Abstract
Background
To investigate the correlation between family history of prostate cancer (PCa) and survival (overall and cancer specific) in patients undergoing treatment for PCa.
Methods
ine thousand four hundred fifty-nine patients with PCa were extracted from the South Australian Prostate Cancer Clinical Outcomes Collaborative (SA-PCCOC) database. Diagnosis occurred after 1998 and treatment before 2014. Cox proportional-hazards modeling was used to assess the effect of family history on overall survival after adjustment for confounders (age at diagnosis, NCCN risk category and year of treatment), and with stratification by primary treatment group. Competing risks regression modelling was used to assess PCa specific mortality.
Results
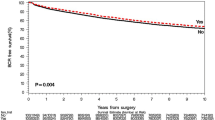
Men with a positive family history of PCa appear to have a lower Gleason score at the time of diagnosis (50% with Gleason < 7, compared to 39% in those without family history) and be diagnosed at a lower age (64 vs 69). Men with a positive family history of PCa appear to have better overall survival outcomes (p < 0.001, log rank test). In analysis adjusting for age at diagnosis, NCCN risk category and year of treatment, family history remained a significant factor when modelling overall survival (HR 0.72 95% CI 0.55–0.95, p = 0.021).
There were no significant differences in treatment subgroups of radical prostatectomy (p = 0.7) and radiotherapy (0.054).
Conclusion
Men with a positive family history of PCa appear to have better overall survival outcomes. This better survival may represent lead time bias and early initiation of PSA screening. Family history of PCa was not associated with different survival outcomes in men who were treated with either radical prostatectomy or radiotherapy.
Similar content being viewed by others
Background
Having a family history of prostate cancer is a known risk factor for developing prostate cancer. Having a single first-degree relative with prostate cancer increases the risk of developing prostate cancer by a factor of 2.1–2.8. Having two affected relatives increases the risk 3.5- fold [1].
Whilst most cases of prostate cancer are associated with somatic mutations, inherited gene changes can increase the risk of development of prostate cancer (for review see [2]). Examples of such genes include tumour suppressor genes, BRCA1 and BRCA2 [3].
Family history has been examined in the context of prostate cancer and overall survival following PSA testing [4]. A number of studies describe whether a positive family history is a prognostic factor following the diagnosis of prostate cancer. Several studies have been published worldwide, mainly examining the relationship between a positive family history and clinical outcomes after radical prostatectomy [5,6,7]. No studies have been published to date in Australia.
The purpose of this study is to examine whether having a family history of prostate cancer affects clinical outcomes in an Australian cohort. Outcomes of interest are overall survival and prostate cancer specific mortality. Subgroup analysis by treatment group (radical prostatectomy and radiation therapy) will be conducted.
Methods
Patients
The South Australia Prostate Cancer Clinical Outcomes Collaborative (SA-PCCOC) database enrolls men with prostate cancer diagnosed in public hospitals and collaborating private institutions in South Australia. It was established in 1998 and recruits men with a histologic diagnosis of prostate cancer including by prostate biopsy and also incidental findings after treatment for other conditions (e.g. transurethral resection of the prostate for treatment of lower urinary tract symptoms).
Men with diagnosis post 1st January 1998 and treatment prior to 1st June 2014 were included. The following data were extracted: age at diagnosis, reason for referral to a specialist for prostate biopsy, PSA measurement at diagnosis, family history of prostate cancer, clinical staging (based on digital rectal examination, laboratory investigations and imaging at the time of diagnosis), Gleason score at the time of diagnosis, primary treatment, treatment year and survival time (the time between date of diagnosis and date of death or censor). A modified (low, intermediate and high risk categories) NCCN risk classification was used to assign risk groups to men based on their PSA levels at diagnosis, diagnostic PSA and Gleason score. This modified score is used for Australian population level studies [8, 9]. Family history was gathered from medical notes and included binary responses relating to grandfather, father, uncle, child and grandchild.
Analysis
Demographic data were tabulated and comparisons made between groups using the Chi squared test (categorical variables) or ANOVA (continuous variables). Overall survival was compared in patients with and without a family history of prostate cancer after diagnosis as well as by primary treatment modality (post radical prostatectomy or post radiotherapy). Survival was plotted using Kaplan-Meier curves and groups compared using a log-rank test. Cox proportional hazards modelling was used to assess overall survival and the effect of a positive family history on survival after adjustment for confounders (age at diagnosis, NCCN [10] staging and year of treatment [continuous variable]). Cumulative incidence plots were used to display death by prostate cancer and other causes. Cause of death data was taken from the South Australian Births, Deaths and Marriages Registry and also the South Australian Cancer Registry. Prostate cancer was attributed as a cause of death where this was documented as a primary or significant contributing cause by the doctor completing the death certificate. Fine and Grey modelling was used to predict prostate cancer specific mortality with adjustment in multivariable analysis as described above. All analyses were conducted in R, and p < 0.05 was taken to indicate statistical significance.
The Southern Adelaide Clinical Human Research Ethics Committee reviewed and approved the SA-PCCOC database, including analysis of data without identifiers, such as this study.
Results
Patient demographics
9459 men were identified after applying exclusion criteria. Patient demographics are shown in Table 1. Men with a positive family history of prostate cancer appear to have a lower Gleason score at the time of diagnosis and be diagnosed at a lower age (p < 0.05).
There was a significant difference in the reasons for referral for biopsy with those having a positive family history being more likely to report having an elevated PSA (68.5% vs 52.6%) and less likely to have prostate related symptoms (14.6% vs 20.1%). The most common relation in those reporting to have a positive family history was parent (56.5%) followed by sibling (33.4%).No men reported having a grandchild with prostate cancer.
There are significant treatment differences between the group of men with and without family history of prostate cancer e.g. hormones 5.5% vs 12.6%, radical prostatectomy 44.5% vs 33.5%, radiation therapy 36.3% vs 28.3% (all p < 0.05). The ‘other’ treatment group included those that could not be classified as one of the above active treatment groups, and included those on observation e.g. watchful waiting or active surveillance.
Overall survival time after diagnosis
In a univariable Log-rank test, men with a family history of prostate cancer appear to have better survival outcomes (p < 0.001, see Fig. 1). In analysis adjusting for age at diagnosis, NCCN risk category and year of treatment, family positive history remained a significant factor when modelling overall survival (Supplementary Table 1, HR 0.74, 95% CI 0.57–0.97, p = 0.027).
In a univariable Log-rank test, men with a family history of prostate cancer who were treated with radical prostatectomy did not appear to have different survival outcomes (p = 0.70, see Fig. 2). This result was unchanged when the cohort was further restricted to those aged 50–70 years at diagnosis with low or intermediate risk disease. In analysis adjusting for age at diagnosis, NCCN risk category and year of treatment, family history was not a significant factor when modelling overall survival (supplementary Table 3, HR 0.89, 95% CI 0.47–1.66, p = 0.70).
In a univariable Log-rank test, men with a family history of prostate cancer who were treated with radiation therapy did not appear to have different survival outcomes (p = 0.054, see Fig. 2b). This result was unchanged when the cohort was further restricted to those aged 50–70 years at diagnosis with low or intermediate risk disease. In analysis adjusting for age at diagnosis, NCCN risk category and year of treatment, family history was not a significant factor when modelling overall survival, in men treated with XRT (supplementary Table 4, HR 0.65, 95% CI 0.41–1.0, p = 0.054).
Age at treatment was associated with overall survival. (radical prostatectomy – HR 1.06, 95% CI 1.03–1.10, p < 0.001; radiation therapy HR 1.06, 95% CI 1.05–1.08, p < 0.001; supplementary Tables 3 and 4).
No differences in prostate cancer specific mortality were detected between men in this cohort with a positive family history of prostate cancer and those without (Fig. 3, p > 0.05, Supplementary Table 2). Subgroup analysis of prostate cancer specific mortality by treatment group was not undertaken due to low event numbers.
Discussion
In our cohort of 9459 men with prostate cancer, a positive family history of the disease was reported in 658 (6.9%). Having a positive family history was associated with significantly longer overall survival; however it was not associated with any change in prostate cancer specific mortality.
Heritability of prostate cancer is estimated to be 57% (95% CI 51–63, [11]) with 100 genetic loci thought to contribute to one third of the genetic component of this disease [12, 13]. While this aspect of the disease – incidence- has been studied extensively there is less literature relating to the impact of heritability and genetics on outcomes of men who are diagnosed. The overall survival advantage observed in our cohort may be related to the fact that patients with a positive family history for prostate cancer appeared to be diagnosed at a younger age and as a result of elevated PSA measurements as opposed to symptomatic presentations. This could be interpreted to mean that these patients are screened more aggressively and from an earlier age. In the literature, Lee et al. [6] have also observed improved disease-free survival in patients reporting a positive family history of prostate cancer compared to those without a family history. This was thought to be related to the earlier age at diagnosis and improved pathologic features in the group with a positive family history rather than to any true biologic differences in their cancers.
Better survival may represent lead time bias and early initiation of PSA screening. The current clinical practice guidelines on PSA testing in Australia do not recommend a national PSA screening program [14]. However, recommendations in the guidelines include offering PSA testing every 2 years for men ages between 40 and 69 in men with a family history of prostate after being informed of the benefits and harms of testing. This recommendation contrasts to men without a positive family history where testing, if it occurs, is recommended to commence at age 50 years. Earlier studies such as Gronberg et al. [15] have shown no difference in overall-survival or prostate cancer specific survival in patients with and without a positive family history. The discrepancy with our work may be explained by the small sample size (302) and historic nature of the cohort (diagnosed between 1958 and 1990). During this time period there have been numerous changes to prostate cancer screening and treatment meaning that this work may not be generalizable to the clinical setting today.
Men with a family history of prostate cancer who were treated with radical prostatectomy did not appear to have different survival outcomes compared with men who did not have a family history. The results of our study are consistent with previous findings such as Bauer et al. [16], Brath et al. [17] and Bagshaw et al. [18], although contradict those reported by Kupelian et al. [19].
To date, there have been few published studies examining survival outcomes following radiation therapy and the impact of family history of prostate cancer [20]. The results of our study suggest that there is no survival difference in men receiving radiation therapy, comparing those with and without a positive family history, which is consistent with literature to date. While some cases of prostate cancer which are diagnosed in the setting of a positive family history are more aggressive, there is also an increase in low risk disease, presumably as a result of increased surveillance and investigation [21]. This later case may account for the results we observe in both surgical and radiotherapy treated cases.
Limitations
Our study has limitations including not having any data for family history of other cancers (e.g. pancreatic, breast, ovarian). These cancers may also be relevant in investigating genes that confer lifetime risks of prostate cancer, such as BRCA1 and BRCA2 [22,23,24]. The demographic and clinical differences between the groups may confound the observations made. While we have used multivariable modelling to adjust for potential confounders, the effect of residual confounding or unmeasured confounding cannot be eliminated, and may also be limited where data is missing. We note also that this cohort is drawn from an Australian cohort and country specific PSA screening and treatment practices may limit generalisability to other settings. Sample size also presents a limitation of the current work. An example of this is in the group of men treated with radiotherapy where the hazards ratio is similar in the subgroup compared with the overall cohort, but the estimate is less precise.
Conclusion
In summary, men with a family history of prostate cancer appear to have better overall survival outcomes. This group of men also appear to have a lower Gleason score at the time of diagnosis and be diagnosed at a lower age. Family history of prostate cancer was not associated with different survival outcomes in men who were treated with either radical prostatectomy or radiotherapy.
Availability of data and materials
Data is available upon request, but access may be subject to ethics and institutional governance approvals.
Abbreviations
- SA-PCCOC:
-
South Australian Prostate cancer clinical outcomes collaborative
- PSA:
-
Prostate specific antigen
- HR:
-
Hazards ratio
- CI:
-
Confidence interval
- PCa :
-
Prostate cancer
- NCCN:
-
National comprehensive cancer network
References
Johns L, Houlston R. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int. 2003;91(9):789–94.
Pomerantz MM, Freedman ML. Genetics of prostate cancer risk. Mt Sinai J Med. 2010;77(6):643–54. https://doi.org/10.1002/msj.20222.
Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed). 2014;6:15–30.
Liss MA, Chen H, Hemal S, Krane S, Kane CJ, Xu J, Kader AK. Impact of family history on prostate cancer mortality in white men undergoing prostate specific antigen based screening. J Urol. 2015;193(1):75–9. https://doi.org/10.1016/j.juro.2014.07.085.
Westerman ME, Gershman B, Karnes RJ, Thompson RH, Rangel L, Boorjian SA. Impact of a family history of prostate cancer on clinicopathologic outcomes and survival following radical prostatectomy. World J Urol. 2016;34(8):1115–22. https://doi.org/10.1007/s00345-015-1738-6.
Lee KL, Marotte JB, Ferrari MK, McNeal JE, Brooks JD, Presti JC. Positive family history of prostate cancer not associated with worse outcomes after radical prostatectomy. Urology. 2005;65(2):311–5.
Bova GS, Partin AW, Isaacs SD, Carter BS, Beaty TL, Isaacs WB, Walsh PC. Biological aggressiveness of hereditary prostate cancer: long-term evaluation following radical prostatectomy. J Urol. 1998;160(3 Pt 1):660–3.
Evans SM, Millar JL, Davis ID, Murphy DG, Bolton DM, Giles GG, Frydenberg M, Andrianopoulos N, Wood JM, Frauman AG, Costello AJ, McNeil JJ. Patterns of care for men diagnosed with prostate cancer in Victoria from 2008 to 2011. Med J Aust. 2013;198(10):540–5. https://doi.org/10.5694/mja12.11241.
Ruseckaite R, Beckmann K, O'Callaghan M, Roder D, Moretti K, Millar J, Evans S. A retrospective analysis of Victorian and south Australian clinical registries for prostate cancer: trends in clinical presentation and management of the disease. BMC Cancer. 2016;16:607. https://doi.org/10.1186/s12885-016-2655-9.
Network. NCC. (2017, February 21). Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, Graff RE, Holst K, Möller S, Unger RH, McIntosh C, Nuttall E, Brandt I, Penney KL, Hartman M, Kraft P, Parmigiani G, Christensen K, Koskenvuo M, Holm NV, Heikkilä K, Pukkala E, Skytthe A, Adami H-O, Kaprio J, Collaboration ftNTSoC (2016) familial risk and heritability of cancer among twins in Nordic Countries. JAMA 315 (1):68–76. doi:https://doi.org/10.1001/jama.2015.17703 JAMA.
Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, Benlloch S, Hazelett DJ, Wang Z, Saunders E, Leongamornlert D, Lindstrom S, Jugurnauth-Little S, Dadaev T, Tymrakiewicz M, Stram DO, Rand K, Wan P, Stram A, Sheng X, Pooler LC, Park K, Xia L, Tyrer J, Kolonel LN, Le Marchand L, Hoover RN, Machiela MJ, Yeager M, Burdette L, Chung CC, Hutchinson A, Yu K, Goh C, Ahmed M, Govindasami K, Guy M, Tammela TL, Auvinen A, Wahlfors T, Schleutker J, Visakorpi T, Leinonen KA, Xu J, Aly M, Donovan J, Travis RC, Key TJ, Siddiq A, Canzian F, Khaw KT, Takahashi A, Kubo M, Pharoah P, Pashayan N, Weischer M, Nordestgaard BG, Nielsen SF, Klarskov P, Roder MA, Iversen P, Thibodeau SN, McDonnell SK, Schaid DJ, Stanford JL, Kolb S, Holt S, Knudsen B, Coll AH, Gapstur SM, Diver WR, Stevens VL, Maier C, Luedeke M, Herkommer K, Rinckleb AE, Strom SS, Pettaway C, Yeboah ED, Tettey Y, Biritwum RB, Adjei AA, Tay E, Truelove A, Niwa S, Chokkalingam AP, Cannon-Albright L, Cybulski C, Wokolorczyk D, Kluzniak W, Park J, Sellers T, Lin HY, Isaacs WB, Partin AW, Brenner H, Dieffenbach AK, Stegmaier C, Chen C, Giovannucci EL, Ma J, Stampfer M, Penney KL, Mucci L, John EM, Ingles SA, Kittles RA, Murphy AB, Pandha H, Michael A, Kierzek AM, Blot W, Signorello LB, Zheng W, Albanes D, Virtamo J, Weinstein S, Nemesure B, Carpten J, Leske C, Wu SY, Hennis A, Kibel AS, Rybicki BA, Neslund-Dudas C, Hsing AW, Chu L, Goodman PJ, Klein EA, Zheng SL, Batra J, Clements J, Spurdle A, Teixeira MR, Paulo P, Maia S, Slavov C, Kaneva R, Mitev V, Witte JS, Casey G, Gillanders EM, Seminara D, Riboli E, Hamdy FC, Coetzee GA, Li Q, Freedman ML, Hunter DJ, Muir K, Gronberg H, Neal DE, Southey M, Giles GG, Severi G, Cook MB, Nakagawa H, Wiklund F, Kraft P, Chanock SJ, Henderson BE, Easton DF, Eeles RA, Haiman CA. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46(10):1103–9. https://doi.org/10.1038/ng.3094.
Amin Al Olama A, Dadaev T, Hazelett DJ, Li Q, Leongamornlert D, Saunders EJ, Stephens S, Cieza-Borrella C, Whitmore I, Benlloch Garcia S, Giles GG, Southey MC, Fitzgerald L, Gronberg H, Wiklund F, Aly M, Henderson BE, Schumacher F, Haiman CA, Schleutker J, Wahlfors T, Tammela TL, Nordestgaard BG, Key TJ, Travis RC, Neal DE, Donovan JL, Hamdy FC, Pharoah P, Pashayan N, Khaw KT, Stanford JL, Thibodeau SN, McDonnell SK, Schaid DJ, Maier C, Vogel W, Luedeke M, Herkommer K, Kibel AS, Cybulski C, Wokolorczyk D, Kluzniak W, Cannon-Albright L, Brenner H, Butterbach K, Arndt V, Park JY, Sellers T, Lin HY, Slavov C, Kaneva R, Mitev V, Batra J, Clements JA, Spurdle A, Teixeira MR, Paulo P, Maia S, Pandha H, Michael A, Kierzek A, Govindasami K, Guy M, Lophatonanon A, Muir K, Vinuela A, Brown AA, Freedman M, Conti DV, Easton D, Coetzee GA, Eeles RA, Kote-Jarai Z. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet. 2015;24(19):5589–602. https://doi.org/10.1093/hmg/ddv203.
Council. NHaMR. [2016, January 20] PSA testing and early management of test- detected prostate cancer. Retrieved from https://issuu.com/prostatecancerfoundationaus/docs/psa-testing-guidelines-overview?e=4459943/32754215.
Grönberg H, Damber L, Tavelin B, Damber JE. No difference in survival between sporadic, familial and hereditary prostate cancer. Br J Urol. 1998;82(4):564–7. https://doi.org/10.1046/j.1464-410x.1998.00801.x.
Bauer JJ, Srivastava S, Connelly RR, Sesterhenn IA, Preston DM, McLeod DG, Moul JW. Significance of familial history of prostate cancer to traditional prognostic variables, genetic biomarkers, and recurrence after radical prostatectomy. Urology. 1998;51(6):970–6.
Brath JM, Grill S, Ankerst DP, Thompson IM, Gschwend JE, Herkommer K. No detrimental effect of a positive family history on long-term outcomes following radical prostatectomy. J Urol. 2016;195(2):343–8.
Bagshaw H, Ruth K, Horwitz EM, Chen DY, Buyyounouski MK. Does family history of prostate cancer affect outcomes following radiotherapy? Radiother Oncol. 2014;110(2):229–34.
Kupelian PA, Klein EA, Witte JS, Kupelian VA, Suh JH. Familial prostate cancer: a different disease? J Urol. 1997;158(6):2197–201.
Bagshaw H, Ruth K, Horwitz EM, Chen DY, Buyyounouski MK. Does family history of prostate cancer affect outcomes following radiotherapy? Radiother Oncol. 2014;110(2):229–34. https://doi.org/10.1016/j.radonc.2013.11.014.
Jansson KF, Akre O, Garmo H, Bill-Axelson A, Adolfsson J, Stattin P, Bratt O. Concordance of tumor differentiation among brothers with prostate cancer. Eur Urol. 2012;62(4):656–61. https://doi.org/10.1016/j.eururo.2012.02.032.
Armstrong N, Ryder S, Forbes C, Ross J, Quek RG. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543–61. https://doi.org/10.2147/clep.S206949.
Jones MR, Kamara D, Karlan BY, Pharoah PDP, Gayther SA. Genetic epidemiology of ovarian cancer and prospects for polygenic risk prediction. Gynecol Oncol. 2017;147(3):705–13. https://doi.org/10.1016/j.ygyno.2017.10.001.
Kryklyva V, Haj Mohammad N, Morsink FHM, Ligtenberg MJL, Offerhaus GJA, Nagtegaal ID, de Leng WWJ, Brosens LAA. Pancreatic acinar cell carcinoma is associated with BRCA2 germline mutations: a case report and literature review. Cancer Biol Ther. 2019;20(7):949–55. https://doi.org/10.1080/15384047.2019.1595274.
Acknowledgements
The authors thank Tina Kopsaftis (SA-PCCOC Clinical Data Coordinator) and Scott Walsh (SA-PCCOC Data Manager) for their technical support in managing and compiling data. We also thank collaborating clinicians for their ongoing contributions and the men who participate in the registry.
Code availability
Code is available upon request.
Funding
Data included in this study were obtained from the South Australian Prostate Cancer Clinical Outcomes Collaborative (SA-PCCOC) database which has received funding from: Movember, the Urological Society of Australia and New Zealand, The Hospital Research Foundation, Mundi Pharma and the SAHMRI SA Cancer Council Beat Cancer initiative. No funders had any influence on the conception of this paper, analysis or reporting.
Author information
Authors and Affiliations
Consortia
Contributions
MA: first draft of manuscript; MB: critical review and clinical interpretation, MO’C: analysis, critical review of manuscript, supervision. All authors have read and approve the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Southern Adelaide Clinical Human Research Ethics Committee reviewed and approved the SA-PCCOC database, including analysis of data without identifiers, such as this study.
Participants in the SA-PCCOC database are involved under an ethics committee approved opt out consent process.
Consent for publication
None required.
Competing interests
No authors declare conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1 Supplementary Table 1
– Cox proportional hazards model – overall survival. Supplementary Table 2 – Fine and Grey model predicting prostate cancer specific mortality. Supplementary Table 3 - Cox proportional hazards model – overall survival – Radical prostatectomy. Supplementary Table 4- Cox proportional hazards model – overall survival – Radiation therapy
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ang, M., Borg, M., O’Callaghan, M.E. et al. Survival outcomes in men with a positive family history of prostate cancer: a registry based study. BMC Cancer 20, 894 (2020). https://doi.org/10.1186/s12885-020-07174-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-07174-9