Abstract
Background
Metastatic colorectal cancer (mCRC) remains a lethal disease. Survival, however, is increasing due to a growing number of treatment options. Yet due to the number of prognostic factors and their interactions, prediction of mortality is difficult. The aim of this study is to provide a clinical model supporting prognostication of mCRC mortality in daily practice.
Methods
Data from 1104 patients with mCRC in three prospective cancer datasets were used to construct and validate Cox models. Input factors for stepwise backward method variable selection were sex, RAS/BRAF-status, microsatellite status, treatment type (no treatment, systemic treatment with or without resection of metastasis), tumor load, location of primary tumor, metastatic patterns and synchronous or metachronous disease. The final prognostic model for prediction of survival at two and 3 years was validated via bootstrapping to obtain calibration and discrimination C-indices and dynamic time dependent AUC.
Results
Age, sidedness, number of organs with metastases, lung as only site of metastasis, BRAF mutation status and treatment type were selected for the model. Treatment type had the most prominent influence on survival (resection of metastasis HR 0.26, CI 0.21–0.32; any treatment vs no treatment HR 0.31, CI 0.21–0.32), followed by BRAF mutational status (HR 2.58, CI 1.19–1.59). Validation showed high accuracy with C-indices of 72.2 and 71.4%, and dynamic time dependent AUC’s of 76.7 ± 1.53% (both at 2 or 3 years), respectively.
Conclusion
The mCRC mortality prediction model is well calibrated and internally valid. It has the potential to support both, clinical prognostication for treatment decisions and patient communication.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is one of the most common malignant diseases in the world and has also one of the highest cancer-related mortality rates [1, 2]. Fortunately, both incidence and mortality from CRC have decreased over the last decades. This is due to several factors, but the most important ones are successful screening and new systemic, as well as progressive surgical treatment options [3]. Nonetheless, metastatic CRC (mCRC) remains a lethal disease that is present in about 20–25% of patients at diagnosis and 30% will experience a metastatic relapse after initial curative surgical treatment, with or without adjuvant chemotherapy [4]. In mCRC, estimating survival is difficult, even for experienced oncologists. This directly influences the quality of the communication with patients as information regarding an accurate prognosis is one of the most important pieces of information provided to patients by oncologists [5]. Sharing information about the course of the disease with patients and estimating survival are challenging issues as is evident by the numerous prognostic and/or predictive factors that have been described over recent years. Socioeconomic factors, supportive treatment enabling adherence to planned treatment procedures, exercise programs and patient reported outcomes play a significant role in prognostication [6]. Other factors of relevance in clinical practice include the tumor load measured by the number of organs involved in metastasis [7, 8], patterns of metastatic spread [9], primary tumor location [10,11,12] and metachronous or synchronous disease [13]. Currently, additional molecular markers and even molecular signatures have enriched the traditional family of markers, adding significantly to the complexity of mortality prognosis [14]. It is now established that BRAF mutated patients show the worst prognosis among all patients [15] and the consensus molecular subtypes give a deeper insight into the biology of the disease [16, 17]. Along with the advent of these modern markers, systemic treatment options targeting angiogenesis, the epidermal growth factor pathway (EGFR) or the BRAF-pathway have been developed that enable the use of individual treatments [18]. For example, metastastic left sided colorectal cancer that is RAS wild-type and treated by an EGFR-antibody combined with chemotherapy shows the longest survival [19]. In addition, patients who are BRAF mutated benefit from targeted treatment rather than from chemotherapy [20].
These improvements in systemic treatments have been accompanied by progressive surgical treatment concepts, which include the resection of metastases, even repeatedly and in more than one organ. For such multimodal treatment concepts, patient selection is the key to success. A multidisciplinarity approach encompassing the experience of specialized oncologists, surgeons and radiologists has become a hallmark of optimal clinical outcomes in the treatment of mCRC resulting in overall survival rates of more than 5 years in the best cases [21]. The complex prognostic interplay between anatomic, pathological, molecular, and clinical factors cannot be captured in clinical trials. Tools allowing a more accurate prognostication, however, are highly desirable. These are not only helpful in clinical practice, but also support the design of new trials and the evaluation of novel treatments that may play a part in improving routine care. There have been a few contributions to date to fill this gap. Two research groups reported a model tested in large patient populations, however they only include patients treated within clinical trials since 1997, thereby not ideally representing a real-life population of colorectal cancer patients treated with modern, multidisciplinary treatments [22, 23]. Mortality determinants were also studied in a real-life cohort treated before 2010 and reported single factors associated with a higher relative risk of early mortality, yet representing a patient population before the era of modern oncology [24, 25]. Ge and colleagues described a nomogram for mCRC patients based on parameters derived from the Surveillance, Epidemiology, and End Results Program (SEER)-database; they identified 13 factors associated with survival at three and 5 years but only treated patients were included and the remarkably high number of prognostic factors limit its clinical utility [26]. Additionally, the vast majority of patients suffering from mCRC will survive approximately two to 3 years, which has been shown for patients treated either in clinical trials [27, 28] or in a real-life setting [9, 29]. To our knowledge, a prognostic model considering this particular survival time in a real-life patient cohort treated by contemporary treatments has not yet been reported.
We therefore aim to validate a mortality prediction model in a representative real-world patient cohort with mCRC by identifying factors directly associated with patient survival at 2 and 3 years. By providing this work, we hope to support oncologists to communicate individualized survival estimation to every patient in daily practice without incurring additional cost.
Methods
Patient sample
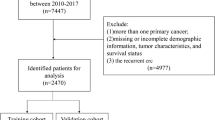
We retrospectively identified 2915 patients diagnosed with colorectal cancer treated at three different oncological centers from January 2006 to March 2020 in Austria. Clinical data were obtained either from a monitored cancer registry (two centers) or by chart review (one center). Patients with metastasized colorectal carcinoma who developed metastases before Dec 31th 2019 were included in the analysis. Further, only adenocarcinoma of the colon or rectum were included and patients without complete follow up data were excluded (Fig. 1). Treatment decisions were based on local guidelines including contemporary systemic treatment with or without resection of metastasis.
Survival analysis and nomogram development
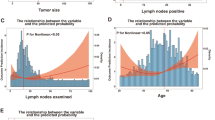
The endpoint of this study was overall survival (OS) defined as the time between first diagnosis of metastatic disease by histology and death. Kaplan-Meier-estimates and curves were used as descriptive measures for survival data. The effects of predictors on overall survival were investigated separately with Kaplan-Meier curves. The reverse Kaplan-Meier method was used for calculation of median follow-up time. A non-normal distribution of continuous variables was assumed and medians with interquartile ranges (IQR) were reported. Correlation coefficients, Cramers V, point-biserial correlation and eta-squared values were used to describe eventual pairwise dependencies between potential predictive parameters. A stepwise backward method according to the Akaike Information Criterion (AIC) was used for automatic variable selection [30]. Cox regression models were used to analyze the effects of predictors in multifactorial models. The following assumptions of Cox regression models were checked: (i) proportionality of hazards by inspecting the Kaplan-Meier curves and by graphically checking independency of residuals over time; (ii) linearity of continuous predictors by plotting marginal residuals with and without different transformations; and (iii) outliers and influential cases were deleted when dfbeta-values were higher than 2/sqrt(N) = 0.06 [31]. Additionally, deviance from symmetry was checked graphically. A nomogram based on the final model was constructed for the probability of survival at 24 and 36 months.
Performance of the nomogram
Validation of the nomogram was done using a bootstrap strategy of 2000 resamples as utilized by similar research [22, 23, 26, 32, 33]. The discrimination ability of the nomogram was evaluated by calculating the concordance index (C-Index). This index should be greater than 50% (100% in a perfect model) [34]. The nomogram was calibrated by bootstrapping (k = 2000) of five groups with differing survival probabilities; predicted survival probability was plotted against the observed survival. The accuracy of the nomogram was quantified and compared using the time dependent AUC-values.
For all analyses the R version 3.6.3 (R core Team, 2020) was used with the packages scoredardModelUtils (pairwise Cramers V), lsr (eta-squared), survival (survival analyses, Kaplan-Meier-analyses, cox-regression, diagnostics), survminer (survival-curves, diagnostics), pec (C-index), rms (calibration, validation, nomogram), timeROC (time dependent AUC-values). A significance level of 5% was assumed in all analyses.
Results
Characteristics of the patient sample
In total, 2915 patients diagnosed with colorectal cancer were identified in three distinct registries. Patients who did not have metastatic disease by Dec 31st 2019 (n = 1796) were excluded from the analysis. Thus, a total of 1119 patients were either synchronously or metachronously metastasized and therefore eligible for inclusion. From this sample 15 patients were excluded due to a histology type other than adenocarcinoma or a lack of sufficient data for survival time calculation. This resulted in a total sample of 1104 patients (Fig. 1). Median age was 67.4 years; most patients had synchronous disease defined as diagnosis of metastasis within the first 6 months after diagnosis of the primary tumor. Molecular markers were not consistently available. When separating at the left flexure, 70% showed a left sided colon or rectum carcinoma and 30% a right sided colon cancer. The majority of patients showed metastases in one organ only at time of diagnosis, which predominantly was the liver (Table 1). The median OS of the whole cohort was 20 months (CI 18.5–22.3). The treatment dependent OS was 6.27 (CI 4.11–7.82) months for patients receiving no treatment at all (best supportive care; BSC) and 26.61 months (CI 24.05–28.45) for patients receiving any treatment, defined as either systemic treatment alone, metastasis resection alone (predominantly in case of metachronous, oligometastatic relapse) or multimodal treatment including metastasis resection and systemic treatment. The resection rate of metastases was 20.7% in the whole population and a proportion of patients (24.8%) received BSC. A total of 67.7% (746 patients) of patients received at least one line of systemic antineoplastic treatment and of these, 62.3% (465 patients) received at least 1 second and 35% (261 patients) at least one third line of treatment.
Prognostic model
In 1104 patients the following parameters were analyzed for statistical significance in a stepwise backward method according to AIC: disease free survival (DFS), rectum or colon, left (LCC) or right (RCC) colon cancer, mutation status in BRAF and RAS, microsatellite status, age, gender, constellation of metastases (single organ, multiple organs), metastatic patterns (detailed information on which organ/organs were affected by metastasis), treatment modality (no treatment, systemic treatment only, multimodal treatment including systemic treatment and metastasis resection or metastasis resection alone if applicable) and receiving systemic treatment with or without monoclonal antibodies. These parameters were selected, because significant correlation with each other was not present and each of it showed significant impact in univariate analysis (data not shown). Treatment type was found to be the most relevant prognostic parameter (HR 0.31, CI 0.26–0.36), especially if a resection of metastases was performed (HR 0.26, HR 0.21–0.32). The presence of a lung metastasis at diagnosis only was of positive prognostic value (HR 0.68, CI 0.53–0.87). A worse mortality outcome was found for having more than two organs affected by metastasis (HR 1.5, CI 1.3–1.74) and patients with RCC fared worse than those with RCC (HR 1.37, CI 1.19–1.58); BRAF mutated tumors showed worst survival (HR 2.58, CI 1.67–3.99). All variables showed statistically significant HR’s (Table 2). The selected parameters were weighted against each other in a nomogram, which finally allows the prediction of survival at 24 or 36 months, respectively (Fig. 2).
Validation and calibration of the prognostic model
Accuracy of the nomogram showed strong internal validity by a time dependent AUC of 76.7 ± 1.53% at 24 months and 77.9 ± 1.72% at 36 months. This was confirmed by the additional calculation of the C-Index, which resulted in a discrimination of 72.2% at 24 months and 71.4% at 36 months (Supplementary Figure 1).
The performance of our model was calibrated by bootstrapping (k = 2000) of five groups, consisting of 215 patients each, with differing survival probabilities. This calculation showed a reliable concordance between predicted and observed survival in the cohort at 24 months and 36 months (Fig. 3).
Discussion
In the presented study a nomogram predicting survival at 24 and 36 months for patients suffering from mCRC was developed and validated. The model is based on the clinical features of 1104 patients and also includes a differentiated view of modern treatment types. The performance of the model in terms of discrimination, calibration and clinical utility is high and reliable.
The selection of variables was based on easily obtained clinical parameters and on treatment types that could potentially be applied to a patient. To our knowledge, this question has not been addressed in this detail in a real-life population treated by contemporary treatment concepts so far. Of the many factors known to be of prognostic relevance, age, number of organs affected by metastasis, lung metastasis only, left or right primary, BRAF mutational status and especially treatment modality were selected for the model after a stepwise exclusion in a multivariate cox-regression model. Age as one of the relevant prognostic factors has also been reported to be associated with the patients’ survival in several other tumor entities [35, 36]. But although it has been reported, the specific mechanisms remain unclear. Chronic inflammation or lower immune responses have been discussed as playing an important role [37, 38]. In our study the survival of patients older than 60 years was worse compared to patients less than 60 years old. This might be due to the higher rate of BSC and less intense treatment regimen found in older patients (data not shown). This correlation has also described in a similar fashion by others [26, 39].
The number of organs involved in metastatic disease and tumor load [40], the pattern of metastasis [9, 41], left or right primary location [11] and BRAF mutational status [42] are known and well described prognostic factors in this population. Among these, in our sample BRAF appeared to have a major impact on survival despite the relatively low number of patients. Furthermore, treatment type was found to have the strongest effect, resulting in the exclusion of known prognostic factors. One of these, for example, was DFS, which potentially discriminates patients with good prognosis from patients with bad prognosis (synchronous vs. metachronous disease; data not shown). We differentiated between patients receiving either best supportive care, systemic treatment only, multimodal treatment including systemic treatment and resection of metastasis or resection of metastasis only. The main reason for best supportive care were comorbidities, which implies a higher risk than benefit from treatment. A considerable fraction of approximately 25% of patients were allocated to this category. Second line treatment was given to 62% of the patients and 35% of patients received a third line therapy. These frequencies are comparable to an analysis of subsequent treatments in the FIRE-3 trial population, where 70 and 43% received second and third line treatment, respectively [43]. The slightly lower rates in our analysis can be explained by obvious differences between our real world cohort and a study population that included selected patients only. Consideration of treatment type in this detail is of high importance as especially multimodal treatment concepts have been proven to be of high efficacy if performed in a multidisciplinary setting [21]. The nomogram shows the weighted impact of the factors on all-cause mortality at 24 and 36 months. The 13 factors associated with survival at 3 and 5 years identified by Ge and colleagues [26] is a fairly high number of variables for use in daily practice and as the median OS in mCRC is found to be around 30 months, the time points allow the prediction of long term survival only. This might have been intentional since the study had only one focus on resection of metastases. Such patients are known for survival times that are beyond the usual median OS. We intended to cover the majority of patients and therefore describe survival at 24 and 36 months, which is fairly around the median OS described in the literature. Most studies investigating the interplay of prognostic relevant factors are limited to patients that received at least one treatment [26] or were even treated within clinical trials [22, 23]. Both are not optimally representative of a real-world scenario. Having this in mind, we also included patients who were considered to be unfit for treatment. This provides information on a representative spectrum of patients, of interest for everyday practice.
When comparing the performance of our models, the most suitable trial is by Ge et al. who reported a C-Index of 0.69 [26]. Another study reporting on a model for prediction of disease free and overall survival after first line treatment with a chemotherapy doublet showed a C-Index of 0.66 [33]. The C-Index of our model was 72% at 24 months and 71% at 36 months, implying an accuracy comparable to these publications. However, for t-year predictions the dynamic time-dependent AUC (dtdAUC) is reported as being more reliable [44]. In our model the tdAUC at 24 months was 77 and 78% for 36 months. These values indicate a high accuracy of the nomogram.
Several potential limitations should be considered when interpreting the results of our study. Foremost, it is a retrospective study, which implies the risk of a selection bias. Unfortunately, the rate of unknown mutation status of RAS and BRAF, as well as information on microsatellite status was high, which may have led to an underestimation of the influence of RAS mutation status and treatment with EGFR antibodies on survival. A prospective evaluation of the presented model and its applicability in daily practice is needed.
Conclusion
In conclusion, we present a clinical model that supports the prediction of the most relevant 2- and 3-year survival in mCRC, focussing on clinicopathological features and on different treatment types covering all conceivable treatment concepts in the modern oncological treatment era. As the patients included in our analysis were treated at three different centers our patient cohort may be considered representative of a real-world scenario. This predictive mortality model may contribute to the difficult but important clinical issue of prognostication in patients with mCRC by supporting communication to the patients and the decisions on treatment strategy.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRC:
-
Colorectal cancer
- mCRC:
-
Metastatic colorectal cancer
- BRAF:
-
B-raf (rapidly accelerated fibrosarcoma), proto-oncogene
- EGFR:
-
Epidermal growth factor receptor
- RAS:
-
Ras (rat sarcoma), proto-oncogene
- SEER:
-
Surveillance, epidemiology, and end fesults program
- IQR:
-
Interquartile ranges
- AIC:
-
Akaike information criterion
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- C-Index:
-
Concordance index
- AUC:
-
Area under the curve
- tdROC:
-
Time dpendent AUC-values
- BSC:
-
Best supportive care
- DFS:
-
Disease free survival
- LCC:
-
Left sided colon cancer
- RCC :
-
Right sided colorectal cancer
- OS:
-
Overall survival
References
Siegel RL. Cancer statitcs. CA Cancer J Clin. 2019;69(1):7–34.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93.
Shah MA, Renfro LA, Allegra CJ, André T, De Gramont A, Schmoll HJ, et al. Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: analysis from modern-era adjuvant studies in the adjuvant colon cancer end points (ACCENT) database. J Clin Oncol. 2016;34(8):843–53.
Mack JW, Smith TJ. Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30:2715–7.
Rutherford C, Campbell R, White K, King M. Patient-reported outcomes as predictors of survival in patients with bowel cancer: a systematic review. Qual Life Res. 2019;28(11):2871–87.
Carpizo DR, D’Angelica M. Liver resection for metastatic colorectal cancer in the presence of extrahepatic disease. Ann Surg Oncol. 2009;16:2411–21.
Lemini R, Attwood K, Almerey T, Gunn J, Yeager TE, Elias AW, et al. Is metastasectomy a worthy option? - The role of surgery in metastatic colon cancer to liver and lungs. J Gastrointest Oncol. 2019;10(6):1032–48.
Prasanna T, Karapetis CS, Roder D, Tie J, Padbury R, Price T, et al. The survival outcome of patients with metastatic colorectal cancer based on the site of metastases and the impact of molecular markers and site of primary cancer on metastatic pattern. Acta Oncol (Madr). 2018;57(11):1438–44.
Reinacher-Schick AC, Noepel-Duennebacke S, Hertel J, Tannapfel A, Arnold D, Hinke A, et al. Localization of the primary tumor (LPT) and maintenance strategies after first line oxaliplatin (Ox), fluoropyrimidine (FP), and bevacizumab (Bev) in metastatic colorectal cancer (mCRC): Results from the AIO 0207 trial. J Clin Oncol. 2017;35(15_suppl):3543.
Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O’Neil BH, et al. Impact of primary (1o) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(15_suppl):3504.
Venook AP, Ou F-S, Lenz H-J, Kabbarah O, Qu X, Niedzwiecki D, et al. Primary (1°) tumor location as an independent prognostic marker from molecular features for overall survival (OS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB / SWOG 80405 (Alliance). J Clin Oncol. 2017;35(15_suppl):3503.
Kumar R, Price TJ, Beeke C, Jain K, Patel G, Padbury R, et al. Colorectal cancer survival: An analysis of patients with metastatic disease synchronous and metachronous with the primary tumor. Clin Colorectal Cancer. 2014;13(2):87–93.
Lenz HJ, Ou FS, Venook AP, Hochster HS, Niedzwiecki D, Goldberg RM, et al. Impact of consensus molecular subtype on survival in patients with metastatic colorectal cancer: Results from CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2019;37:1876–85.
Barras D. BRAF mutation in colorectal cancer: an update. Biomark Cancer. 2015;7s1:BIC.S25248.
Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17;79–92.
Martini G, Dienstmann R, Ros J, Baraibar I, Cuadra-Urteaga JL, Salva F, et al. Molecular subtypes and the evolution of treatment management in metastatic colorectal cancer. Ther Adv Med Oncol. 2020;12:1–18.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Targeted Ther. 2020;5:22–51.
Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with ras wild-type metastatic colorectal cancer retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201.
Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N Engl J Med. 2019;381(17):1632–43.
Modest DP, Denecke T, Pratschke J, Ricard I, Lang H, Bemelmans M, et al. Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer—central evaluation of FIRE-3. Eur J Cancer. 2018;88:77–86.
Sjoquist KM, Renfro LA, Simes RJ, Tebbutt NC, Clarke S, Seymour MT, et al. Personalizing survival predictions in advanced colorectal cancer: The ARCAD nomogram project. J Natl Cancer Inst. 2018;110(6):638–48.
Renfro LA, Goldberg RM, Grothey A, Sobrero A, Adams R, Seymour MT, et al. Clinical calculator for early mortality in metastatic colorectal cancer: an analysis of patients from 28 clinical trials in the aide et recherche en cancérologie digestive database. J Clin Oncol. 2017;35(17):1929–37.
McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112:S108–15.
Downing A, Aravani A, MacLeod U, Oliver S, Finan PJ, Thomas JD, et al. Early mortality from colorectal cancer in England: a retrospective observational study of the factors associated with death in the first year after diagnosis. Br J Cancer. 2013;108(3):681–5.
Ge H, Yan Y, Xie M, Guo L, Tang D. Construction of a nomogram to predict overall survival for patients with M1 stage of colorectal cancer: a retrospective cohort study: short title: a nomogram to predict OS for patients with M1 stage of colorectal cancer. Int J Surg. 2019;72:96–101.
Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer a randomized clinical trial. JAMA - J Am Med Assoc. 2017;317(23):2392–401.
Simkens LHJ, Van Tinteren H, May A, Ten Tije AJ, Creemers GJM, Loosveld OJL, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015.
Heervä E, Lavonius M, Jaakkola P, Minn H, Ristamäki R. Overall survival and metastasis resections in patients with metastatic colorectal cancer using electronic medical records. J Gastrointest Cancer. 2018;49(3):245–51.
Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw. 2012;50(11):1-23.
Patterson HD, Belsley DA, Kuh E, Welsch RE. Regression diagnostics: identifying influential data and sources of collinearity. Biometrics. 1981;37(4):862.
Wang Z, Wang Y, Yang Y, Luo Y, Liu J, Xu Y, et al. A competing-risk nomogram to predict cause-specific death in elderly patients with colorectal cancer after surgery (especially for colon cancer). World J Surg Oncol. 2020;18(1):1–13.
Degeling K, Wong HL, Koffijberg H, Jalali A, Shapiro J, Kosmider S, et al. Simulating progression-free and overall survival for first-line doublet chemotherapy with or without bevacizumab in metastatic colorectal cancer patients based on real-world registry data. Pharmacoeconomics. 2020;38(11):1263–75.
Gerds TA, Kattan MW, Schumacher M, Yu C. Estimating a time-dependentconcordance index for survival prediction models with covariate dependent censoring. Stat Med. 2013;32(13):2173–84.
Wray CJ, Phatak UR, Robinson EK, Wiatek RL, Rieber AG, Gonzalez A, et al. The effect of age on race-related breast cancer survival disparities. Ann Surg Oncol. 2013;20(8):2541–7.
Shen W, Sakamoto N, Yang L. Cause-specific mortality prediction model for patients with basaloid squamous cell carcinomas of the head and neck: a competing risk analysis. J Cancer. 2018;9(21):4009–17.
Hugo HJ, Saunders C, Ramsay RG, Thompson EW. New insights on COX-2 in chronic inflammation driving breast cancer growth and metastasis. J Mammary Gland Biol Neoplasia. 2015;20:109–19.
Yang J, Li X, Liu XP, Liu Y. The role of tumor-associated macrophages in breast carcinoma invasion and metastasis. Int J Clin Exp Pathol. 2015;8(6):6656–64.
Hu CY, Bailey CE, You YN, Skibber JM, Rodriguez-Bigas MA, Feig BW, et al. Time trend analysis of primary tumor resection for stage iv colorectal cancer less surgery, improved survival. JAMA Surg. 2015;150(3):245–51.
Elias D, Liberale G, Vernerey D, Pocard M, Ducreux M, Boige V, et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12(11):900–9.
Prasanna T, Craft PS, Chua YJ, Karapetis CS, Gibbs P, Wong R, et al. The outcome of patients (pts) with metastatic colorectal cancer (mCRC) based on site of metastases (mets) and the impact of molecular markers and site of primary cancer on metastatic pattern. J Clin Oncol. 2017;35(15_suppl):3560.
Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Research article combined BRAF, EGFR, and MEK inhibition in patients with BRAF V600E -mutant colorectal cancer. Cancer Discov. 2018;8(4):428–43.
Modest DP, Stintzing S, Von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: First-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. 2015;33(32):3718–26.
Blanche P, Kattan MW, Gerds TA. The c-index is not proper for the evaluation of t-year predicted risks. Biostatistics. 2019;20(2):347–57.
Acknowledgements
The authors thank D. Zauner, M. Pötscher and F. Knotz for their support with data management. The abstract of this paper has been presented at the Virtual Congress of the European Society of Medical Oncology (ESMO) in October 2020 (abstract #505P).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HR: idea for the project, concept design, data structuring, planned and did analysis of the data, drafted and revised the manuscript. DNB: data structuring of the cohort at the Wilhelminenspital Vienna, revision of the manuscript. DF and CH: data acquisition of the cohort at the Wilhelminenspital Vienna, revision of the manuscript. HVW: substantial revision of the manuscript, deeply involved in concept design. SMG: data acquisition and data structuring at the Ordensklinikum Linz, analyzed data, revision of the manuscript. GP: Data acquisition and structuring of the cohort at Klinikum Wels-Grieskirchen, revision of the manuscript. JT: concept design and revision of the manuscript. All authors substantially contributed to discussion and interpretation of the data. The final version was approved by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our research project was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Permission for analysis of the clinical data was given by the regulatory heads of the respective hospitals, after the project was approved by the ethics committee of the Land Oberoesterreich (Ethics Committee Land Oberösterreich, 1001/2020, February 12th 2020) and the ethics committee of Vienna (Ethics Committee Vienna, EK 19–253-VK, January 2nd 2020). According to these votes an informed consent for participation was not necessary. This is based on the fact, that all data analyzed were obtained within the routine care procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rumpold, H., Niedersüß-Beke, D., Heiler, C. et al. Prediction of mortality in metastatic colorectal cancer in a real-life population: a multicenter explorative analysis. BMC Cancer 20, 1149 (2020). https://doi.org/10.1186/s12885-020-07656-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-07656-w