Abstract
Background
Treatment of acute myeloid leukemia (AML) in elderly patients remains a great challenge. In this prospective single arm study (ChiCTR-OPC-15006492), we evaluated the efficacy and safety of a novel consolidation therapy with low-dose decitabine (LD-DAC) priming with intermediate-dose cytarabine (ID-Ara-C) followed by umbilical cord blood (UCB) infusion in elderly patients with AML.
Methods
A total of 25 patients with a median age of 64-years-old (60–74-years-old) who achieved complete remission (CR) after induction chemotherapy were enrolled in the study.
Results
The 2-year actual overall survival (OS) rate and leukemia-free survival (LFS) was 68.0 and 60.0%, respectively. The hematological and non-hematological toxicity were mild to moderate, and only one patient died in remission due to infection with possible acute graft versus host disease (aGVHD). Compared to a concurrent cohort of patients receiving conventional consolidation therapy, the study group tended to have an improved OS and LFS (p = 0.046 and 0.057, respectively), while the toxicity was comparable between the two groups.
Conclusions
This study suggested the novel combination of LD-DAC, ID-Ara-C, and UCB infusion might be an optimal consolidation therapy for elderly patients with AML, and a prospective phase III randomized study is warranted to confirm this observation.
Trial registration
This single-arm phase II clinical trial in elderly AML patients was registered prospectively at www.chictr.org.cn (identifier: ChiCTR-OPC-15006492) on June 2, 2015.
Similar content being viewed by others
Background
Acute myeloid leukemia (AML) is a common type of leukemia in adults, especially in older adults [1]. Although the overall survival (OS) of patients with AML has improved in the past three decades, this improvement has been limited to younger patients [2]. Several underlying factors contribute to poor outcomes, such as unfavorable cytogenetic abnormalities or mutation profiles, drug resistance, and intolerance of standard chemotherapy in elderly AML patients [3, 4]. Several attempts have been made to optimize the consolidation chemotherapy, but the overall outcome remains unsatisfactory [5,6,7,8,9,10]. Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) has become the standard therapeutic option for AML patients after complete remission. However, not all patients are eligible for HSCT, and a sizable proportion of patients may eventually die of transplantation-related mortality. Also, in a clinical setting, AML patients ≥60 years old are rarely provided with the option of HSCT. As a result, it is crucial to develop novel post-remission treatment strategies in elderly patients with AML.
Outside the setting of classical allo-HSCT, infusion of HLA-mismatched granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood cells combined with intensive chemotherapy has demonstrated promising clinical outcomes in AML patients [11]. Nevertheless, verification of these results is warranted. T cells in umbilical cord blood (UCB), an alternative source for allo-HSCT, were reported to possess superior anti-tumor effects compared with adult peripheral T cells [12]. In 2012, Majhail et al. [13] reported that UCB is a feasible option for AML/myelodysplastic syndrome (MDS) patients in the absence of suitable donors. Later, the same group retrospectively investigated the outcome of 10 AML/MDS patients older than 70-years-old who received UCB transplantation and reported a 2-year OS of 60%, similar to those who received HLA full-matched sibling donor transplantation [14].
Hypomethylating agents (HMAs), such as decitabine (DAC) or azacytidine, have demonstrated encouraging results in the treatment of myelodysplasia syndrome and more recently AML in elderly patients, especially among those who are not eligible for intensive chemotherapy. In addition, the combination of HMAs and chemotherapy agents may have synergistic effects in the induction of leukemia cell apoptosis, while augmenting natural killer (NK) cell responsiveness [15] and tumor specific cytotoxic T lymphocyte responses [16], as demonstrated in animal models and in human cells. Clinical studies have also shown that administration of decitabine prior to chemotherapy increases responsiveness [17] and results in higher complete remission (CR) rates [18]. Therefore, the addition of HMAs to cytoreductive treatment before stem cell infusion might improve the overall outcome.
Based on the aforementioned studies, we developed a novel consolidation therapy consisting of low-dose decitabine (LD-DAC) and priming intermediate-dose cytarabine (ID-Ara-C) combined with UCB infusion in elderly patients with AML. Here, we report its efficacy and safety in a single-arm phase II study.
Methods
Study enrollment and oversight
This was an investigator-initiated, prospective, nonrandomized, single-arm phase II clinical trial in elderly AML patients registered at www.chictr.org.cn (identifier: ChiCTR-OPC-15006492). The study was approved by the Human Ethics Committee of the Ruijin Hospital and was in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. Decitabine was provided free of charge by Chiatai Tianqing Pharma (China), which played no role in the study design, data collection, analysis, or writing of the manuscript.
Elderly patients with newly diagnosed AML, including MDS transformed AML or secondary AML, were eligible to participate in the study. Patients with acute promyelocytic leukemia or with a blast crisis of chronic myeloid leukemia were excluded. The induction chemotherapy consisted of daunorubicin hydrochloride (45 mg/m2) or idarubicin hydrochloride (8 mg/m2) for 3 days in combination with cytarabine (100 mg/m2) for 7 days, which was given for 1 or 2 cycles. After induction therapy, only those patients with documented CR defined according to standard criteria were enrolled in the study. Other inclusion criteria included an age of 60–75-years-old with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–3.
Interventions
Treatment protocol
The post-remission therapy included two cycles of low-dose decitabine [15 mg/m2 intravenously over 4 h for 5 consecutive days (day 1–5)], intermediate-dose cytarabine [1.0 g/m2 at q12 h for 2 days (day 6–7)] followed by infusion of one unit of UCB on day 9. Decitabine was provided free of charge by Chiatai Tianqing Pharma (China), which played no role in the study design, data collection, analysis, or writing of the manuscript. No immunosuppression was given as prophylaxis for graft versus host disease (GVHD), unless acute GVHD (aGVHD) was documented or clinically diagnosed. Infection prophylaxis and other support treatments, such as G-CSF, was administered according to a regular transplantation program. The comorbidities were assessed with the hematopoietic cell transplantation comorbidity index (HCT-CI) before each cycle of treatment. The second cycles of treatment were repeated in up to 2-month intervals. After two courses of post-remission therapy, patients received 1 course of Etoposide (100 mg intravenously for 5 consecutive days) and cytarabine (100 mg intravenously for 5 consecutive days), followed by 2 cycles of 6-mercaptopurine (25 mg/d d1–14), all-trans-retinoic acid (20 mg bid d29–56), and vitamin D3 (125 iu/d d57–84) sequential treatment as maintenance treatment.
The standard care for AML patients achieving first CR (CR1) at Ruijin Hospital is three courses of chemotherapy with Ara-C 0.5–1.0 g/m2 intravenously every 12 h for 3 days. We also collected clinical follow-up data of 24 elderly AML patients who received this traditional chemotherapy after CR1 (traditional chemotherapy group, TCG) for comparison of the clinical outcomes.
Selection of umbilical cord blood
High-resolution HLA typing for HLA-A, B, and DR loci were performed in all enrolled patients. The UCB units were obtained from the cord blood bank at the China Cord Blood Bank Network if they [1] were serologically matched for four or five of six HLAs, and [2] contained at least 3 × 107 nucleated cells/kg of recipient body weight before freezing (Additional file 1: Table S1).
Chimerism analysis
After treatment, peripheral-blood cells were obtained from all participants and then tested for chimerism by standard cytogenetic analysis and a semi-quantitative PCR-based analysis of the short tandem repeats with a sensitivity of 1%.
Monitoring of minimal residual disease
The detailed MRD detection process was described previously [19]. Monoclonal antibodies against 20 antigens as follows: CD34, CD38, CD117, HLA-DR, CD13, CD33, CD14, CD15, CD64, CD11b, CD7, CD56, CD2, CD4, CD19, MPO, TdT, cyCD3, cyCD79a, and CD45, were utilized. Leukemia associated immunophenotyping (LAIPs) was classified at diagnosis with different surface antigens. A cut-off value of 0.1% was set as minimal residual disease (MRD).
Study endpoints
The primary endpoint of our study is the 2-year leukemia-free survival (LFS). Secondary end points consisted of the 2-year OS, the incidence of hematological and non-hematological toxicity, median time to the recovery of neutropenia or platelets, incidence of aGVHD or chronic GVHD (cGVHD), 2-year incidence of treatment-related mortality (TRM), and documentation of chimerism in blood mononucleated cells. The LFS, OS, TRM, aGVHD and cGVHD were evaluated according to published criteria [20,21,22]. The time to hematopoietic recovery was determined as the duration from the end of chemotherapy to the time when the neutrophil count was > 0.5 × 109/L and when the platelet count was > 20 × 109/L without transfusion.
Study design and statistical analysis
We used the Simon’s two-stage optimal design for this phase II study [23]. We expected this novel treatment protocol might decrease or delay relapse and result in an improved LFS. A 25% increase in LFS was of interest to further a large-scale phase III clinical trial. Based on the study design, 11 patients will be accrued in the first phase, and if 6 patients or less remained in continuous remission, the study will be stopped. Otherwise, 14 additional patients will be accrued for a total of 25 patients. The null hypothesis will be rejected if 17 or more patients remain in LFS at 1 year. This design yields a type I error rate of 0.05 and power of 0.8. Severe toxicity of the treatment is closely monitored. The accrual of patients will be halted if excessive numbers of patients die in remission during the treatment until the last follow-up, that is, if the number of patients died in remission is equal to or exceeds bn out of n patients with full follow-up according to the Pocock-type stopping boundary (as shown in Additional file 2: Table S2) [24]. Data in the study were statistically analyzed using the Statistical Package for Social Science (SPSS version 22.0). Survival curves were plotted using the Kaplan-Meier method. A p value of less than 0.05 was considered statistically significant.
Results
Patients’ characteristics
From January 2015 to May 2017, a total of 25 patients 60–74-years-old with AML in CR1 were enrolled in the study according to the patient’s willingness to participate in this study (Table 1). The diagnoses were defined according to the French-American-British and World Health Organization criteria [20]. Cytogenetic studies on pretreatment bone marrow samples were performed according to the International System of Human Cytogenetic Nomenclature [25]. Screening for molecular markers AML1-ETO, CBFβ-MYH11, NPM1, FLT3-ITD, FLT3-TKD, CEBPA, MLL-PTD, TET2, N-RAS, and DNMT3A was performed, and the prognostic risk groups were defined according to the NLE 2017 criteria [26].
Twenty-four patients in TCG were also listed in Table 1. Overall, there was no significant difference in the patients’ characteristics except for consolidation chemotherapy.
Overall outcome
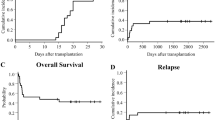
Upon the latest follow-up schedule as of May 2019, all patients have been followed-up for at least 2 years or met the primary endpoint. Fifteen of 25 patients remained in CR1, while 10 patients relapsed at a median of 16.5 months (range 4–32). Eight patients died of relapsed AML, and only one patient died of infection with possible aGVHD on day 20 after the first cycle of UCB treatment, which was characterized by persistent fever with antibiotics coverage, skin rash, liver function damage, and eventually development of multi-organ failure (MOF). The median OS and LFS for all patients was 31.9 months (range 4–53 months) and 29 months (range 4–53 months), respectively, with an actual 2-year OS and LFS at 68.0 and 60.0%, respectively.
As to the overall outcome, the actual 2-year OS (45.8%) and LFS (37.5%) in the TCG was inferior to the study group (p = 0.046 and 0.057, respectively) as shown in Fig. 1.
Hematological and non-hematological toxicities
The grade III to IV hematological toxicities including neutropenia and thrombocytopenia were common after treatment in both groups (as shown in Table 2). However, no hemorrhage was documented, and severe infection was diagnosed only in few cases (n = 2 in UCB and n = 3 in TCG). Also, the study group had an earlier hematological recovery than TCG. The median time to the recovery of the neutrophils was 11.9 days and 15.3 days, respectively, in UCB and TCG groups (p = 0.028). The recovery of platelets was also faster in the UCB group (11.5 days) compared to the TCG (18.8 days, p = 0.009).
Non-hematological toxicities were documented in up to 20% of patients, but were usually mild to moderate. In the UCB group, 1 patient experienced liver function damage, 5 experienced mucositis disorder, 2 had skin rash, and 3 were diagnosed cardiac disorder. Severe infection (sepsis) was documented in only 2 patients in the UCB group, and 1 patient developed infection together with clinically diagnosed aGVHD and eventually died of MOF, as shown in Table 2. Non-hematological toxicities were comparable between the UCB group and TCG.
Chimerism and GVHD
The chimerism was regularly tested on day 7 after UBC infusion. Of the 25 patients, only one patient (4%) had an established mixed chimerism level at 56.7%. For the remaining 24 patients, 20 (83.3%) had a very low level of micro-chimerism, with a range of 0.003–0.171%. For GVHD, only the aforementioned patient with a high level of mixed chimerism developed clinical signs of grade III aGVHD after UCB infusion and eventually died of infection and MOF 20 days after UCB infusion. No definite clinical aGVHD or cGVHD was observed in any other patients.
Treatment outcome by MRD level
In this study, we monitored the treatment response in patients by detecting LAIPs in bone marrow through flow cytometry after induction therapy, each cycle of consolidation therapy, and every 3 months afterwards. Patients with low MRD (≤ 0.1%) after induction therapy tend to have a better LFS in the study group compared to patients with an MRD level over 0.1% (76.9% vs. 41.7%, p = 0.173). Intriguingly, for patients maintaining a high MRD level (≥ 0.1%) after CR1, the 2-year LFS was significantly higher in the UCB group (41.7%) compared to those treated with conventional consolidation therapy (9.1%, p = 0.008)
Discussion
Although 50–60% of elderly AML patients can achieve CR with induction therapy, the consolidation was not well established. Conventional chemotherapy tends to have a poor outcome with a 2-year OS of about 10–20%. Juliusson et al. [27] reported the use of cytarabine in elderly AML patients as a consolidation therapy, and the 2-year OS and LFS remained at 25 and 22%, respectively. Recently, allo-HSCT in elderly AML patients became more prevalent in a clinical setting. Kasanon et al. [28] reported a 3-year OS of 38% in elderly AML patients receiving T-cell-repleted haploidentical HSCT.
Recent studies reported that cord blood T cells exert superior anti-tumor effects compared with adult peripheral blood T cells [12]. The novel micro-transplantation strategy with the infusion of G-CSF-mobilized HLA-mismatched donor peripheral-blood stem cells following high-dose cytarabine has also shown an encouraging outcome in the treatment AML, including elderly AML, with a 2-year OS and LFS of 50.2 and 42.1%, respectively [29].
Inspired by these reports of cellular therapy, we evaluated the efficacy and safety of a novel consolidation regimen consisting of low-dose decitabine priming with an intermediate dose cytarabine followed by UCB infusion. The inclusion of decitabine in the protocol was based on the evidence of previous studies. First, it has been shown that the sequence and combination of decitabine and cytarabine present synergy in the induction of cell apoptosis in leukemia cell lines [30]. Second, in myeloid leukemia cell lines, transient, low-dose decitabine exposure has been shown to induce CD80 gene expression in a variety of human leukemia cells, which provides evidence that epigenetic modulation can induce the expression of a major T cell co-stimulatory molecule on cancer cells, may overcome immune tolerance, and induce an efficient anti-tumor response [16]. More recently, in mice challenged with myeloid leukemia celli line THP-1, decitabine was the only hypomethylation agent that may enhance the anti-AML effect of CD34+ derived NK cells. In a clinical setting, two retrospective studies demonstrated that decitabine combined with cytarabine or low-dose chemotherapy, followed by the infusion of G-CSF mobilized peripheral blood, potentially improved the treatment outcomes in elderly AML patients [31, 32]. Interestingly, recent study reported that T cells from UCB possess more anti-leukemia potential than T cells from adult peripheral T cells. On the other hand, UCB may exert effects in promoting hematopoiesis, modulating NK cell responsiveness [33, 34], and was associated with a reduced risk of GVHD compared to mobilized peripheral blood, while maintaining similar graft-versus-leukemia effects (GVL) in HSCT settings [34]. Another important reason to choose UCB rather than G-CSF mobilized peripheral blood from haplo-identical family donors is to save the potential donors for future salvage of allo-HSCT.
In the present study, we reported a 2-year OS of 68.0% and a 2-year LFS of 60.0% in older patients with AML, while only 1 patient died in remission. The hematological and non-hematological toxicities were mostly mild to moderate, which were also comparable to conventional consolidation chemotherapy. Of greater interest, we noticed that patients receiving the novel protocol tend to have a more rapid recovery of platelet counts and significantly higher platelet levels after recovery. The explanation might be the protective effects of LD-DAC on megakaryocytes and its maturation and platelet production, as shown in the mouse model, or possible unidentified effects conferred by UCB [35]. This may provide an important advantage in the novel regimen, because the improvement of both OS and LFS may be attributed to the fact that the novel consolidation was more tolerable to elderly patients, while the standard consolidation chemotherapy may cause slow hematopoietic recovery, leading to a delay in the treatment schedule and/or dose reduction, thus impeding the overall outcome. Therefore, we considered that the novel treatment is feasible and perhaps the more favorable choice for the treatment of elderly patients with AML.
As MRD monitoring plays an important role in AML [36,37,38,39], we also regularly monitored the bone marrow (BM) MRD level by flow cytometry. Patients with low bone marrow MRD levels (≤ 0.1%) immediately after induction therapy tend to have a better LFS. However, this difference is not statistically significant, probably due to the limited number of patients. Of note, when we compared the outcome of patients who failed to obtain the low MRD level after induction therapy, the 2-year LFS was significantly higher in the study group (41.7%) compared to those treated with conventional consolidation therapy (9.1%, p = 0.008). Therefore, our data suggest that patients with higher MRD levels after induction therapy can still benefit from LD-DAC combined chemotherapy with UCB infusion. During subsequent MRD monitoring after 2 cycles, LD-DAC chemotherapy combined with UCB treatment showed a reduced relapse potential in patients who achieved low MRD levels. This observation suggests LD-DAC chemotherapy combined with UCB infusion may achieve promising outcomes in patients who achieved low MRD levels early after induction or consolidation therapy. However, longer follow-up is required to confirm this observation.
Conclusion
Based on the study design, our data suggests that LD-DAC combined with ID-Ara-C with UCB infusion has the potential to improve the overall outcome in elderly AML patients. One limitation of the study is the small sample size and relative short follow-up. A prospective, randomized, phase 3 confirmatory study with a sufficient number of patients with long follow-up is warranted to further confirm our observation; a multi-center study is underway to compare the long-term outcomes of the novel approach versus conventional chemotherapy consolidation in elderly AML patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- aGVHD:
-
Acute graft versus host disease
- Allo-HSCT:
-
Allogeneic hematopoietic stem cell transplantation
- AML:
-
Acute myeloid leukemia
- BM:
-
Bone marrow
- cGVHD:
-
Chronic GVHD
- CR:
-
Complete remission
- DAC:
-
Decitabine
- ECOG:
-
Eastern Cooperative Oncology Group
- G-CSF:
-
Ranulocyte colony-stimulating factor
- GVHD:
-
Graft versus host disease
- GVL:
-
Graft-versus-leukemia effect
- HCT-CI:
-
Hematopoietic cell transplantation comorbidity index
- HMAs:
-
Hypomethylating agents
- ID-Ara-C:
-
Intermediate-dose cytarabine
- LAIPs:
-
Leukemia associated immunophenotyping
- LD-DAC:
-
Low-dose decitabine
- LFS:
-
Leukemia-free survival
- MDS:
-
Myelodysplastic syndrome
- MOF:
-
Multi-organ failure
- MRD:
-
Minimal residual disease
- NK:
-
Natural killer
- OS:
-
Overall survival
- PS:
-
Performance status
- TRM:
-
Treatment-related mortality
- UCB:
-
Umbilical cord blood
References
Juliusson G, Lazarevic V, Horstedt A, et al. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119(17):3890–9.
Thein MS, Ershler WB, Jemal A, et al. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over three decades. Cancer. 2013;119(15):2720–7.
Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5.
Rao AV, Valk PJ, Metzeler KH, et al. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(33):5580–6.
Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–9.
Krug U, Röllig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376(9757):2000–8.
Löwenberg B, Ossenkoppele GJ, Van PW, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235.
Mcclune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(11):1878.
Kersey JH. The role of allogeneic-cell transplantation in leukemia. N Engl J Med. 2010;363(22):2158.
Devine SM, Owzar K, Blum W, et al. Phase II study of allogeneic transplantation for older patients with acutemyeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from Cancer and leukemia group B 100103 (Alliance for clinical trials in oncology)/blood and marrow transplant clinical trial network 0502. J Clin Oncol. 2015;33(35):4167–75.
Ai H, Guo M, Chao NJ. Study limitations in HLA-mismatched microtransplant in older patients newly diagnosed with acute myeloid leukemia—reply. JAMA Oncol. 2018;4(6):891.
Hiwarkar P, Qasim W, Ricciardelli I, et al. Cord blood T cells mediate enhanced anti-tumor effects compared with adult peripheral blood T cells. Blood. 2015;126(26):2882–91.
Majhail NS, Brunstein CG, Shanley R, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012;47(4):494.
Sandhu KS, Brunstein C, Defor T, et al. Umbilical cord blood transplantation outcomes in acute myelogenous leukemia/myelodysplastic syndromes patients ≥70 years old. Biol Blood Marrow TransplantJ Am Soc Blood Marrow Transplant. 2016;22(2):390.
Schmiedel BJ, Arélin V, Gruenebach F, et al. Azacytidine impairs NK cell reactivity while decitabine augments NK cell responsiveness toward stimulation. Int J Cancer. 2011;128(12):2911–22.
Wang LX, Mei ZY, Zhou JH, et al. Low dose decitabine treatment induces CD80 expression in cancer cells and stimulates tumor specific cytotoxic T lymphocyte responses. PLoS One. 2013;8(5):e62924.
Scandura JM, Roboz GJ, Moh M, et al. Phase 1 study of epigenetic priming with decitabine prior to standard induction chemotherapy for patients with AML. Blood. 2011;118(6):1472.
Jianyong L, Yaoyu C, Yu Z, et al. Efficacy and safety of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in newly diagnosed elderly patients with acute myeloid leukemia. Oncotarget. 2015;6(8):6448–58.
Sui J-N, Chen Q-S, Zhang Y-X, Sheng Y, Wu J, Li J-M, Weng X-Q, Chen B. Identifying leukemia-associated Immunophenotype-based individualized minimal residual disease in acute myeloid leukemia and its prognostic significance. Am J Hematol. 2019;94(5):528–38. https://doi.org/10.1002/ajh.25431.
Cheson BD, Bennett JM, Kopecky KJ, et al. International working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. Revised recommendations of the international working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21(24):4642–9.
Przepiorka D,Weisdorf D. Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–8.
Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, I: diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56.
Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10.
Ivanova A, Qaqish BF, Schell MJ. Continuous toxicity monitoring in phase II trials in oncology. Biometrics. 2015;61(2):540–5.
Shaffer LG, McGowan-Jordan J, Schmid M. ISCN 2013: An international system for human cytogenetic nomenclature. 2013. Unionville: S Karger; 2012.
Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood. 2009;113(18):4179–87.
Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of Nonmyeloablative HLA-Haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(28):3152–61.
Guo M, Chao NJ, Li JY, et al. HLA-mismatched microtransplant in older patients newly diagnosed with acute myeloid leukemia: results from the microtransplantation interest group. JAMA Oncol. 2018;4(1):54.
Qin T, Youssef EM, Jelinek J, et al. Effect of cytarabine and decitabine in combination in human leukemic cell lines. Clin Cancer Res. 2007;13(14):4225–32.
Cox ST, Laza-Briviesca R, Pearson H, et al. Umbilical cord blood plasma contains soluble NKG2D ligands that mediate loss of natural killer cell function and cytotoxicity. Eur J Immunol. 2015;45(8):2324–34.
Rieber N, Gille C, Köstlin N, et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin Exp Immunol. 2014;174(1):45–52.
Li WY, Wang Y, Chen SN, et al. Consolidation therapy with decitabine and intermediate-dose cytarabine followed by HLA-mismatched peripheral blood stem cells infusion for older patients with acute myeloid leukemia in first remission[J]. Leukemia Lymphoma. 2017:1-7.
Lu RN, Miao KR, Zhang R, et al. Haploidentical hematopoietic stem cell transplantation following myeloablative conditioning regimens in hematologic diseases with G-CSF-mobilized peripheral blood stem cells grafts without T cell depletion: a single center report of 38 cases. Med Oncol. 2014;31(8):81.
Wang J, Yi Z, Wang S, et al. The effect of decitabine on megakaryocyte maturation and platelet release. Thromb Haemost. 2011;105(02):337–43.
Al-Mawali A, Gillis D, Lewis I. The role of multiparameter flow cytometry for detection of minimal residual disease in acute myeloid leukemia. Am J Clin Pathol. 2009;131(1):16–26.
Terwijn M, van Putten WL, Kelder A, van der Velden VH, Brooimans RA, Pabst T, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31(31):3889–97.
Freeman SD, Virgo P, Couzens S, Grimwade D, Russell N, Hills RK, et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol. 2013;31(32):4123–31.
Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol. 2013;10(8):460–71.
Acknowledgements
We would like to express appreciation to the research assistants for their diligence and attentiveness to detail and the outstanding clinical care delivered by all staff members.
Funding
The design of the study was supported by the National Natural Science Foundation of the People’s Republic of China (No. 81500162, No. 81770144, No. 81870110) and Shanghai Collaborative Innovation Program on Regenerative Medicine and Stem Cell Research (2019CXJQ01). The collection, analysis, and interpretation of data was supported by the Clinical Research Plan of SHDC (16CR1034B) and Clinical Research Center of Shanghai Jiao Tong University School of Medicine (DLY201513).
Author information
Authors and Affiliations
Contributions
JH and JL designed the study and served as principal investigators. XL and YL carried out the research, enrolled patients, included the initial data, and reviewed the manuscript. YD, RR, WW and HZ collected, analyzed, and interpreted the data. JH, YZ and XL carried out statistical analysis. XL and YD wrote the manuscript, and all authors contributed to the final draft.All authors read and approved the final manuscript
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Human Ethics Committee of the Ruijin Hospital and was in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients, including the patient who died during the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Decitabine was provided free of charge by Chiatai Tianqing Pharma (China), which played no role in the study design, data collection, analysis, or writing of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. HLA match status from patient to donor. (DOCX 17 kb)
Additional file 2:
Table S2. Continuous monitoring for severe toxicity by Pocock-type boundary. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, X., Dong, Y., Li, Y. et al. Low-dose decitabine priming with intermediate-dose cytarabine followed by umbilical cord blood infusion as consolidation therapy for elderly patients with acute myeloid leukemia: a phase II single-arm study. BMC Cancer 19, 819 (2019). https://doi.org/10.1186/s12885-019-5975-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-5975-8