Study Group. Smoking and alcohol and subsequent risk of myelodysplastic syndromes in Japan: the Japan public health Centre-based prospective study. Br J Haematol. 2017b;178(5):747–55. https://doi.org/10.1111/bjh.14749.
Wakabayashi I, Sakamoto K, Masui H, Yoshimoto S, Kanamaru A, Kakishita E, Hara H, Shimo-oku M, Nagai K, Shimo-oka M [corrected to Shimo-oku M] (1994) A case-control study on risk factors for leukemia in a district of Japan. Intern Med 33(4):198–203.
West RR, Stafford DA, Farrow A, Jacobs A. Occupational and environmental exposures and myelodysplasia: a case-control study. Leuk Res. 1995;19(2):127–39.
Wong O, Harris F, Yiying W, Hua F. A hospital-based case-control study of acute myeloid leukemia in Shanghai: analysis of personal characteristics, lifestyle and environmental risk factors by subtypes of the WHO classification. Regul Toxicol Pharmacol. 2009;55(3):340–52. https://doi.org/10.1016/j.yrtph.2009.08.007.
Xu X, Talbott EO, Zborowski JV, Rager JR. Cigarette smoking and the risk of adult leukemia: results from the three Mile Island cohort study. Arch Environ Occup Health. 2007;62(3):131–7. https://doi.org/10.3200/AEOH.62.3.131-137.
Mundt KA, Gentry PR, Dell LD, Rodricks JV, Boffetta P. Six years after the NRC review of EPA's draft IRIS toxicological review of formaldehyde: regulatory implications of new science in evaluating formaldehyde leukemogenicity. Regul Toxicol Pharmacol. 2018;92:472–90. https://doi.org/10.1016/j.yrtph.2017.11.006.
Acknowledgments
The authors gratefully acknowledge the valuable contributions and insights of Dr. Michael J. Thirman, University of Chicago Medical Center, on clinical and research aspects of myeloid malignancy and leukemia etiology. The authors also acknowledge the valuable assistance with the tables and calculations provided by Dr. Lynne P. Marshall, Cardno ChemRisk. The authors also thank the peer-reviewers for their careful review of the manuscript and insightful comments, which helped strengthen the manuscript.
Funding
This research was sponsored in part by a grant to Ramboll US Corporation from the Foundation for Chemistry Research and Initiatives (FCRI), a tax-exempt organization established by the American Chemistry Council (ACC) under Section 501(c)(3) of the Internal Revenue Code. The sponsors had no role in the design, conduct, analysis, interpretation or reporting.
Author information
Authors and Affiliations
Contributions
KAM, LD, PB and WJT substantially contributed to the conception and design of the work; EMB, HNL, CKL and VJD substantially contributed to the search, review, data extraction and statistical analysis; all authors contributed to the interpretation of findings, drafting and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No human subjects were involved in this work. All data used were obtained from public sources, primarily the published scientific literature, and therefore consent to use these data was not required.
Consent for publication
Not applicable.
Competing interests
KAM, EMB, HNL, CKL and WJT are employed by Cardno ChemRisk and LD is employed by Ramboll US Consulting Inc., consulting firms that provide scientific advice to the government, corporations, law firms, and various scientific/professional organizations. Their contributions to the manuscript were part of their regular responsibilities with their respective employers. PB and VJD are academic professionals who generously contributed their time and expertise in preparing and reviewing the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mundt, K.A., Dell, L.D., Boffetta, P. et al. The importance of evaluating specific myeloid malignancies in epidemiological studies of environmental carcinogens. BMC Cancer 21, 227 (2021). https://doi.org/10.1186/s12885-021-07908-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-07908-3